Synthesis of 2-Phenylazonaphtho[1,8-ef][1,4]diazepines and 9-(3-Arylhydrazono)pyrrolo[1,2-a]perimidines as Antitumor Agents
Abstract
:1. Introduction
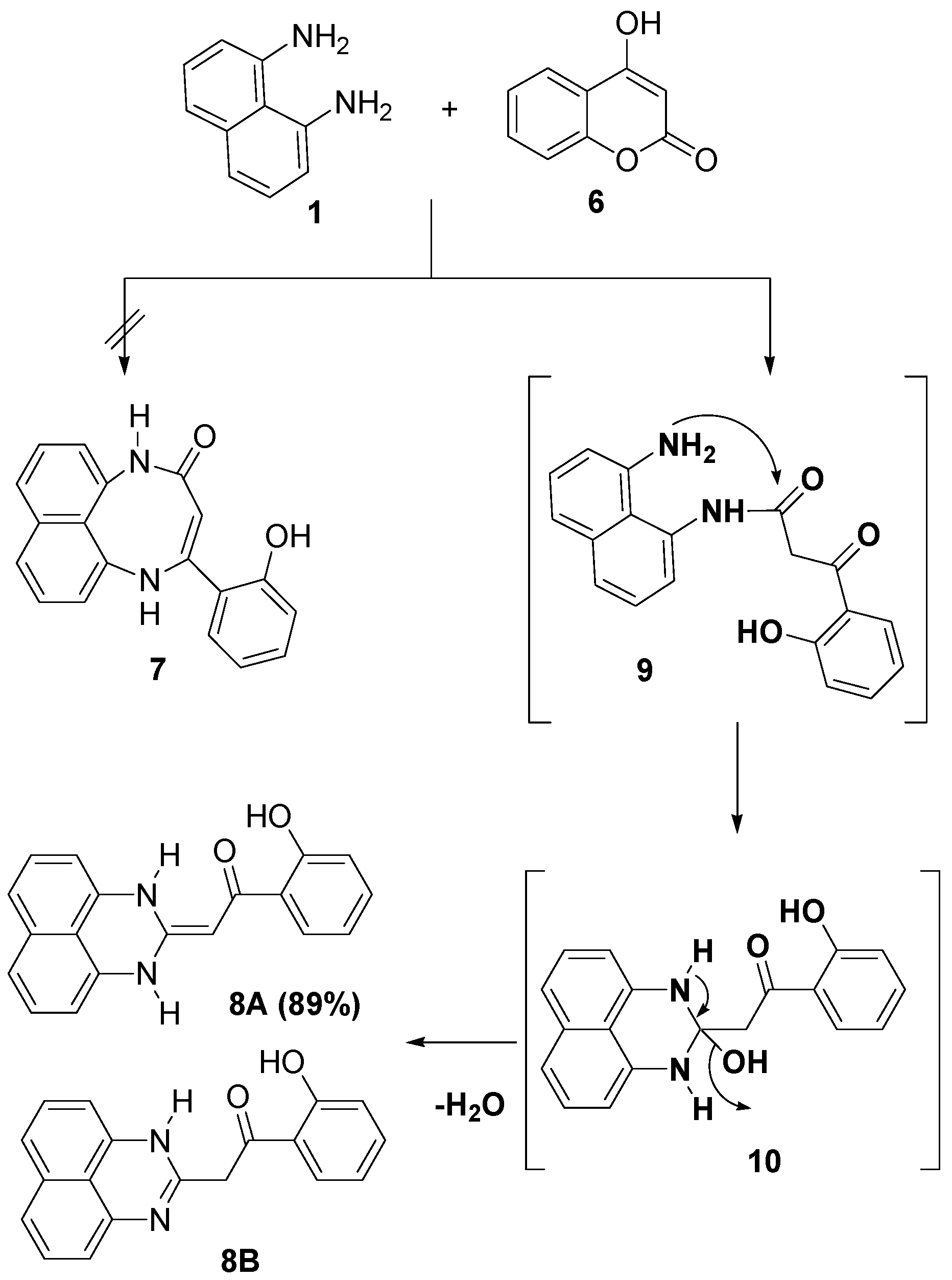
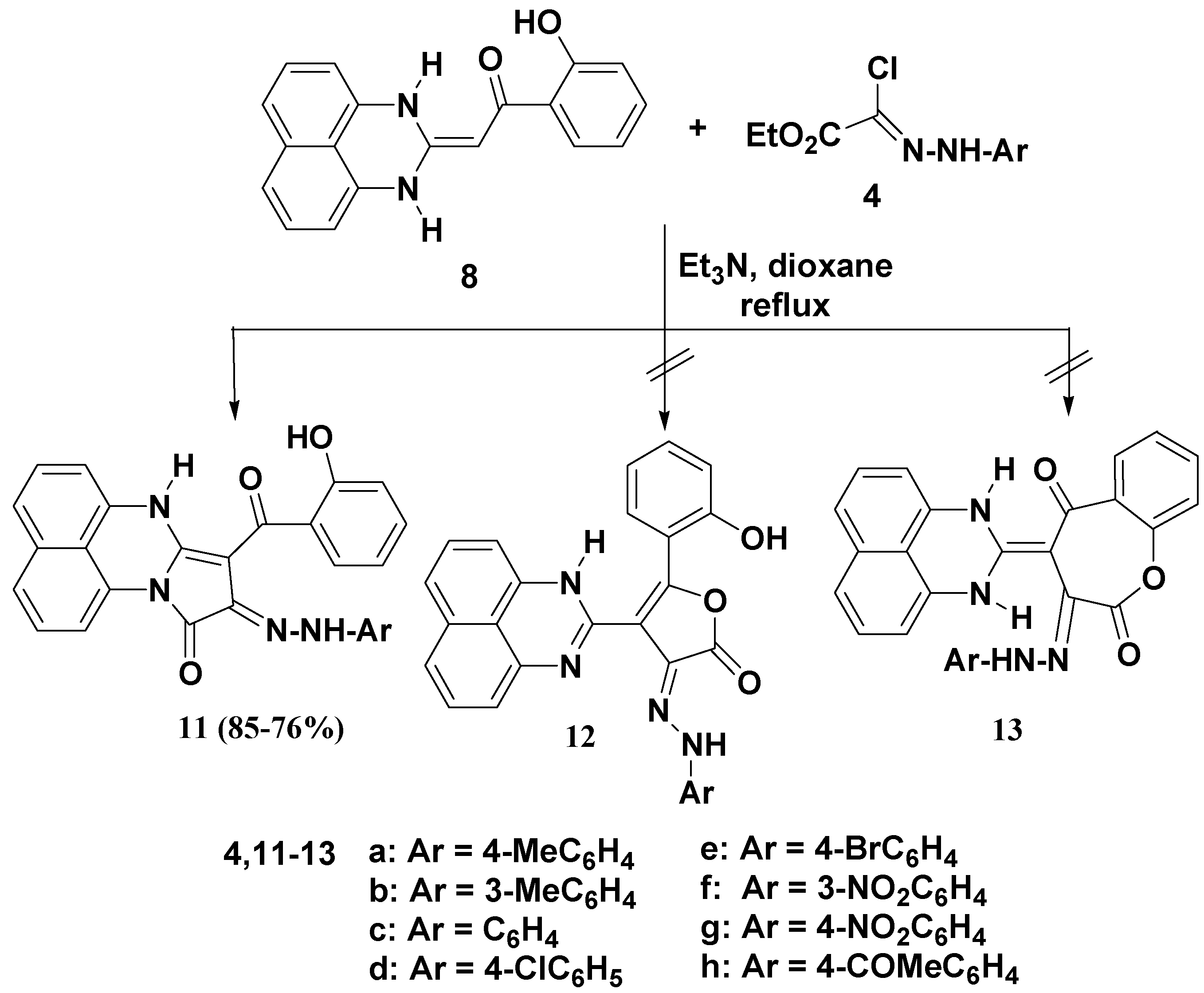
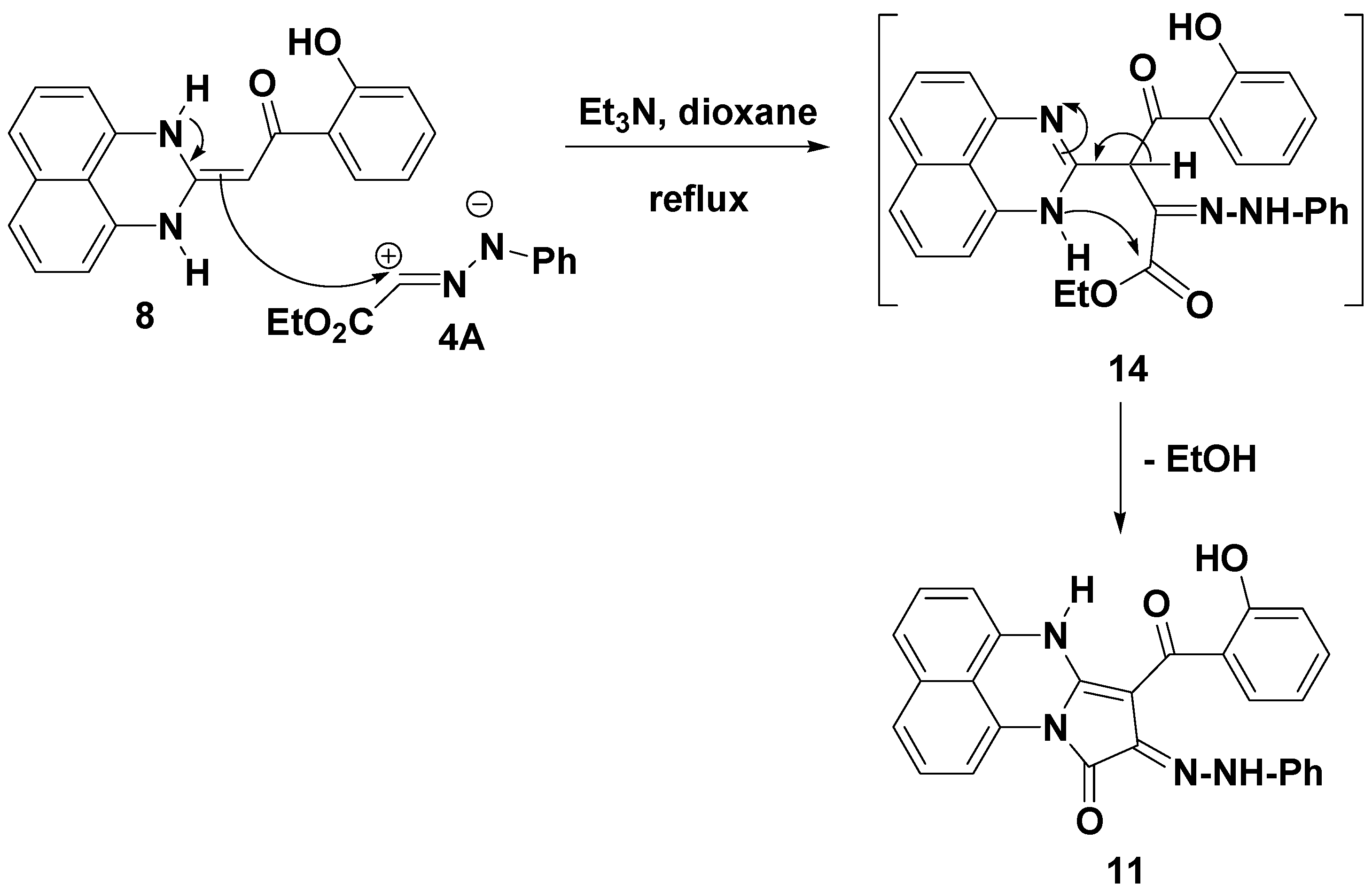
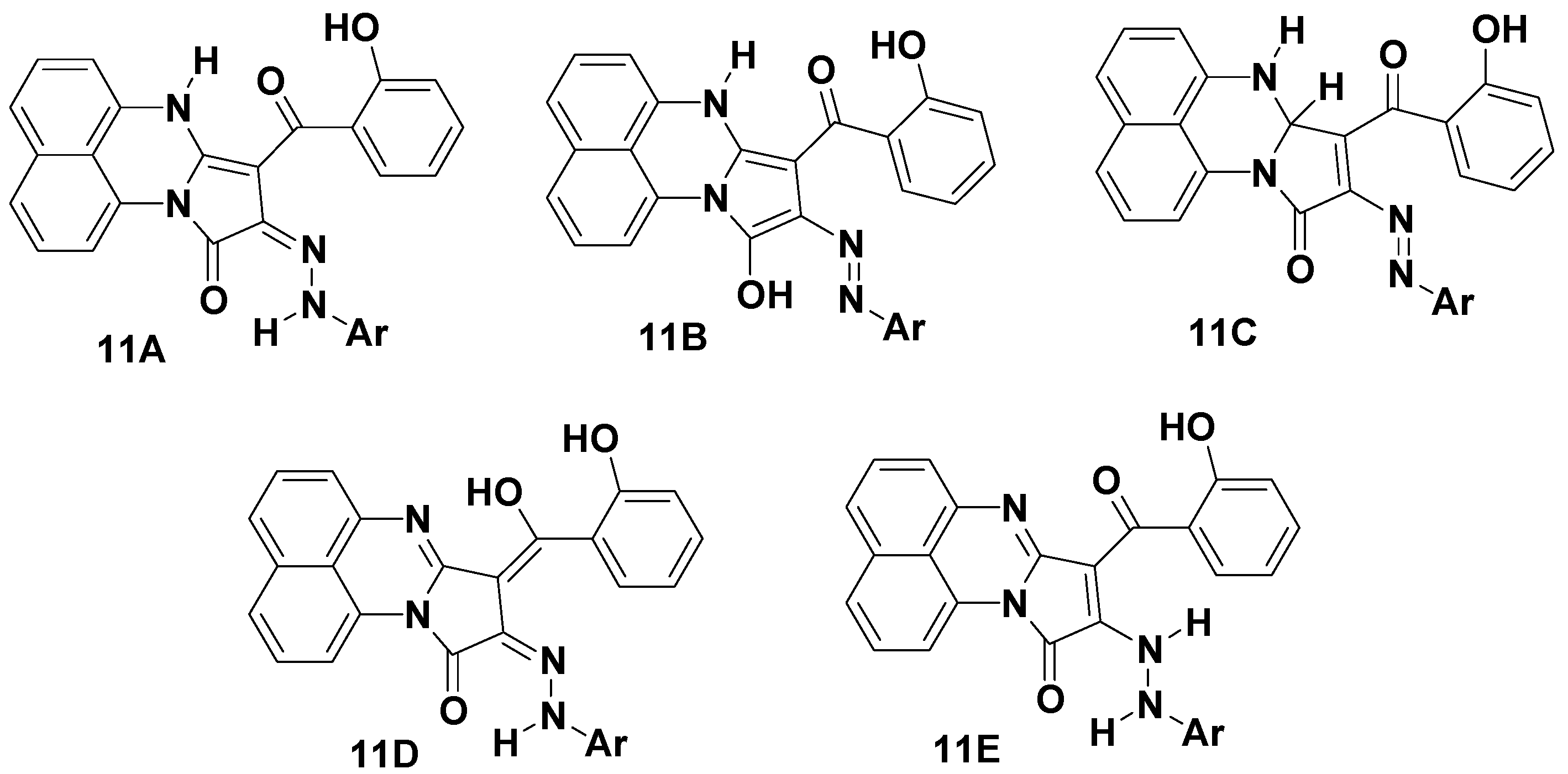
2. Results and Discussion
2.1. Chemistry

| Compd. No. | λmax (log ε) | Compd. No. | λmax (log ε) |
|---|---|---|---|
| 3a | 359 (4.25) | 5a | 378 (4.45) |
| 3b | 349 (3.89) | 5b | 385 (4.33) |
| 3c | 347 (3.59) | 11a | 484 (4.24), 346 (4.19), 269 (4.24) |
| 3d * | 348 (4.30) | 11b | 476 (4.01), 340 (4.10), 233 (4.28) |
| 3e | 349 (3.87) | 11c ** | 473 (4.15), 351 (4.32), 231 (4.38) |
| 3f | 349 (3.88) | 11d | 472 (4.24), 339 (4.15), 232 (4.35) |
| 3g | 345 (3.89) | 11e | 472 (4.0), 337 (3.89), 267 (4.01) |
| 3h | 381 (3.76) | 11f | 490 (4.66), 357 (4.57), 231 (4.89) |
| 3i | 359 (4.19) | 1g | 509 (4.05), 361 (4.11), 262 (4.13) |
| 3j | 351 (4.05) | 11h | 478 (4.10), 336 (3.79), 296 (3.80) |
2.2. Results of Biological Activity
2.2.1. Effect on the Growth of Human Cancer Cell Lines
| Product | Inhibition parameters | TK-10 | MCF-7 | UACC-62 |
|---|---|---|---|---|
| 3c | GI50 | 62.44 ± 3.58 | 80.66 ± 5.24 | >100 |
| TGI | >100 | >100 | >100 | |
| LC50 | >100 | >100 | >100 | |
| 3d | GI50 | >100 | 95.48 ± 11.62 | >100 |
| TGI | >100 | >100 | >100 | |
| LC50 | >100 | >100 | >100 | |
| 3f | GI50 | 83.72 ± 9.73 | >100 | >100 |
| TGI | >100 | >100 | >100 | |
| LC50 | >100 | >100 | >100 | |
| 3i | GI50 | 96.73 ± 11.27 | 81.29 ± 8.28 | 41.97 ± 4.97 |
| TGI | >100 | >100 | 86.66 ± 7.14 | |
| LC50 | >100 | >100 | >100 | |
| 3j | GI50 | 71.06 ± 4.87 | 63.87 ± 3.49 | >100 |
| TGI | >100 | >100 | >100 | |
| LC50 | >100 | >100 | >100 | |
| 8 | GI50 | 72.08 ± 6.94 | 42.25 ± 4.11 | >100 |
| TGI | >100 | >100 | >100 | |
| LC50 | >100 | >100 | >100 | |
| 11c | GI50 | 16.39 ± 1.79 | 12.67 ± 1.08 | 43.78 ± 1.05 |
| TGI | 25.28 ± 2.39 | 21.87 ± 2.10 | >100 | |
| LC50 | 38.99 ± 3.01 | 37.77 ± 3.91 | >100 |
2.2.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
| Comp. No. | ORAC Value | Comp. No. | ORAC Value |
|---|---|---|---|
| 3d | 0.61 ± 0.04 | 11c | 0.86 ± 0.09 |
| 3f | 0.74 ± 0.07 | 11d | 0.43 ± 0.59 |
| 3h | 0.54 ± 0.06 | 11b | 0.51 ± 0.10 |
| 3i | 0.97 ± 0.10 | 11f | 0.60 ± 0.07 |
| 3j | 0.68 ± 0.03 | 11e | 0.62 ± 0.09 |
| 3c | 0.81 ± 0.05 | 11g | 0.42 ± 0.05 |
| 3e | 0.65 ± 0.07 | 11a | 0.53 ± 0.08 |
| 8 | 0.72 ± 0.06 | 11h | 0.59 ± 0.06 |
3. Experimental
3.1. General Information
3.2. General Procedure for the Reaction of Hydrazonoyl Chlorides with 1,8-Diaminonaphthalene
3.3. ORAC Assay
3.4. Human Cell Lines for Cytotoxicity Assays
3.5. Testing Procedure and Data Processing
3.6. Sulphorhodamine B Method
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pandya, A.B.; Prajapati, D.G.; Pandya, S.S. Synthesis of novel Naphthalene COX inhibitors for anti-inflammatory activity. J. Appl. Pharm. Sci. 2012, 2, 226–232. [Google Scholar]
- Mkpenie, V.; Ebong, G.; Obot, I.B.; Abasiekong, B. Evaluation of the effect of azo group on the biological activity of 1-(4-methylphenylazo)-2-naphthol. E-J. Chem. 2008, 5, 431–434. [Google Scholar] [CrossRef]
- Strom, M.B.; Haug, B.E.; Rekdal, O.; Skar, M.L.; Stensen, W.; Svendsen, J.S. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell Biol. 2002, 80, 65–74. [Google Scholar] [CrossRef]
- Antonini, I.; Polucci, P.; Magnano, A.; Sparapani, S.; Martelli, S. Rational design, Synthesis, and biological evaluation of bis(pyrimido[5,6,1-de]acridines) and bis(pyrazolo[3,4,5-kl]acridine-5-carboxamides) as new anticancer agents. J. Med. Chem. 2004, 47, 5244–5250. [Google Scholar] [CrossRef]
- Akita, M.; Seto, H.; Aoyama, R.; Kimura, J.; Kobayashi, K. Novel rearrangements in the reactions directed toward preparation of spiro-N,N-ketals: Reactions of naphthalene-1,8-diamine with ninhydrin and isatin. Molecules 2012, 17, 13879–13890. [Google Scholar] [CrossRef]
- El-Shaieb, K.M. Condensation of 1-(dicyanomethylene)acenaphthene-2-one with aromatic diamines. J. Chin. Chem. Soc. 2008, 55, 1150–1155. [Google Scholar]
- Cheeseman, G.W.H.; Eccleshall, S.A. Synthesis of pyrrolo[1,2-a]naphtho[1,8-ef][1,4]diazepines. J. Heterocycl. Chem. 1986, 23, 65–67. [Google Scholar] [CrossRef]
- ngören, S.H. Transformation of furan-2,3-diones with 1,8-diaminonaphthalene to naphtho-[1,8-ef][1,4]diazepin-2(1H)-ones. Synth. Comm. 2009, 39, 3657–3662. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Paknia, F.; Narayan, A.; Mathew, T.; Olah, G.A. Synthesis of perimidine and 1,5-benzodiazepine derivatives using tamed Brønsted acid, BF3–H2O. J. Fluor. Chem. 2013, 152, 99–105. [Google Scholar] [CrossRef]
- Luthin, D.R.; Rabinovich, A.K.; Bhumralkar, D.R.; Youngblood, K.L.; Bychowski, R.A.; Dhanoa, D.S.; May, J.M. Synthesis and biological activity of oxo-7H-benzo[e]perimidine-4-carboxylic acid derivatives as potent, nonpeptide corticotropin releasing factor (CRF) receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 765–770. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Dalnikovskaya, V.V. Perimidines. Russ. Chem. Rev. 1981, 50, 816–835. [Google Scholar] [CrossRef]
- Claramunt, R.M.; Dotor, J.; Elguero, J. Structure, Reactivity and synthesis of perimidines derivatives (Dihydroperimidines and perimidinones). Ann. Quim. 1995, 91, 151–183. [Google Scholar]
- Herbert, J.M.; Woodgate, P.D.; Denny, W.A. Potential antitumor agents. 53. Synthesis, DNA binding properties, and biological activity of perimidines designed as minimal DNA-intercalating agents. J. Med. Chem. 1987, 30, 2081–2086. [Google Scholar] [CrossRef]
- Brana, M.F.; Garrido, M.; Rodriguez, M.; Morcillo, M.J.; Alvarez, Y.; Valladares, Y.; Klebe, G. Synthesis, Structure and cytostatic activity of a series of 2-substituted perimidines. Eur. J. Med. Chem. 1990, 25, 209–215. [Google Scholar] [CrossRef]
- Stefańska, B.; Dzieduszycka, M.; Bontemps-Gracz, M.M.; Borowski, E.; Martelli, S.; Supino, R.; Pratesi, G.; de Cesare, M.A.; Zunino, F.; Kuśnierczyk, H.; et al. 8,11-Dihydroxy-6-[(aminoalkyl)amino]-7H-benzo[e]perimidin-7-ones with activity in multidrug-resistant cell lines: Synthesis and antitumor evaluation. J. Med. Chem. 1999, 42, 3494–3501. [Google Scholar] [CrossRef]
- Bu, X.; Deady, L.W.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. Synthesis and cytotoxic activity of 7-Oxo-7H-dibenz[f,ij]isoquinoline and 7-Oxo-7H-benzo[e]perimidine derivatives. J. Med. Chem. 2001, 44, 2004–2014. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Mahmoud, H.K. Synthesis of pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5-ones 3 as potential antimicrobial agents. Arch. Pharm. Chem. Life Sci. 2013, 346, 392–402. [Google Scholar] [CrossRef]
- Kima, S.-H.; Kimb, J.-H.; Cuia, J.-Z.; Galc, Y.-S.; Jind, S.-H.; Koh, K. Absorption spectra, aggregation and photofading behaviour of near-infrared absorbing squarylium dyes containing perimidine moiety. Dyes Pigments 2002, 55, 1–7. [Google Scholar] [CrossRef]
- Wan, X.; Lv, X.; He, G.; Yu, A.; Chen, Y. Synthesis of neutral stable polyradicals and their application on photovoltaic devices. Eur. Polym. J. 2011, 47, 1018–1030. [Google Scholar] [CrossRef]
- Bazinet, P.; Yap, G.P.A.; Richeson, D.S. Constructing a stable carbene with a novel topology and electronic framework. J. Am. Chem. Soc. 2003, 125, 13314–13315. [Google Scholar] [CrossRef]
- Herbst, W.; Hunger, K. Industrial Organic Pigments, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2004; p. 9. [Google Scholar]
- Nagao, Y.; Tsuda, K.; Kozawa, K.; Uchida, T. Synthesis and properties of benzimidazole and naphthoimidazole derivatives of perylenedicarboximide. Heterocycles 2001, 54, 757–764. [Google Scholar] [CrossRef]
- Alfredo, N.V.; Likhatchev, D.; Ramirez, S.B.; Vazquez, J.R.; Valverde, G.C.; Alexandrova, L. Highly effective low temperature route to pyrroloperimidines synthesis and their copolymerization with styrene and methyl methacrylate. Polymer 2008, 49, 3654–3662. [Google Scholar] [CrossRef]
- Mizuguchi, J. Crystal structure and electronic characterization of trans and cis perinone pigments. J. Phys. Chem. B 2004, 108, 8926–8930. [Google Scholar] [CrossRef]
- Abdel Hafez, N.A.; Farghaly, T.A.; Al-Omar, M.A.; Abdalla, M.M. Synthesis of bioactive polyheterocyclic ring systems as 5α-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 4838–4844. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Gomha, S.M.; Abbas, E.M.; Abdalla, M.M. Hydrazonoyl halides as precursors for new fused heterocycles of 5α-reductase inhibitors. Arch. Pharm. 2012, 345, 117–122. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; El-Zahabi, H.S.A.; Dawood, K.M. Microwave-assisted synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur. J. Med. Chem. 2010, 45, 2427–2432. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Mekawey, A.A.I.; Dawood, K.M. Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur. J. Med. Chem. 2009, 44, 3637–3644. [Google Scholar] [CrossRef]
- Dawood, K.M.; Abdel-Gawad, H.; Ellithey, M.; Mohamed, H.A.; Hegazi, B. Synthesis, Anticonvulsant, and anti-inflammatory activities of some new benzofuran-based heterocycles. Arch. Pharm. Chem. Life Sci. 2006, 339, 133–140. [Google Scholar] [CrossRef]
- Dawood, K.M.; Abdel-Gawad, H.; Ragab, E.A.; Ellithey, M.; Mohamed, H.A. ynthesis, Anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg. Med. Chem. 2006, 14, 3672–3680. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdallah, M.A.; Aziz, A.M.R. Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole derivatives. Molecules 2012, 17, 14625–14636. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Farghaly, T.A.; Abdallah, M.A.; Abdalla, M.M.; Abd El-Aziz, M.R. New pyrazoles incorporating pyrazolylpyrazole moiety: Synthesis, Anti-HCV and antitumor activity. Eur. J. Med. Chem. 2010, 45, 1042–1050. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdalla, M.M. Synthesis, Tautomerism, Antimicrobial, Anti-HCV, Anti-SSPE, Antioxidant and antitumor activities of arylazobenzosuberones. Bioorg. Med. Chem. 2009, 17, 8012–8019. [Google Scholar] [CrossRef]
- Karcı, F.; Sener, N.; Yamac, M.; Sener, I.; Demircalı, A. The synthesis, antimicrobial activity and absorption characteristics of some novel heterocyclic disazo dyes. Dyes Pigments 2009, 80, 47–52. [Google Scholar] [CrossRef]
- Hamdi, M.; Grech, O.; Sakellariou, R.; Speziale, V. New method of synthesis of 1,5-benzodiazepin-2-ones from 4-hydroxycoumarin. J. Heterocycl. Chem. 1994, 31, 509–511. [Google Scholar] [CrossRef]
- Hamdi, N.; Lidrissi, C.; Saoud, M.; Nievas, R.A.; Zarrouk, H. Synthesis of some new biologically active coumarin derivatives. Chem. Heterocycl. Compd. 2006, 42, 320–325. [Google Scholar] [CrossRef]
- Üngören, S.H.; Koca, T.; Yilmaz, F. Preparation of perinones via a novel multicomponent synthesis of isoindole scaffold. Tetrahedron 2011, 67, 5409–5414. [Google Scholar] [CrossRef]
- Paragamian, V.; Baker, M.B.; Puma, B.M.; Reale, J. A study of the synthesis and some reactions of perimidines. J. Heterocycl. Chem. 1968, 5, 591–597. [Google Scholar] [CrossRef]
- Cado, F.; Di-Martino, J.; Jacquault, P.; Bazureau, J.P.; Hamelin, J. Amidine-enediamine tautomerism: Addition of isocyanates to 2-substituted 1H-perimidines. Some syntheses under microwave irradiation. Bull. Soc. Chim. Fr. 1996, 133, 587–595. [Google Scholar]
- Shawali, A.S.; Zayed, M.M.; Farghaly, T.A. Synthesis and biological activity of new 1H-pyrazolo[3,4-b]quinoxalines (Flavazoles). J. Heterocycl. Chem. 2005, 42, 185–189. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A Review of reactive oxygen species scavenging, glutathione peroxidase, and metal binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Monks, A.; Scudeiro, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3, 5, 8 and 11 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farghaly, T.A.; Abbas, E.M.H.; Dawood, K.M.; El-Naggar, T.B.A. Synthesis of 2-Phenylazonaphtho[1,8-ef][1,4]diazepines and 9-(3-Arylhydrazono)pyrrolo[1,2-a]perimidines as Antitumor Agents. Molecules 2014, 19, 740-755. https://doi.org/10.3390/molecules19010740
Farghaly TA, Abbas EMH, Dawood KM, El-Naggar TBA. Synthesis of 2-Phenylazonaphtho[1,8-ef][1,4]diazepines and 9-(3-Arylhydrazono)pyrrolo[1,2-a]perimidines as Antitumor Agents. Molecules. 2014; 19(1):740-755. https://doi.org/10.3390/molecules19010740
Chicago/Turabian StyleFarghaly, Thoraya A., Eman M. H. Abbas, Kamal M. Dawood, and Tarek B. A. El-Naggar. 2014. "Synthesis of 2-Phenylazonaphtho[1,8-ef][1,4]diazepines and 9-(3-Arylhydrazono)pyrrolo[1,2-a]perimidines as Antitumor Agents" Molecules 19, no. 1: 740-755. https://doi.org/10.3390/molecules19010740
APA StyleFarghaly, T. A., Abbas, E. M. H., Dawood, K. M., & El-Naggar, T. B. A. (2014). Synthesis of 2-Phenylazonaphtho[1,8-ef][1,4]diazepines and 9-(3-Arylhydrazono)pyrrolo[1,2-a]perimidines as Antitumor Agents. Molecules, 19(1), 740-755. https://doi.org/10.3390/molecules19010740




