Three-Component Reaction of Tautomeric Amidines with 3-Ferrocenylmethylidene-2,4-pentanedione. Formation of Polymeric Coordination Complexes of Potassium Ferrocenyl-(hexahydro)pyrimidoxides
Abstract
:1. Introduction
2. Results and Discussion
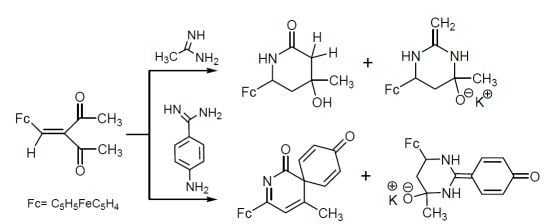
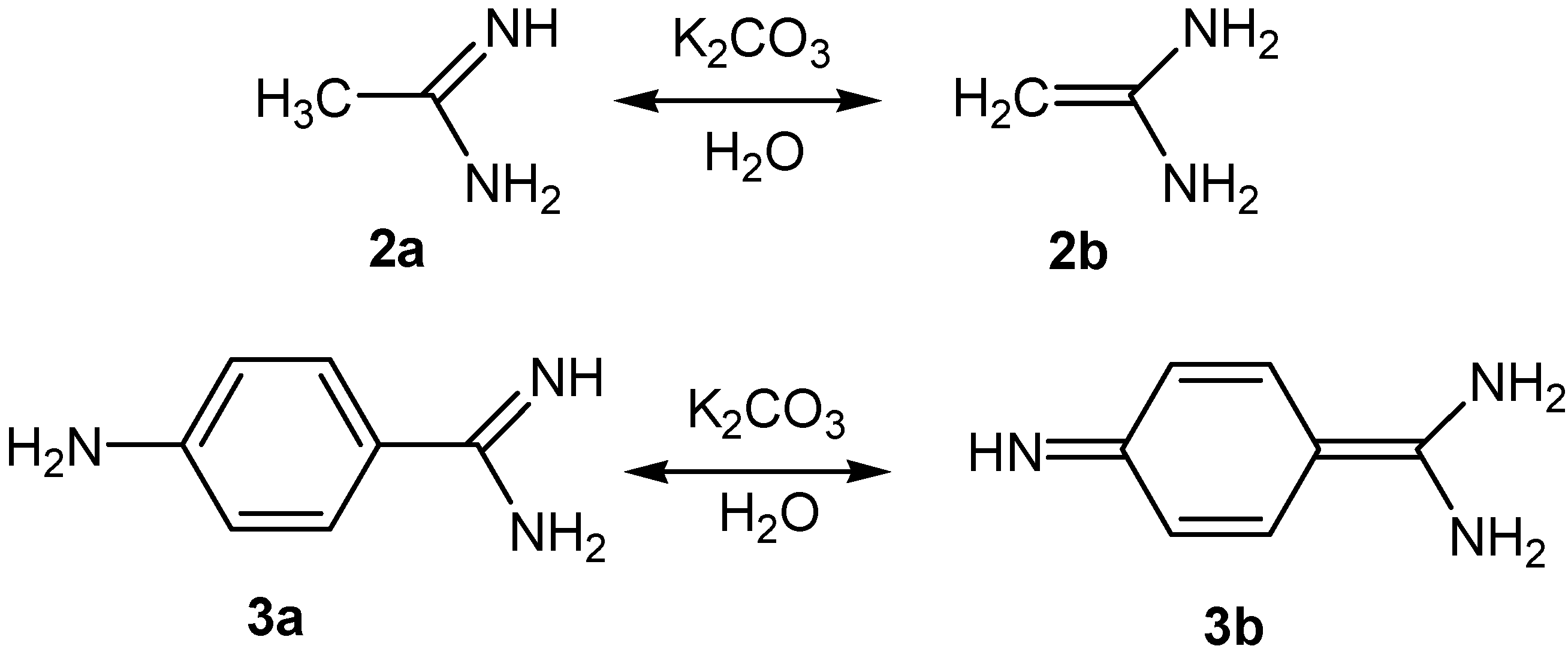
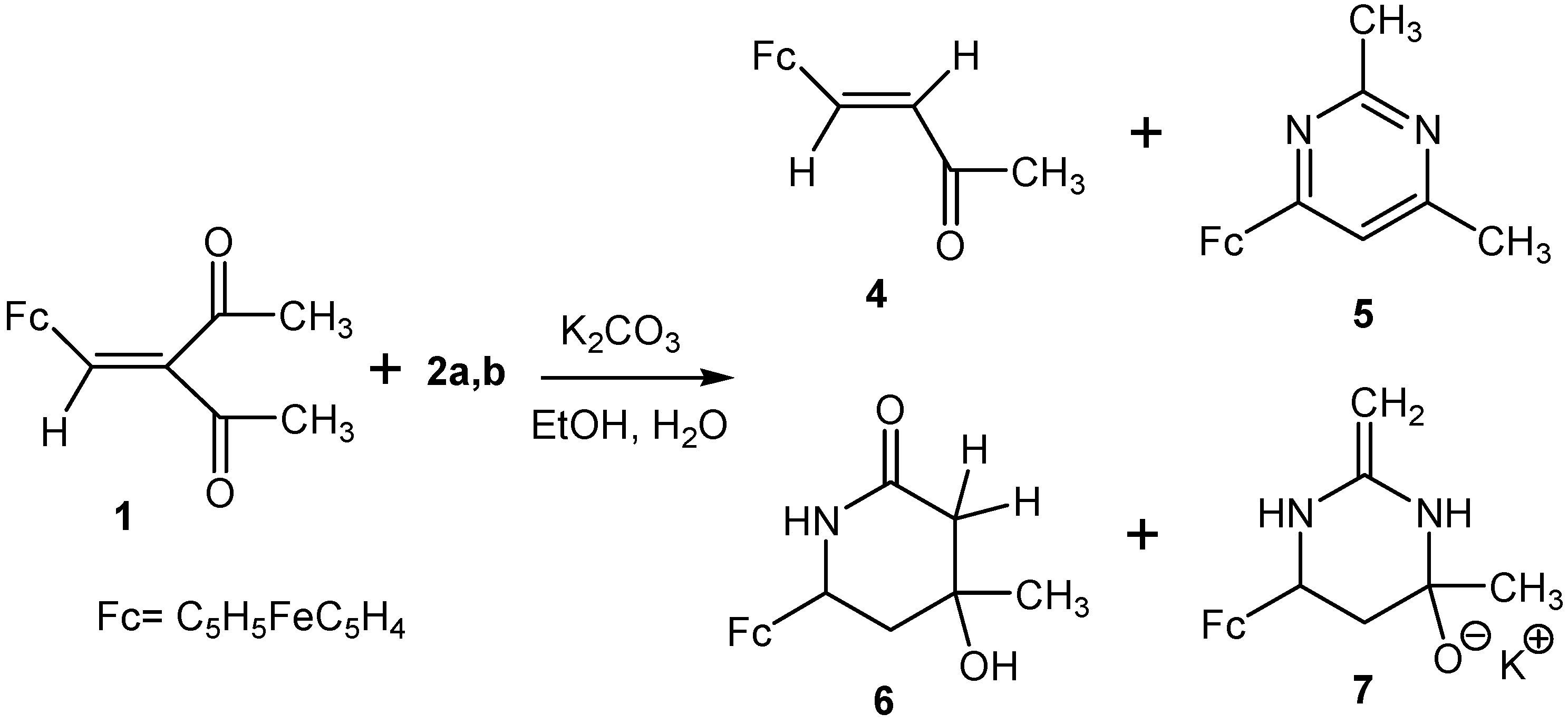
2.1. Reactions of Acetamidine and p-Aminobenzamidine with 3-Ferrocenylmethylidene-2,4-pentanedione
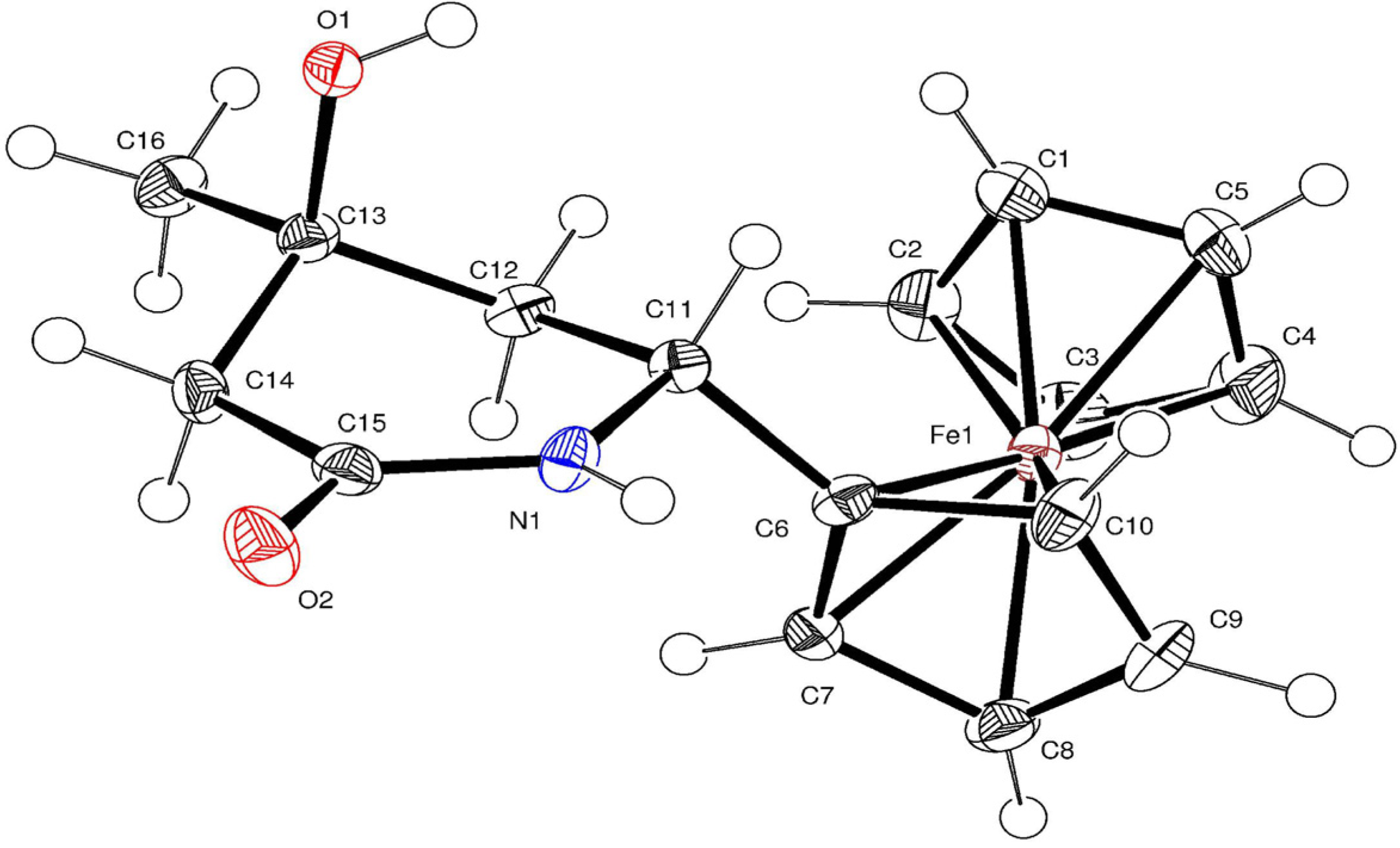
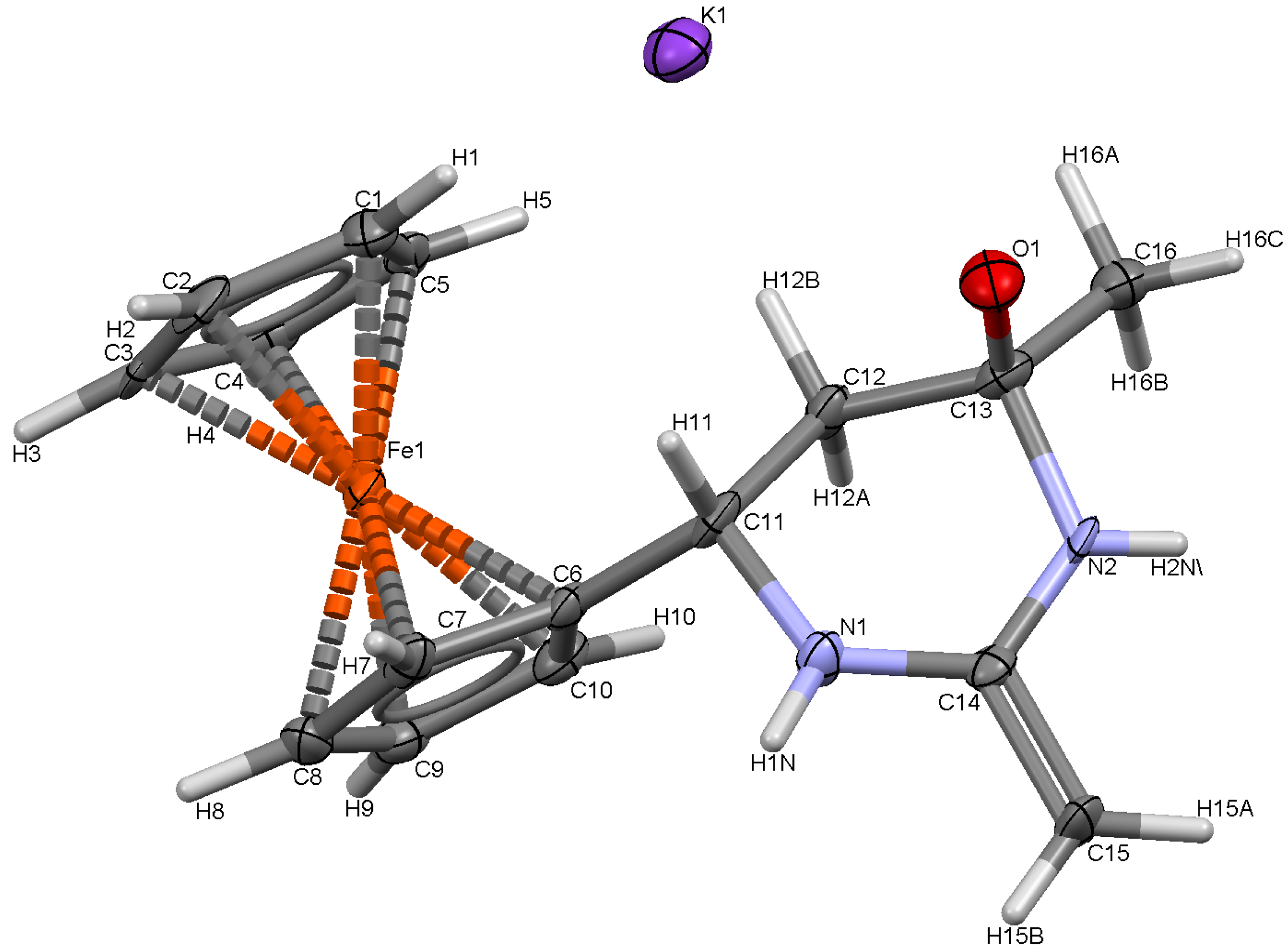
| Selected bond lengths (Å) | Selected bond angles (°) | ||
|---|---|---|---|
| 6 | |||
| C(11)-N(1) | 1.476(3) | N(1)-C(11)-C(12) | 111.22(19) |
| C(11)-C(12) | 1.520(3) | C(13)-C(12)-C(11) | 113.77 (18) |
| C(12)-C(13) | 1.517(3) | O(1)-C(13)-C(12) | 111.54(19) |
| C(13)-O(1) | 1.439(3), | O(1)-C(13)-C(14) | 105.81(18) |
| C(15)-N(1) | 1.329(3) | C(12)-C(13)-C(14) | 108.05 (19) |
| C(13)-C(14) | 1.524(3) | C(15)-C(14)-C(13) | 112.94(19) |
| C(14)-C(15) | 1.506(3) | O(2)-C(15)-N(1) | 121.3(2) |
| C(15)-O(2) | 1.247(3). | N(1)-C(15)-C(14) | 117.9(2) |
| 7 | |||
| N(1)-C(11) | 1.482(6) | N(1)-C(11)-C(12) | 109.6(4) |
| C(11)-C(12) | 1.522(6) | C(13)-C(12)-C(11) | 112.7(4) |
| C(12)-C(13) | 1.520(7) | O(1)-C(13)-N(2) | 105.1(4) |
| C(13)-O(1) | 1.418(6) | O(1)-C(13)-C(12) | 111.3(4) |
| C(13)-N(2) | 1.469(6) | N(2)-C(13)-C(12) | 106.8(4) |
| C(14)-N(2) | 1.305(6) | N(2)-C(14)-N(1) | 120.4(4) |
| C(14)-N(1) | 1.327(6) | N(2)-C(14)-C(15) | 120.5(4) |
| C(14)-C(15) | 1.487(6) | N(1)-C(14)-C(15) | 119.1(4) |
| O(1)-K(1) | 3.072(4) | C(13)-O(1)-K(1) | 119.5(3) |
| K(1)-N(1)#1 | 3.136(4) | O(1)-K(1)-N(1)#1 | 97.31(10) |
| N(1)-K(1)#1 | 3.136(4) | O(1)-K(1)-N(2)#2 | 138.86(11) |
| K(1)-N(2)#2 | 3.147(5) | N(1)#1-K(1)-N(2)#2 | 92.44(11) |
| N(2)-K(1)#3 | 3.147(5) | C(14)-N(2)-C(13) | 123.1(4) |
| C(13)-C(16)) | 1.502(7) | C(14)- N(1)- K(1)#1 | 117.0(3) |
| C(1)-Fe(1) | 2.044(5) | C(11)- N(1)- K(1)#1 | 116.9(3) |
| N(1)- H(N1) | 0.91(5) | C(14)- N(2)- K(1)#3 | 121.2(3) |
| N(2)- H(N2) | 0.76(5) | C(13)- N(2)- K(1)#3 | 115.4(3) |
| 8 | |||
| N(1)-C(11) | 1.347(5) | N(1)-C(11)-C(12) | 121.4(3) |
| C(11)-C(12) | 1.400(5) | C(13)-C(12)-C(11) | 116.9(3) |
| C(12)-C(13) | 1.389(6) | N(2)-C(13)-C(12) | 121.8(3) |
| C(13)-N(2) | 1.347(5) | N(2)-C(13)-C(21) | 117.5(3) |
| C(14)-N(2) | 1.340(5) | N(2)-C(14)-N(1) | 125.3(3) |
| C(14)-N(1) | 1.341(5) | N(2)-C(13)-C(15) | 118.7(3) |
| C(14)-C(15) | 1.470(5) | C(14)-N(1)-C(11) | 117.3(3) |
| C(18)-N(3) | 1.377(5) | C(14)-N(2)-C(13) | 117.2(3) |

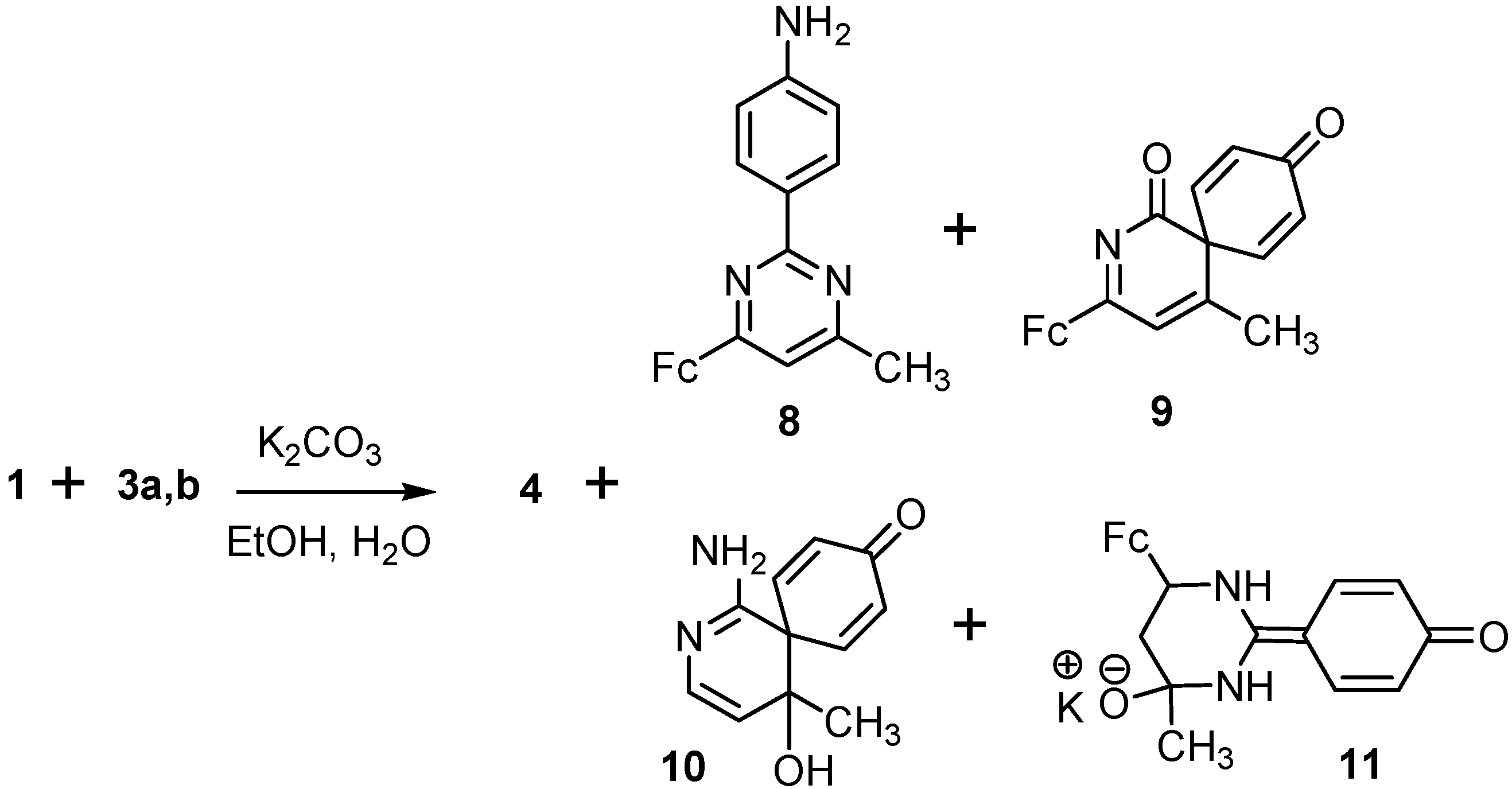

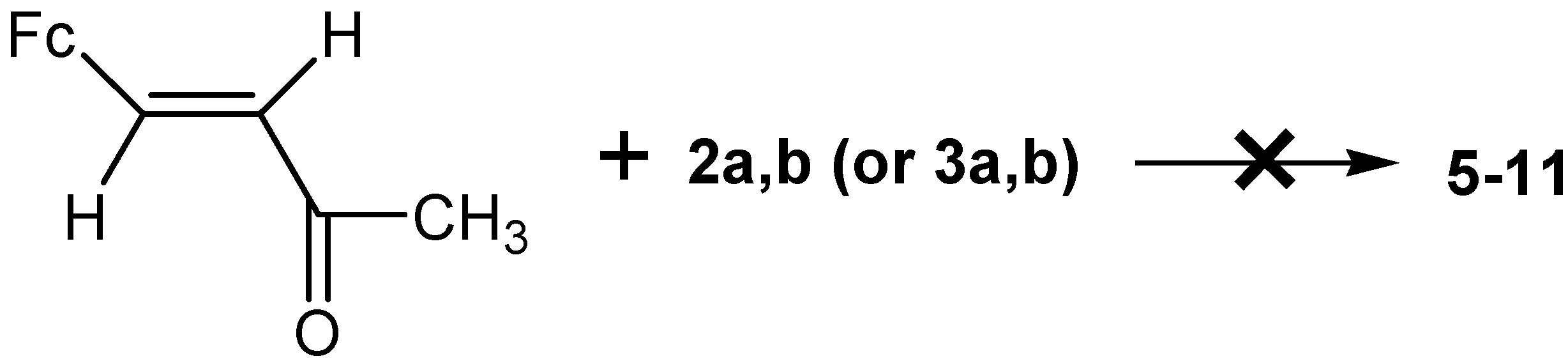
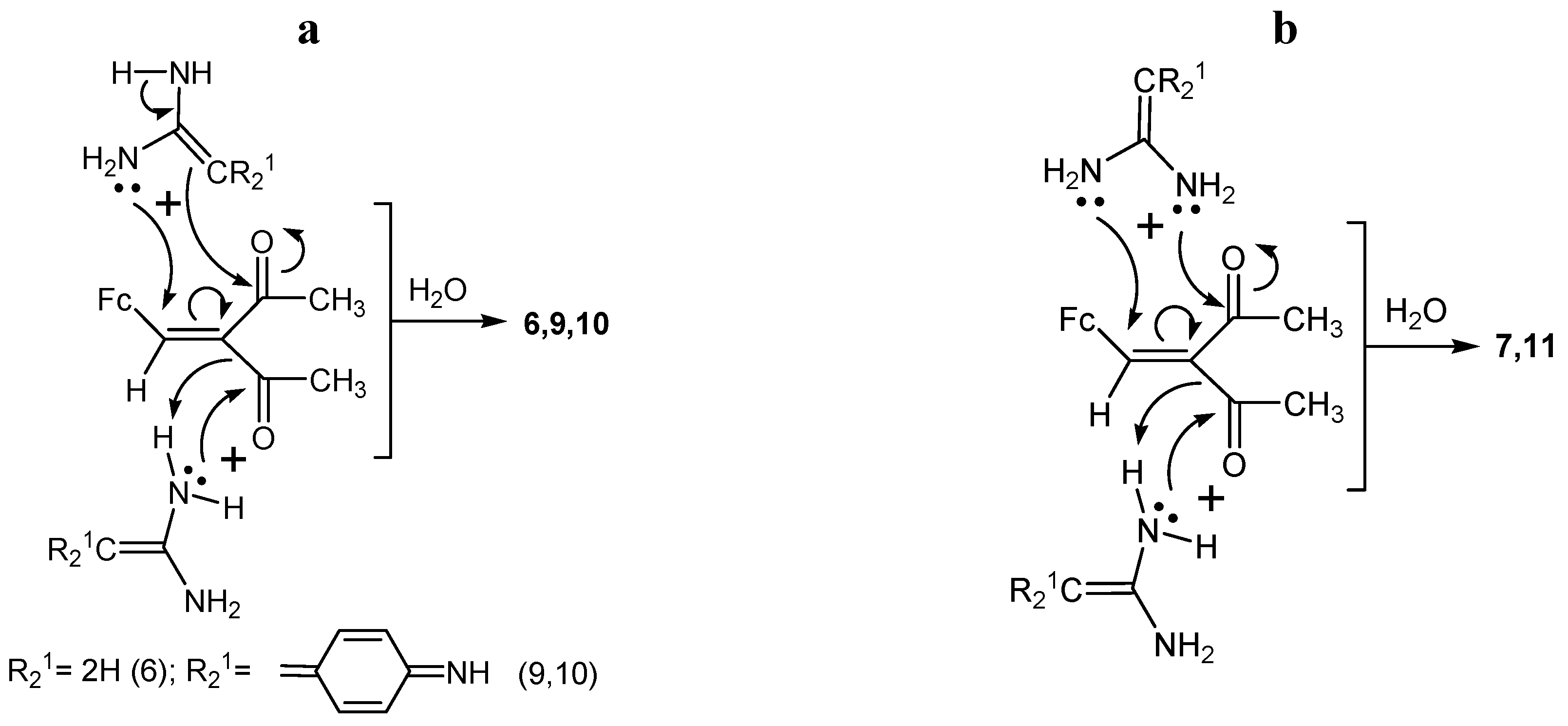
2.2. Posible Mechanisms of the Reactions of Acetamidine and p-Aminobenzamidine with 3-Ferrocenyl-methylidene-2,4-pentanedione
- (1)
- Chalcone 4 is formed in the studied reactions as a result of one-pot nucleophilic attack of amidine nitrogens on the carbonyl carbon of the acetyl group in β-diketone 1 (Scheme 5a).
- (2)
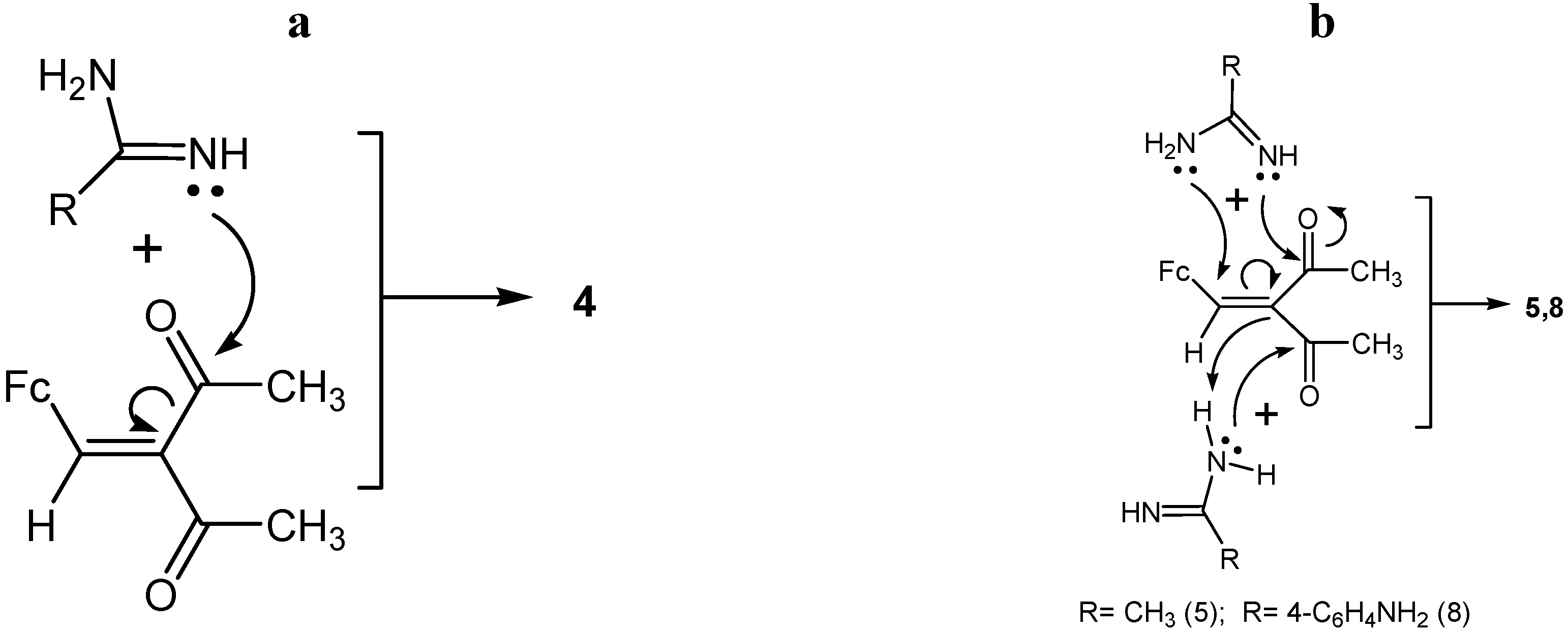
3. Experimental
3.1. General
3.2. Reactions of 3-Ferrocenylmethylidenepentane-2,4-dione (1) with Acetamidine Hydrochloride (2) (General Procedure)
3.3. Reactions of 3-Ferrocenylmethylidene-pentane-2,4-dione (1) with p-Aminobenzamidine Dihydrochloride (3)
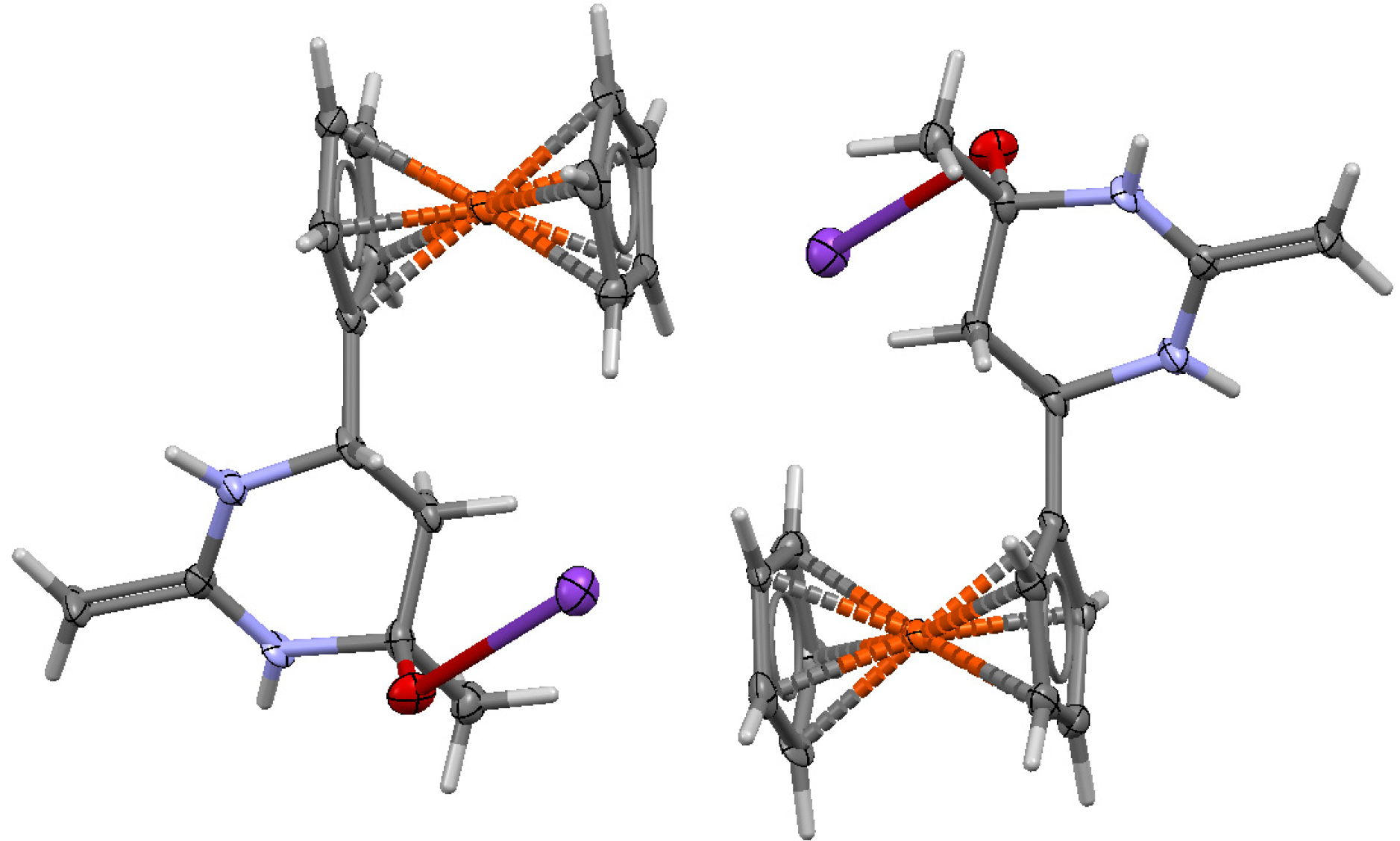
3.4. Crystal Structures of 6, 7 and 8
4. Conclusions
Supplementary Materials
Acknowledgements
Conflicts of Interest
References
- Metallocenes; Togni, A.; Halterman, R.L. (Eds.) Wiley-VCH: New York, NY, USA, 1998; Volume 1, Chapter 1.
- Stepnicka, P. Ferrocenes.: Ligands., Materials and Biomolecules; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Bildstein, B.; Skibar, W.; Schweiger, M.; Kopacka, H.; Wurst, K. In situ synthesis of the first C7 cumulene (Fc)2 C=C=C=C=C=C=C(Fc)2 via deprotonation of its conjugate acid [(Fc)2C7H(Fc)2]+BF4− (Fc-ferrocenyl). J. Organomet. Chem. 2001, 622, 135–142. [Google Scholar] [CrossRef]
- Kaifer, A.E.; Mendoza, S. Comprehensive Supramolecular Chemistry; Elsevier: Oxford, UK, 1996; Volume 1. [Google Scholar]
- Heo, R.W.; Somoza, F.B.; Lee, T.R. Soluble conjugated polymers that contain ferrocenylene units in the backbone. J. Am. Chem. Soc. 1998, 120, 1621–1622. [Google Scholar] [CrossRef]
- Tolbert, L.M.; Zhao, X.; Ding, Y.; Bottomley, L.A. Bis(ferroceny1)polymethine cations. A prototype molecular wire with redox-active end groups. J. Am. Chem. Soc. 1995, 117, 12891–12892. [Google Scholar] [CrossRef]
- Schvekhgeimer, M.-G.A. Heterylferrocenes. Synthesis and use. Russ. Chem. Rev. 1996, 65, 41–79. [Google Scholar] [CrossRef]
- Klimova, E.I.; Ruíz-Ramírez, L.; Martínez-García, M.; Meleshonkova, N.N. Synthesis of biologically active compounds based on 2-ferrocenylmethylene-3-quinuclidone. Russ. Chem. Bull. 1996, 45, 2602–2609. [Google Scholar] [CrossRef]
- Snegur, L.V.; Boev, V.I.; Yu, S.N.; Ilyin, M.M.; Davankov, V.A.; Starikova, Z.A.; Yanovsky, A.I.; Kolomiets, A.F.; Babin, V.N. Synthesis and structure of biologically active ferrocenylalkyl polyfluoro benzimidazoles. J. Organomet. Chem. 1999, 580, 26–35. [Google Scholar] [CrossRef]
- Klimova, E.; Klimova, T.; Ramírez, A.T.; Nieto, C.A.; Moreno, E.R.; Zea, C.D.; Martínez-García, M. Synthesis of 3-Ferrocenyl-3,3a,4,5-tetrahydro-2H-benzo[g]indazoles. Heterocycles 2004, 63, 1045–1056. [Google Scholar] [CrossRef]
- Vázquez, L.E.A.; Klimova, E.; Klimova, T.; Álvarez, T.C.; Ruíz, R.L.; Toscano, R.A.; Martínez, G.M. Synthesis of ferrocenyl-1-(4-pyridylmethyl)- and ferrocenyl-1-[2-hydroxy-1,2-bis(4-pyridyl)ethyl]pyrazoles. Synthesis 2004, 15, 2471–2478. [Google Scholar]
- Klimova, E.I.; Vázquez, L.E.A.; Klimova, T.; Álvarez, T.C.; Toscano, R.A.; Martínez, G.M. Synthesis of ferrocenylpyrazole derivatives. J. Heterocycl. Chem. 2005, 42, 265–271. [Google Scholar] [CrossRef]
- Martínez, M.J.M.; Vázquez, L.E.A.; Moreno, E.R.; Flores-Alamo, M.; Klimova, E.I. Formation of pyridazino[4,5-c]pyridazine derivatives upon [4+2]-cycloaddition of 4-phenyl-1,2,4-triazoline-3,5-dione to cross-conjugated monoferrocenyltrienes. J. Heterocycl. Chem. 2006, 43, 1115–1121. [Google Scholar] [CrossRef]
- Ratković, Z.; Juranić, Z.D.; Stanojković, T.; Manojlović, D.; Vukićević, R.D.; Radulović, N.; Joksović, M.D. Synthesis, Characterization, Electrochemical studies and antitumor activity of some new chalcone analogues containing ferrocenyl pyrazole moiety. Bioorg. Chem. 2010, 38, 26–32. [Google Scholar] [CrossRef]
- Parveen, H.; Hayat, F.; Salahuddin, A.; Azarn, A. Synthesis, characterization and biological evaluation of novel 6-ferrocenyl-4-aryl-2-substituted pyrimidine derivatives. Eur. J. Med. Chem. 2010, 45, 3497–3503. [Google Scholar] [CrossRef]
- Klimova, E.I.; Sánchez, G.J.J.; Klimova, T.; Ramírez, A.T.; Vázquez, L.E.A.; Flores-Alamo, M.; Martínez, G.M. Synthesis and biological evaluation of novel ethyl 2-amino-6-ferrocenyl-1,6-dihydropyrimidine-5-carboxylates and ethyl 2-amino-6-ferrocenylpyrimidine-5-carboxylates. J. Organomet. Chem. 2012, 708–709, 37–45. [Google Scholar]
- Klimova, E.I.; Sotelo Dominguez, V.H.; Sánchez, G.J.J.; Klimova, T.; Backinowsky, L.V.; Flores-Alamo, M.; Martínez, G.M. 1,3-Insertion of amidines into ethyl E-2-acyl-3-ferrocenylacrylates. Mendeleev. Commun. 2011, 21, 1–3. [Google Scholar] [CrossRef]
- Klimova, E.I.; Klimova, T.; Flores-Alamo, M.; Méndez, S.J.M.; Ruiz, R.L.; Backinowsky, L.V.; Martínez, G.M. The formation of 3-ferrocenylpyrazole-4-carboxylates and alkylhydrazine insertion products from α-ferrocenylmethylidene-β-oxocarboxylates. J. Heterocycl. Chem. 2011, 48, 441–448. [Google Scholar] [CrossRef]
- Postnov, V.N.; Klimova, E.I.; Pushin, A.N.; Meleshonkova, N.N. The interaction of the 1,3-bis-(p-methoxyphenyl)allylic cation with ferrocenyl-1,3-butadienes. Metalloorg. Khim. 1992, 5, 564–569. [Google Scholar]
- Postnov, V.N.; Polivin, Y.N.; Sazonova, V.A. Fragmentation of β-dicarbonyl compounds with ferrocenyl group. Dokl. Akad. Nauk. SSSR 1983, 271, 1402–1404. [Google Scholar]
- Chang, C.H.; Porter, R.F.; Bauer, S.H. Structures of azomethane, 1,1,1-trifluoroazomethane, and hexafluoroazomethane, determined by electron diffraction. J. Am. Chem. Soc. 1970, 92, 5313–5318. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97; Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1994. [Google Scholar]
- Sample Availability: Samples of the compounds 6–8 are available from the authors.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Klimova, E.I.; Flores-Alamo, M.; Klimova, T.; Maya, S.C.; Beletskaya, I.P. Three-Component Reaction of Tautomeric Amidines with 3-Ferrocenylmethylidene-2,4-pentanedione. Formation of Polymeric Coordination Complexes of Potassium Ferrocenyl-(hexahydro)pyrimidoxides. Molecules 2014, 19, 41-54. https://doi.org/10.3390/molecules19010041
Klimova EI, Flores-Alamo M, Klimova T, Maya SC, Beletskaya IP. Three-Component Reaction of Tautomeric Amidines with 3-Ferrocenylmethylidene-2,4-pentanedione. Formation of Polymeric Coordination Complexes of Potassium Ferrocenyl-(hexahydro)pyrimidoxides. Molecules. 2014; 19(1):41-54. https://doi.org/10.3390/molecules19010041
Chicago/Turabian StyleKlimova, Elena I., Marcos Flores-Alamo, Tatiana Klimova, Sandra Cortez Maya, and Irina P. Beletskaya. 2014. "Three-Component Reaction of Tautomeric Amidines with 3-Ferrocenylmethylidene-2,4-pentanedione. Formation of Polymeric Coordination Complexes of Potassium Ferrocenyl-(hexahydro)pyrimidoxides" Molecules 19, no. 1: 41-54. https://doi.org/10.3390/molecules19010041
APA StyleKlimova, E. I., Flores-Alamo, M., Klimova, T., Maya, S. C., & Beletskaya, I. P. (2014). Three-Component Reaction of Tautomeric Amidines with 3-Ferrocenylmethylidene-2,4-pentanedione. Formation of Polymeric Coordination Complexes of Potassium Ferrocenyl-(hexahydro)pyrimidoxides. Molecules, 19(1), 41-54. https://doi.org/10.3390/molecules19010041