Effect Analysis of Mineral Salt Concentrations on Nosiheptide Production by Streptomyces actuosus Z-10 Using Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Critical Salts by PBD
| Runs | Real Levels (Coded Levels) | Nsh Production (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CaCO3 (g/L) | Na2SO4(g/L) | K2HPO4·3H2O (g/L) | MnSO4·H2O (g/L) | MgSO4·7H2O (g/L) | FeSO4·7H2O (g/L) | CuSO4·5H2O (g/L) | ZnSO4·7H2O (g/L) | ||
| 1 | 7.5 (+) | 1.0 (−) | 0.5 (+) | 0.1 (−) | 1.0 (−) | 0.01 (−) | 0.03 (+) | 0.09 (+) | 840.3 ± 21.3 |
| 2 | 7.5 (+) | 5.0 (+) | 0.1 (−) | 0.5 (+) | 1.0 (−) | 0.01 (−) | 0.01 (+) | 0.09 (+) | 1258.6 ± 30.6 |
| 3 | 2.5 (−) | 5.0 (+) | 0.5 (+) | 0.1 (−) | 5.0 (+) | 0.01 (−) | 0.01 (−) | 0.03 (−) | 1121.5 ± 33.6 |
| 4 | 7.5 (+) | 1.0 (−) | 0.5 (+) | 0.5 (+) | 1.0 (−) | 0.05 (+) | 0.01 (−) | 0.03 (−) | 940.3 ± 26.2 |
| 5 | 7.5 (+) | 5.0 (+) | 0.1 (−) | 0.5 (+) | 5.0 (+) | 0.01 (−) | 0.03 (+) | 0.03 (−) | 1341.2 ± 31.5 |
| 6 | 7.5 (+) | 5.0 (+) | 0.5 (+) | 0.1 (−) | 5.0 (+) | 0.05 (+) | 0.01 (−) | 0.09 (+) | 1128.5 ± 27.9 |
| 7 | 2.5 (−) | 5.0 (+) | 0.5 (+) | 0.5 (+) | 1.0 (−) | 0.05 (+) | 0.03 (+) | 0.03 (−) | 1160.4 ± 22.3 |
| 8 | 2.5 (−) | 1.0 (−) | 0.5 (+) | 0.5 (+) | 5.0 (+) | 0.01 (−) | 0.03 (+) | 0.09 (+) | 1100.9 ± 25.4 |
| 9 | 2.5 (−) | 1.0 (−) | 0.1 (−) | 0.5 (+) | 5.0 (+) | 0.05 (+) | 0.01 (−) | 0.09 (+) | 1029.6 ± 31.5 |
| 10 | 7.5 (+) | 1.0 (−) | 0.1 (−) | 0.1 (−) | 5.0 (+) | 0.05 (+) | 0.03 (+) | 0.03 (−) | 951.3 ± 29.7 |
| 11 | 2.5 (−) | 5.0 (+) | 0.1 (−) | 0.1 (−) | 1.0 (−) | 0.05 (+) | 0.03 (+) | 0.09 (+) | 833.7 ± 23.6 |
| 12 | 2.5 (−) | 1.0 (−) | 0.1 (−) | 0.1 (−) | 1.0 (−) | 0.01 (−) | 0.01 (−) | 0.03 (−) | 801.7 ± 19.8 |
| Variables # | Effect | Coefficient | SE-Coefficient | T-Value | p-Value |
|---|---|---|---|---|---|
| Constant | 1042.33 | 18.16 | 57.41 | 0.000 | |
| X1 | 68.73 | 34.37 | 18.16 | 1.89 | 0.155 |
| X2 | 196.63 | 98.32 | 18.16 | 5.42 | 0.012 * |
| X3 | 12.63 | 6.32 | 18.16 | 0.35 | 0.751 |
| X4 | 192.33 | 96.17 | 18.16 | 5.30 | 0.013 * |
| X5 | 139.67 | 69.83 | 18.16 | 3.85 | 0.031 * |
| X6 | −70.07 | −35.03 | 18.16 | −1.93 | 0.149 |
| X7 | −8.73 | −4.37 | 18.16 | −0.24 | 0.825 |
| X8 | −20.80 | −10.40 | 18.16 | −0.57 | 0.607 |
| R2 | 96.38% | ||||
| Adj R2 | 86.74% |
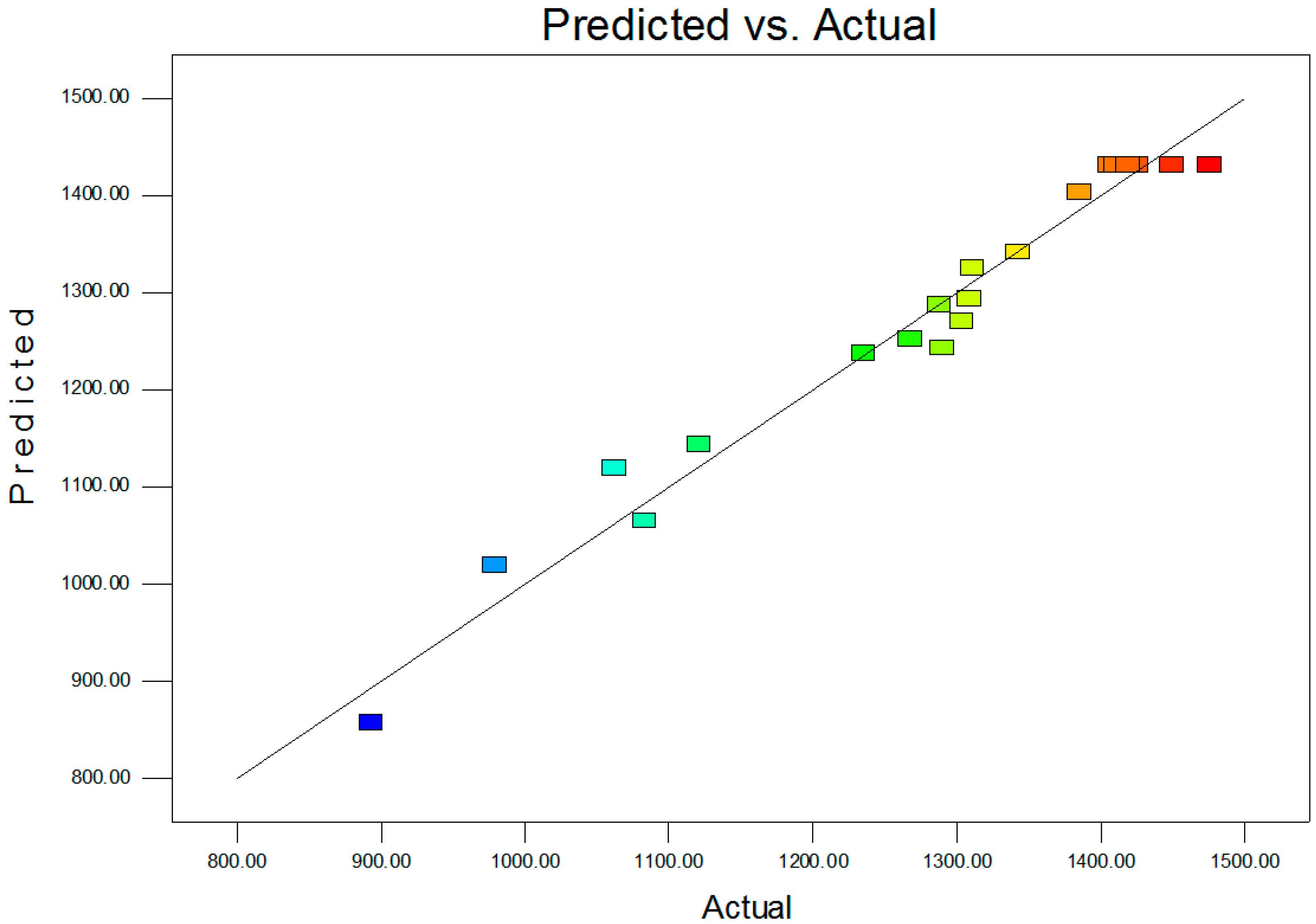
2.2. Statistical Optimization with CCD
| Runs | Real Levels (Coded Levels) | Nsh Production (mg/L) | |||
|---|---|---|---|---|---|
| Na2SO4(g/L) | MnSO4·H2O (g/L) | MgSO4·7H2O (g/L) | Actual | Predicted | |
| 1 | 1.05 (−1) | 0.08 (−1) | 0.84 (−1) | 892.4 ± 25.8 | 857.6 |
| 2 | 3.94 (1) | 0.08 (−1) | 0.84 (−1) | 1303.5 ± 37.7 | 1271.5 |
| 3 | 1.05 (−1) | 0.31 (1) | 0.84 (−1) | 1083.2 ± 41.9 | 1065.7 |
| 4 | 3.94 (1) | 0.31 (1) | 0.84 (−1) | 1310.8 ± 40.9 | 1325.5 |
| 5 | 1.05 (−1) | 0.08 (−1) | 3.15 (1) | 1290.3 ± 33.4 | 1243.5 |
| 6 | 3.94 (1) | 0.08 (−1) | 3.15 (1) | 1308.5 ± 37.6 | 1293.9 |
| 7 | 1.05 (−1) | 0.31 (1) | 3.15 (1) | 1342.2 ± 35.7 | 1342.2 |
| 8 | 3.94 (1) | 0.31 (1) | 3.15 (1) | 1235.7 ± 29.8 | 1238.4 |
| 9 | 0 (−1.732) | 0.2 (0) | 2.0 (0) | 978.3 ± 26.8 | 1019.8 |
| 10 | 5.0 (1.732) | 0.2 (0) | 2.0 (0) | 1287.2 ± 31.4 | 1288.4 |
| 11 | 2.5 (0) | 0 (−1.732) | 2.0 (0) | 1061.7 ± 22.4 | 1120.1 |
| 12 | 2.5 (0) | 0.4 (1.732) | 2.0 (0) | 1267.9 ± 39.8 | 1252.3 |
| 13 | 2.5 (0) | 0.2 (0) | 0 (−1.732) | 1120.1 ± 27.3 | 1144.6 |
| 14 | 2.5 (0) | 0.2 (0) | 4.0 (1.732) | 1385.2 ± 30.8 | 1403.4 |
| 15 | 2.5 (0) | 0.2 (0) | 2.0 (0) | 1475.9 ± 30.5 | 1431.3 |
| 16 | 2.5 (0) | 0.2 (0) | 2.0 (0) | 1406.2 ± 33.4 | 1431.3 |
| 17 | 2.5 (0) | 0.2 (0) | 2.0 (0) | 1411.1 ± 28.4 | 1431.3 |
| 18 | 2.5 (0) | 0.2 (0) | 2.0 (0) | 1449.2 ± 33.1 | 1431.3 |
| 19 | 2.5 (0) | 0.2 (0) | 2.0 (0) | 1425.7 ± 25.3 | 1431.3 |
| 20 | 2.5 (0) | 0.2 (0) | 2.0 (0) | 1419.5 ± 28.3 | 1431.3 |
| Term # | DF | Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|
| Model | 9 | 4.975 × 105 | 36.85 | <0.0001 ** |
| X2 | 1 | 84154.24 | 56.11 | <0.0001 ** |
| X4 | 1 | 20394.91 | 13.60 | 0.0042 ** |
| X5 | 1 | 78146.16 | 52.10 | <0.0001 ** |
| X2X4 | 1 | 11873.40 | 7.92 | 0.0184 * |
| X2X5 | 1 | 66066.12 | 44.05 | <0.0001 ** |
| X4X5 | 1 | 5995.12 | 4.00 | 0.0735 |
| X22 | 1 | 1.344 × 105 | 89.61 | <0.0001 ** |
| X42 | 1 | 1.051 × 105 | 70.08 | <0.0001 ** |
| X52 | 1 | 43262.75 | 28.84 | 0.0003 ** |
| Residual | 10 | 14999.35 | ||
| lack-of-fit | 5 | 11481.14 | 3.26 | 0.1101 |
| Pure error | 5 | 3518.21 | ||
| Corrected Total | 19 | 5.125 × 105 | ||
| R2 | 0.9707 | PredR2 | 0.8176 | |
| AdjR2 | 0.9444 | Adeq Precision | 20.948 |
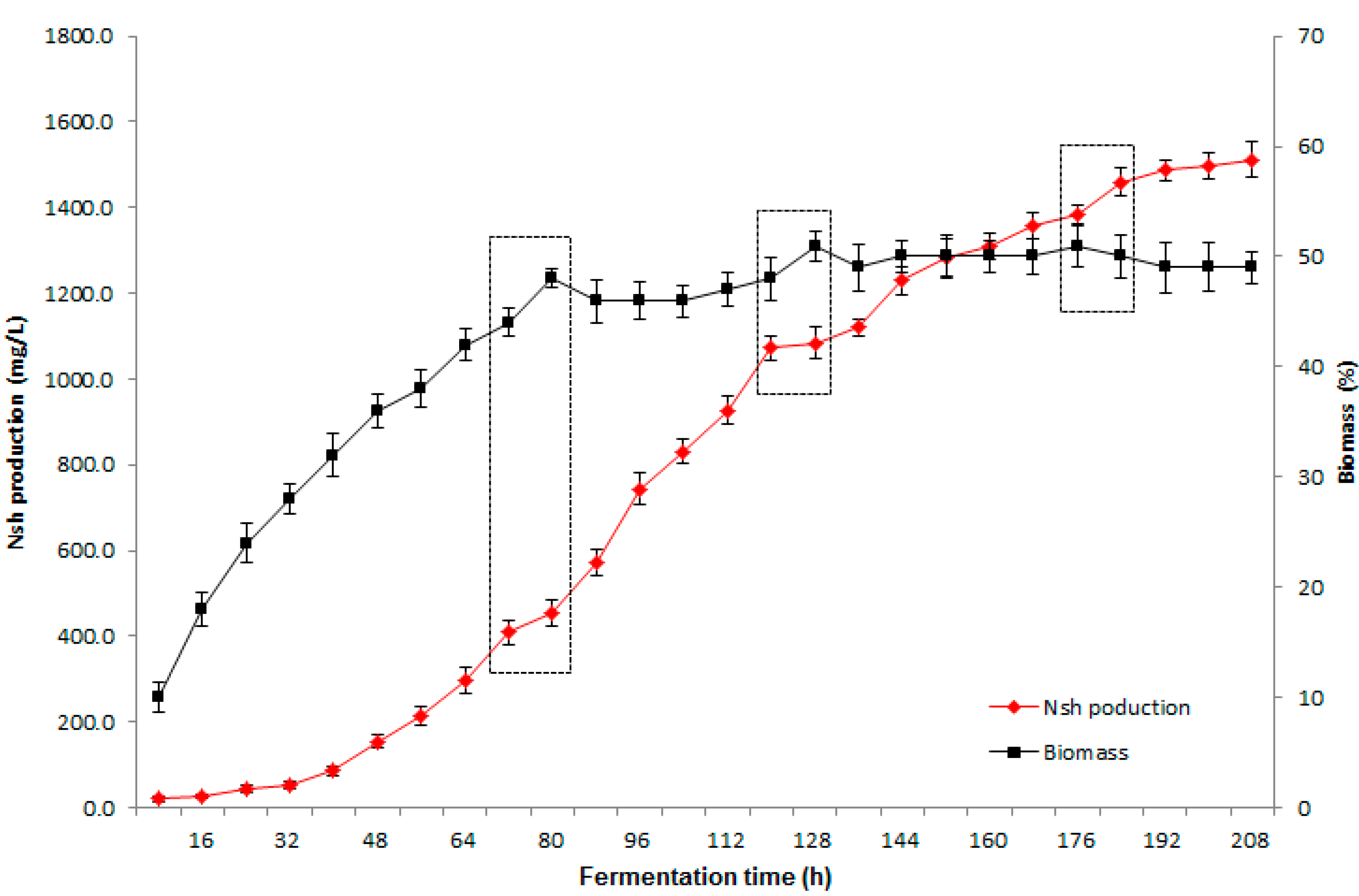
2.3. Verification of Optimal Conditions in Scale-up Fermentation

3. Experimental Section
3.1. Microorganisms and Chemicals
3.2. Fermentation Conditions
3.3. Plackett-Burman Design
3.4. Central Composite Design

3.5. Scale-up Fermentation Process
3.6. Analysis of Nsh Concentration

3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prange, T.; Ducruix, A.; Pascard, C.; Lunel, J. Structure of nosiheptide, a polythiazole-containing antibiotic. Nature 1977, 265, 189–190. [Google Scholar]
- Baumann, S.; Schoof, S.; Bolten, M.; Haering, C.; Takagi, M.; Shin-ya, K.; Arndt, H.D. Molecular determinants of microbial resistance to thiopeptide antibiotics. J. Am. Chem. Soc. 2010, 132, 6973–6981. [Google Scholar]
- Krinke, L.; Jamroz, D. Effects of feed antibiotic avoparcine on organ morphology in broiler chickens. Poult. Sci. 1996, 75, 705–710. [Google Scholar]
- Nikaido, H. Multidrug resistance in bacteria. Ann. Rev. Biochem. 2009, 78, 119–146. [Google Scholar]
- Kelly., W.L.; Pan, L.; Li, C. Thiostrepton biosynthesis: Prototype for a new family of bacteriocins. J. Am. Chem. Soc. 2009, 131, 4327–4334. [Google Scholar]
- Lu, J.Y.; Riedrich, M.; Mikyna, M.; Arndt, H.D. Aza-Wittig-supported synthesis of the A ring of nosiheptide. Angew. Chem. Int. Ed. Engl. 2009, 48, 8137–8140. [Google Scholar]
- Schoof, S.; Arndt, H.D. D-cysteine occurrence in thiostrepton may not necessitate an epimerase. Chem. Commun. 2009, 14, 7113–7115. [Google Scholar]
- Kimber, M.C.; Moody, C.J. Construction of macrocyclic thiodepsipeptides: Synthesis of a nosiheptide “southern hemisphere” model system. Chem. Commun. 2008, 13, 591–593. [Google Scholar]
- Martin, T.; Laguerre, C.; Hoarau, C.; Marsais, F. Highly efficient borylation Suzuki coupling process for 4-bromo-2-ketothiazoles: Straightforward access to micrococcinate and saramycetate esters. Org. Lett. 2009, 11, 3690–3693. [Google Scholar]
- Lu, J.Y.; Riedrich, M.; Wojtas, K.P.; Arndt, H.D. Assembly of the Nosiheptide A-Ring: A fruitful lesson. Synthesis 2013, 45, 1300–1311. [Google Scholar]
- Niu, D.P.; Wang, F.L.; Zhang, L.L.; He, D.K.; Jia, M.X. Neural network ensemble modeling for nosiheptide fermentation process based on partial least squares regression. Chemom. Intell. Lab. 2011, 105, 125–130. [Google Scholar]
- He, S.; Wang, H.Q.; Wu, B.; Zhou, H.; Zhu, P.; Yang, R.; Yan, X.J. Response surface methodology optimization of fermentation conditions for rapid and efficient accumulation of macrolactin A by marine Bacillus amyloliquefaciens ESB-2. Molecules 2013, 18, 408–417. [Google Scholar]
- Sansonetti, S.; Curcio, S.; Calabrò, V.; Iorio, G. Optimization of ricotta cheese whey (RCW) fermentation by response surface methodology. Bioresour. Technol. 2010, 101, 9156–9162. [Google Scholar]
- Liu, J.L.; Zheng, S.L.; Fan, Q.J.; Yuan, J.C.; Yang, S.M.; Kong, F.L. Optimization of high-pressure ultrasonic-assisted simultaneous extraction of six major constituents from Ligusticum chuanxiong rhizome using response surface methodology. Molecules 2014, 19, 1887–191. [Google Scholar]
- Zhao, H.L.; Yang, J.; Chen, X.L.; Su, H.N.; Zhang, X.Y.; Huang, F.; Zhou, B.C.; Xie, B.B. Optimization of fermentation conditions for the production of the M23 protease Pseudoalterin by deep-sea Pseudoalteromonas sp. CF6-2 with artery powder as an inducer. Molecules 2014, 19, 4779–4790. [Google Scholar]
- Nwodo, U.U.; Okoh, A.I. Mixed culture fermentation and media optimization by response surface model: Streptomyces and brachybacterium species in bioflocculant production. Molecules 2014, 19, 11131–11144. [Google Scholar]
- Nwodo, U.U.; Green, E.; Mabinya, L.V.; Okaiyeto, K.; Rumbold, K.; Obi, L.C.; Okoh, A.I. Bioflocculant production by a consortium of Streptomyces and Cellulomonas species and media optimization via surface response model. Colloids Surf. B Biointerfaces 2014, 116, 257–264. [Google Scholar]
- Weinberg, E.D. Mineral element control of microbial secondary metabolism. In Microorganisms and Minerals; Weinberg, E.D., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1977; pp. 289–316. [Google Scholar]
- Mirhosseini, H.; Tan, C.P.; Taherian, A.R.; Boo, H.C. Modeling the physicochemical properties of orange beverage emulsion as function of main emulsion components using response surface methodology. Carbohydr. Polym. 2009, 75, 512–520. [Google Scholar]
- Raza, W.; Wu, H.S.; Shen, Q.R. Use of response surface methodology to evaluate the effect of metal ions by Paenibacillus polymyxa. Bioresour. Technol. 2010, 101, 1904–1912. [Google Scholar]
- Lei, J.; He, W.Q.; Wang, Y.G. Regulatory mechanism of morphological differentiation and secondary metabolism in Streptomyces. Pharm. Biotechnol. 2007, 14, 225–229. [Google Scholar]
- Wang, Y.J.; Xue, F.; Wu, Y.F.; Xue, Y.P.; Zheng, Y.G. Development of macrolide lactone antibiotic brefeldin A fermentation process with Eupenicillium brefeldianum ZJB082702. J. Biosci. Bioeng. 2012, 114, 262–267. [Google Scholar]
- Mander, P.; Choi, Y.H.; Seong, J.H.; Na, B.H.; Cho, S.S.; Lee, H.J.; Yoo, J.C. Statistical optimization of a multivariate fermentation process for enhancing antibiotic activity of Streptomyces sp. CS392. Arch. Pharm. Res. 2013, 36, 973–980. [Google Scholar]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar]
- Niu, D.P.; Jia, M.X.; Wang, F.L.; He, D.K. Optimization of nosiheptide fed-batch fermentation process based on hybrid model. Ind. Eng. Chem. Res. 2013, 52, 3373–3380. [Google Scholar]
- Zhou, P.; Li, X.; Li, J.Y.; Jiang, Y. Study on enhancing production of nosiheptide. Chin. J. Pharm. 1995, 26, 438–440. [Google Scholar]
- Wan, R.; Liu, X.H.; Li, J.Y.; Zhou, P. Breeding of S. actuosus and optimize fermentation conditions. Fudan Univ. Med. Sci. 2004, 31, 579–581. [Google Scholar]
- Cover, W.H.; Kirpekar, A.C.; George, H. A calcium inhibition of efrotomycin production by Nocardia lactamdurans. J. Ind. Microbiol. Biotechnol. 1991, 7, 41–44. [Google Scholar]
- Wolf, F.I.; Cittadini, A. Magnesium in cell proliferation and differentiation. Front. Biosci. 1999, 4, 607–617. [Google Scholar]
- Kang, Y.; Wang, Y.H.; Zhuang, Y.P.; Chu, J.; Zhang, S.L. Influence of Mn2+ on the biotechmycin fermentation. Acta Microbiol. Sin. 2005, 45, 81–85. [Google Scholar]
- Choi, S.B.; Lew, L.C.; Hor, K.C.; Liong, M.T. Fe2+ and Cu2+ increase the production of hyaluronic acid by Lactobacilli via affecting different stages of the pentose phosphate pathway. Appl. Biochem. Biotechnol. 2014, 173, 129–142. [Google Scholar]
- Liao, R.J.; Duan, L.; Lei, C.; Pan, H.X.; Ding, Y.; Zhang, Q.; Chen, D.J.; Shen, B.; Yu, Y.; Liu, W. Thiopeptide biosynthesis featuring ribosomally synthesized precursor peptides and conserved posttranslational odifications. Chem. Biol. 2009, 16, 141–147. [Google Scholar]
- Yu, Y.I.; Duan, L.; Zhang, Q.; Liao, R.J.; Ding, Y.; Pan, H.X.; Wendt-Pienkowski, E.; Tang, G.L.; Shen, B.; Liu, W. Nosiheptide biosynthesis featuring a unique indole side ring formation on the characteristic thiopeptide framework. ACS Chem. Biol. 2009, 4, 855–864. [Google Scholar]
- Yu, Y.; Guo, H.; Zhang, Q.; Duan, L.; Ding, Y.; Liao, R.J.; Lei, C.; Shen, B.; Liu, W. NosA catalyzing carboxyl-terminal amide formation in nosiheptide maturation via an enamine dealkylation on the serine-extended precursor peptide. J. Am. Chem. Soc. 2010, 132, 16324–16326. [Google Scholar]
- Zhang, X.C.; Fen, M.Q.; Shi, X.L.; Bai, L.Q.; Zhou, P. Overexpression of yeast S-adenosylmethionine synthetase metK in Streptomyces actuosus leads to increased production of nosiheptide. Appl. Microbiol. Biotechnol. 2008, 78, 991–995. [Google Scholar]
- Sample Availability: Sample of nosiheptide is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Liu, X.; Zhang, P.; Zhou, P.; Shi, X. Effect Analysis of Mineral Salt Concentrations on Nosiheptide Production by Streptomyces actuosus Z-10 Using Response Surface Methodology. Molecules 2014, 19, 15507-15520. https://doi.org/10.3390/molecules191015507
Zhou W, Liu X, Zhang P, Zhou P, Shi X. Effect Analysis of Mineral Salt Concentrations on Nosiheptide Production by Streptomyces actuosus Z-10 Using Response Surface Methodology. Molecules. 2014; 19(10):15507-15520. https://doi.org/10.3390/molecules191015507
Chicago/Turabian StyleZhou, Wei, Xiaohui Liu, Pei Zhang, Pei Zhou, and Xunlong Shi. 2014. "Effect Analysis of Mineral Salt Concentrations on Nosiheptide Production by Streptomyces actuosus Z-10 Using Response Surface Methodology" Molecules 19, no. 10: 15507-15520. https://doi.org/10.3390/molecules191015507
APA StyleZhou, W., Liu, X., Zhang, P., Zhou, P., & Shi, X. (2014). Effect Analysis of Mineral Salt Concentrations on Nosiheptide Production by Streptomyces actuosus Z-10 Using Response Surface Methodology. Molecules, 19(10), 15507-15520. https://doi.org/10.3390/molecules191015507





