A New Isoflavonoid from Seeds of Lepidium sativum L. and Its Protective Effect on Hepatotoxicity Induced by Paracetamol in Male Rats
Abstract
:1. Introduction
2. Results and Discussion
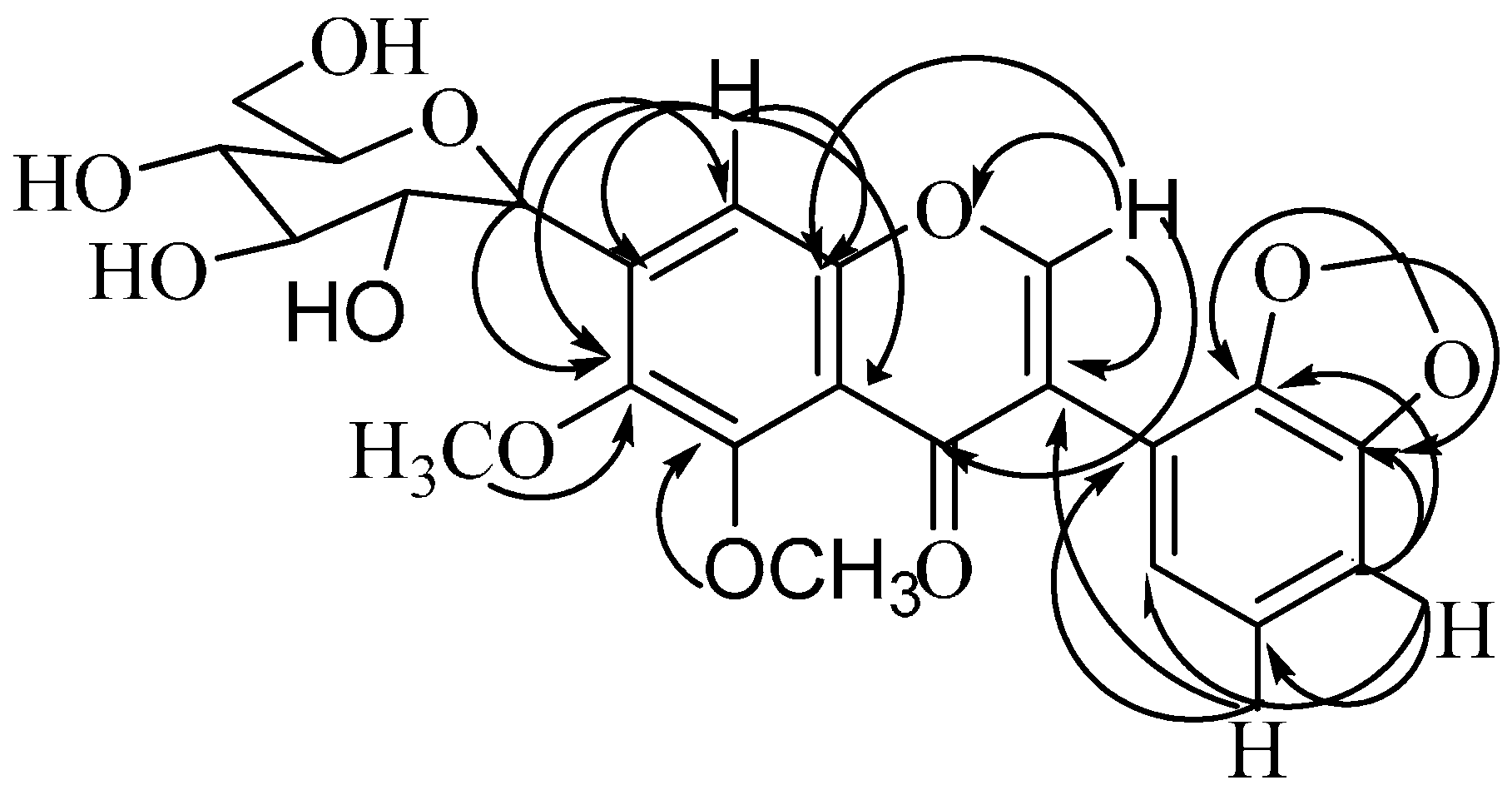
2.1. Chemistry
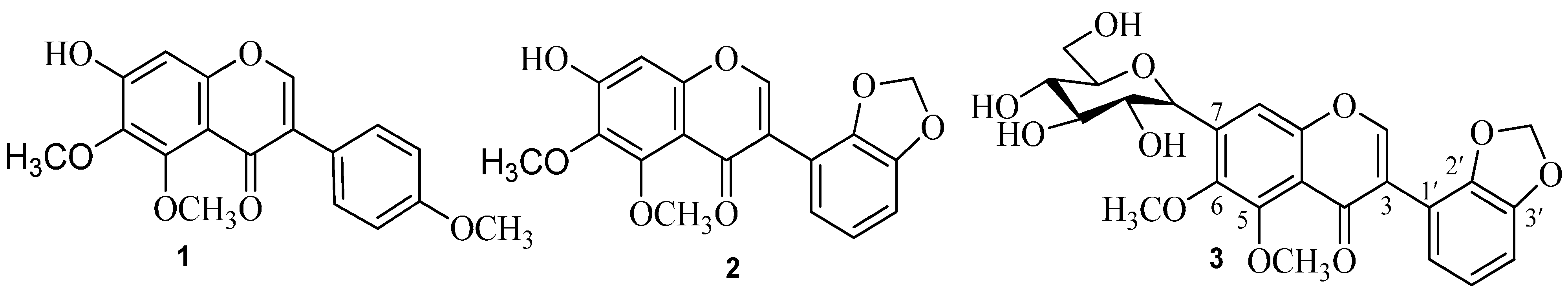
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | |
| 2 | 8.68s | 153.1 | 8.51s | 153.6 | 8.5s | 153.2 |
| 3 | - | 124.7 | - | 120.9 | - | 121.7 |
| 4 | - | 174.5 | - | 180.6 | - | 180.5 |
| 5 | - | 153.2 | - | 153.6 | - | 157.2 |
| 6 | - | 137.2 | - | 137.5 | - | 144.2 |
| 7 | - | 157.3 | - | 157.1 | - | 131.3 |
| 8 | 6.14s | 96.1 | 6.15s | 94.7 | 6.78s | 107.8 |
| 9 | - | 151.3 | - | 151.9 | - | 150.3 |
| 10 | - | 113.2 | - | 113.9 | - | 116.3 |
| 1' | - | 124.8 | - | 120.9 | - | 120.8 |
| 2' | 7.52 (d2.0) | 130.1 | - | 149.1 | - | 147.8 |
| 3' | 6.94 (d8.0) | 114.7 | - | 148.7 | - | 148.2 |
| 4' | - | 159.3 | 6.75 (d1.99) | 114.3 | 6.76 (d1.95) | 114.5 |
| 5' | 6.96 (d2,4) | 114.5 | 7.18 (d2.0) | 121.5 | 6.98 (d2.0) | 121.3 |
| 6' | 7.52 (d2.9) | 130.6 | 6.85 (d1.95) | 122.6 | 7.15 (d1.95) | 122.3 |
| 1'' | - | - | - | - | 4.82 (d,8.9) | 73.7 |
| 2'' | - | - | - | - | 3.75 (dd8.9,9.2) | 84.2 |
| 3'' | - | - | - | - | 3.37 (t8,9) | 72.2 |
| 4'' | - | - | - | - | 3.41 (t8.9) | 71.3 |
| 5'' | - | - | - | - | 2.94 (ddd6.2, 8.8) | 84.6 |
| 6'' | - | - | - | - | 3.81 (d12.3) 3.54 (dd6.2, 12.5) | 62.2 |
| OCH3-5 | 3.83s | 62.2 | 3.84s | 61.3 | 3.79s | 61.8 |
| OCH3-6 | 3.83s | 61.1 | 3.85s | 62.2 | 3.83s | 60.6 |
| OCH3-4' | 3.86s | 55.5 | - | - | - | |
| OCH2O-3',4' | - | - | 6.15s | 101.3 | 6.07s | 101.6 |
| OH-7 | 5.33s | - | 5.35s | - | - | - |
2.2. Biology Assays
2.2.1. Effect of Compounds 1, 2 and 3 on Liver Function
| Group | ALT(U/L) | AST(U/L) | ALP(U/L) | GGT(U/L) |
|---|---|---|---|---|
| Control Group 1 | 40 ± 1.5 | 43 ± 2.1 | 33 ± 2.4 | 60 ± 1.4 |
| Group2: Paracetamol | 70 ± 1.6 * | 80 ± 2.5 * | 71 ± 2.2 * | 117 ± 2.5 * |
| Group 3: Paracetamol + compound 1 | 60 ± 1.45 *# | 60 ± 2.2 *# | 53 ± 1.9 *# | 91 ± 2.4 *# |
| Group 4: Paracetamol + compound 2 | 61 ± 1.33 *# | 62.2 ± 2.3 *# | 54 ± 1.8 *# | 95 ± 2.7 *# |
| Group 5: Paracetamol + compound 3 | 50 ± 1.2 # | 53.5 ± 2.1 # | 39 ± 2.1 # | 72 ± 1.6 # |
2.2.2. Effect of Compounds 1, 2 and 3 on Oxidative Stress Parameters
| Group | TAC (mM/L) | MDA nmole/g tissue | SOD units/g tissue | GPx units/g tissue | CAT mole/min/g tissue | GSH μmol/ mg protein | GST units/g tissue |
|---|---|---|---|---|---|---|---|
| Group 1: Control | 1.2 ± 0.04 | 160 ± 3.23 | 60 ± 1.25 | 150 ± 1.5 | 0.8 ± 0.02 | 60 ± 1.3 | 2.2 ± 0.11 |
| Group 2: Paracetamol | 0.52 ± 0.02 * | 547 ± 9.46 * | 24 ± 2.4 * | 112 ± 1.2 * | 0.42 ± 0.01 * | 35 ± 0.85 * | 1.1 ± 0.01 * |
| Group 3: Paracetamol + compound 1 | 0.61 ± 0.03 *# | 320 ± 8.5 *# | 30 ± 2.1 *# | 118 ± 1.5 *# | 0.56 ± 0.01 *# | 40 ± 0.65 *# | 1.34 ± 0.01 *# |
| Group 4: Paracetamol + compound 2 | 0.65 ± 0.03 *# | 323 ± 9.11 *# | 33 ± 2.2 *# | 115 ± 1.4 *# | 0.55 ± 0.01 *# | 42 ± 0.54 *# | 1.37 ± 0.01 *# |
| Group 5: Paracetamol + compound 3 | 0.73 ± 0.02 # | 211 ± 5.22 # | 36 ± 1.5 # | 120 ± 6.5 # | 0.621 ± 0.01 # | 45.5 ± 1.2 # | 1.50 ± 0.8 # |
2.2.3. Histopathology
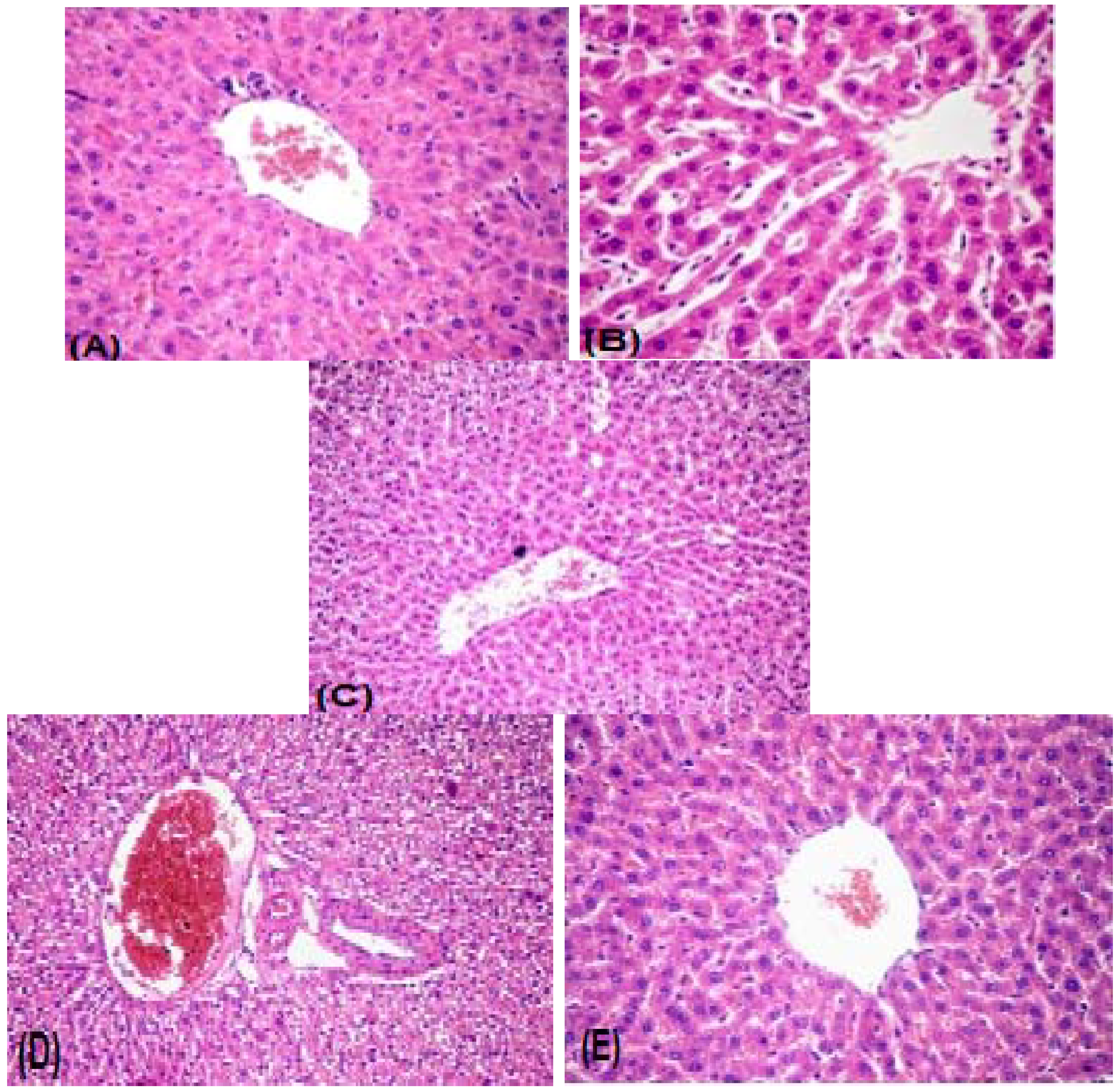
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Plant Extract
3.4. 7-Hydroxy-4',5,6-trimethoxyisoflavone (1)
3.5. 7-Hydroxy-5,6-dimethoxy-2',3'-methylenedioxyisoflavone (2)
3.6. 5,6-Dimethoxy-2',3'-methylenedioxy-7-C-β-d-glucopyranosylisoflavone (3)
3.7. Animals
3.8. Animal Treatments
3.9. Sample Preparation
3.10. Biochemical Analysis
3.11. Preparation of Tissue Homogenates
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loatfy, B. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Broun, A.F.; Massey, R.E. Flora of the Sudan; Wellington House, Buckingham Gate: London, UK, 1929; p. 5. [Google Scholar]
- Merzouki, A.; Ed-derfoufi, F.; Molero, M.J. Contribution to the knowledge of Rifian traditional medicine. II: Flok Medicine in ksar lakbir district (NW Morocco). Fitoterapia 2002, 71, 278–307. [Google Scholar] [CrossRef]
- Maghrani, M.; Zeggwah, N.; Michel, J.; Eddouks, M. Antihypertensive effect of Lepidium sativum in spontaneously hypertensive reats. J. Ethnopharmacol. 2005, 100, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Washington, M.C. Effect of ascorbic acid and curcumin on quercetin-induced nuclear DNA damage, lipid peroxidation and protein degradation. Cancer Lett. 1992, 63, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E.; Garcia-Romera, I.; Ocampo, J.I.; Carbone, V.; Mari, A.; Malorni, A.; Sannino, F.; de Martino, A.; Capasso, R. Chemical characterizationand effects on Lepidium Sativum of the native and biomediated components of dry olive mill residue. Chemosphere 2007, 69, 229–239. [Google Scholar] [CrossRef]
- Horie, T.; Shibata, K.; Yamashita, K.; Fujii, K.; Tsukayama, M.; Ohtsuru, Y. Studies of the selective o-alkylation and dealkylation of flavonoids. A convenient method for synthesizing 6- and 8-methoxylated 5,7 dihydroxyisoflavones. Chem. Pharm. Bull. 1998, 46, 222–230. [Google Scholar] [CrossRef]
- Roitman, J.N.; James, L.F. Chemistry of toxic range plants. Highly oxygenated fiavonol methyl ethers from Gutierrezia micmcephala. Phytochemistry 1985, 24, 835–848. [Google Scholar] [CrossRef]
- Kingston, D.G.I. Mass spectrometry of organic compounds—VI: Electron-impact spectra of flavonoid compounds. Tetrahedron 1971, 27, 2691–2700. [Google Scholar] [CrossRef]
- Markham, K.R.; Geiger, H. The Flavonoids: Advances in Research Since 1986; Harborne, J.B., Ed.; CRC Press: London, UK, 1994; p. 447. [Google Scholar]
- Andreas, K.; Susann, D.; Thomas, H.; Adiaratou, T.; Berit, S.P. Synthesis and characterization of branched polysaccharides by reaction of cellulose with 2,3,4,6-tetraacetyl-1-bromo-α D glucopyranoside. Arkivoc 2012, 3, 76–89. [Google Scholar]
- Minami, M.; Yoshikawa, H. A simplified assay method of superoxide dismutase activity of clinical use. Clin. Chem. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Calvert, D.J.; Cambie, R.C.; Davis, B.R. 13C-NMR spectra of polymethoxy-polymethylenedioxyflavonols. Org. Magn. Reson. 1979, 12, 583–586. [Google Scholar] [CrossRef]
- Miserez, F.; Potterat, O.; Marston, A.; Mungai, G.M.; Hostettmann, K. Flavonol glycosides from Vernonia galamensis ssp. nairobiensis. Phytochemistry 1996, 43, 283–286. [Google Scholar] [CrossRef]
- Sakeran, M.I.; Zidan, N.; Rehman, H.; Aziz, A.T.; Saggu, S. Abrogation by Trifolium alexandrinum root extract on hepatotoxicity induced by acetaminophen in rats. Redox Rep. 2014, 19, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Adnyaa, I.K. Hepatoprotective effect of commbretum quadrang, ulare and its constituents. Biol. Pharm. Bull. 2000, 23, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.T. Antioxidant constituent of almond [Ptunus dulicis (mill) D.A. Webb.] hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.H.; Shylesh, B.S.; Padikkala, J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia 2001, 72, 272–277. [Google Scholar] [CrossRef] [PubMed]
- XWIN-NMR Software, Version 2.6; Bruker Co.: Oxford, UK, 2002.
- Ritman, S.; Frankel, S. A colorimeteric determination of GOT and GPT. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [PubMed]
- Bessey, O.A.; Lowry, O.H.; Brock, M.J. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 1964, 164, 321–329. [Google Scholar]
- Rosalki, S.B. Gamma-glutamyltranspeptidase. In Advances in Clinical Chemistry; Bodanski, O., Latner, A.L., Eds.; Academic Press: New York, NY, USA, 1975; Volume 17, pp. 53–107. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Xu, J.B.; Yuan, X.F.; Lang, P.Z. Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometery. Chin. Environ. Chem. 1997, 16, 73–76. [Google Scholar]
- Marklund, A.; Marklund, G. Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P.J.; Chalk, B.T. Clinical Test: Histopathology; Wolfe Medical Publications: London, UK, 1986. [Google Scholar]
- SPSS Software for Windows, Version 16; IBM Corporation: Armonk, NY, USA, 2010.
- Sokal, R.P.; Rohlf, F.J. The Principles and Practice of Statistics in Biological Research, 1st ed.; Freeman WH: San Francisco, CA, USA, 1969; pp. 469–484. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 2 and 3 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakran, M.; Selim, Y.; Zidan, N. A New Isoflavonoid from Seeds of Lepidium sativum L. and Its Protective Effect on Hepatotoxicity Induced by Paracetamol in Male Rats. Molecules 2014, 19, 15440-15451. https://doi.org/10.3390/molecules191015440
Sakran M, Selim Y, Zidan N. A New Isoflavonoid from Seeds of Lepidium sativum L. and Its Protective Effect on Hepatotoxicity Induced by Paracetamol in Male Rats. Molecules. 2014; 19(10):15440-15451. https://doi.org/10.3390/molecules191015440
Chicago/Turabian StyleSakran, Mohamed, Yasser Selim, and Nahla Zidan. 2014. "A New Isoflavonoid from Seeds of Lepidium sativum L. and Its Protective Effect on Hepatotoxicity Induced by Paracetamol in Male Rats" Molecules 19, no. 10: 15440-15451. https://doi.org/10.3390/molecules191015440
APA StyleSakran, M., Selim, Y., & Zidan, N. (2014). A New Isoflavonoid from Seeds of Lepidium sativum L. and Its Protective Effect on Hepatotoxicity Induced by Paracetamol in Male Rats. Molecules, 19(10), 15440-15451. https://doi.org/10.3390/molecules191015440




