Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Linker Synthesis

2.2. Characterization
2.2.1. Elemental Analysis
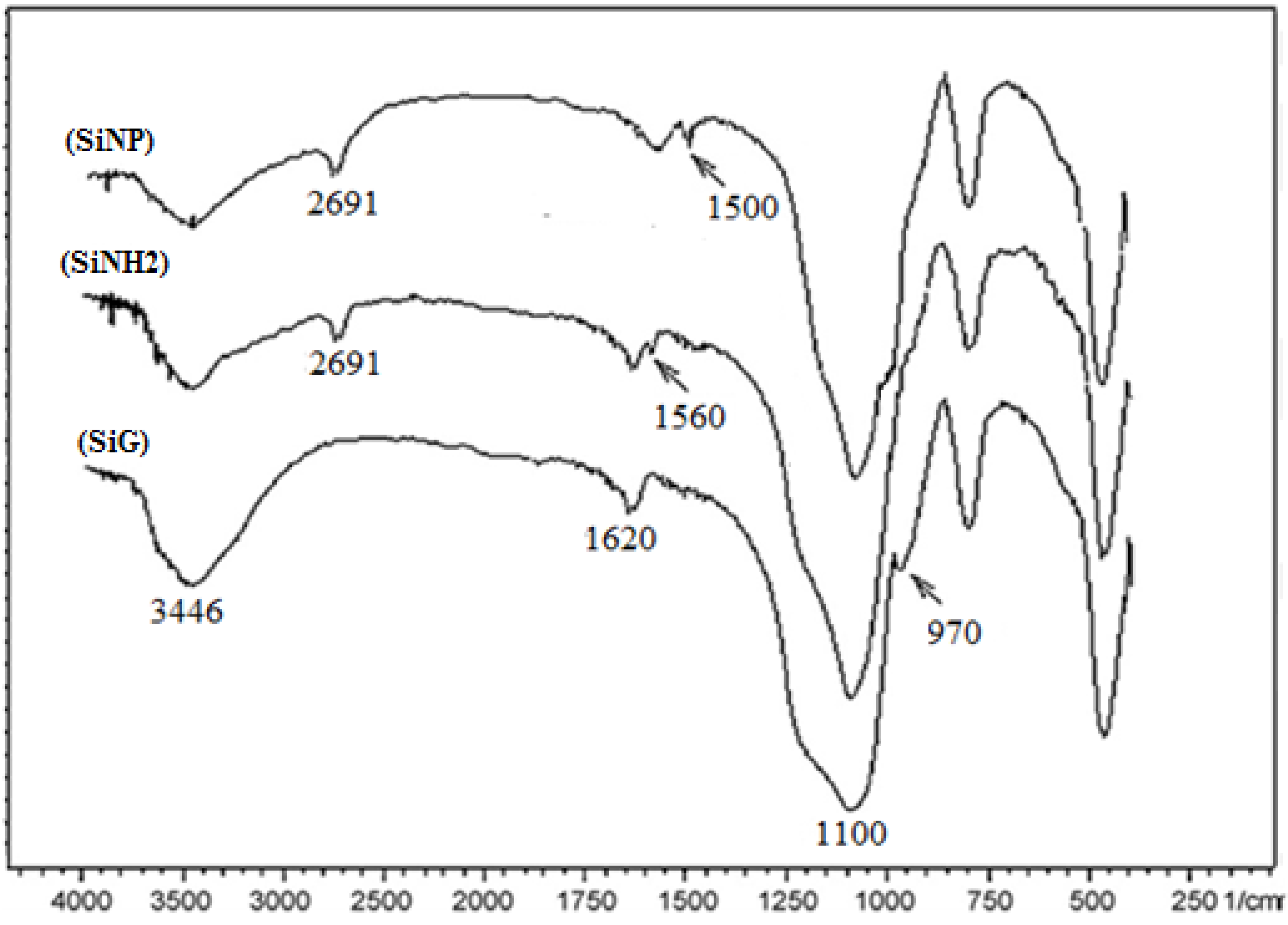
2.2.2. FT-IR Characterization
2.2.3. Scanning Electron Micrographs
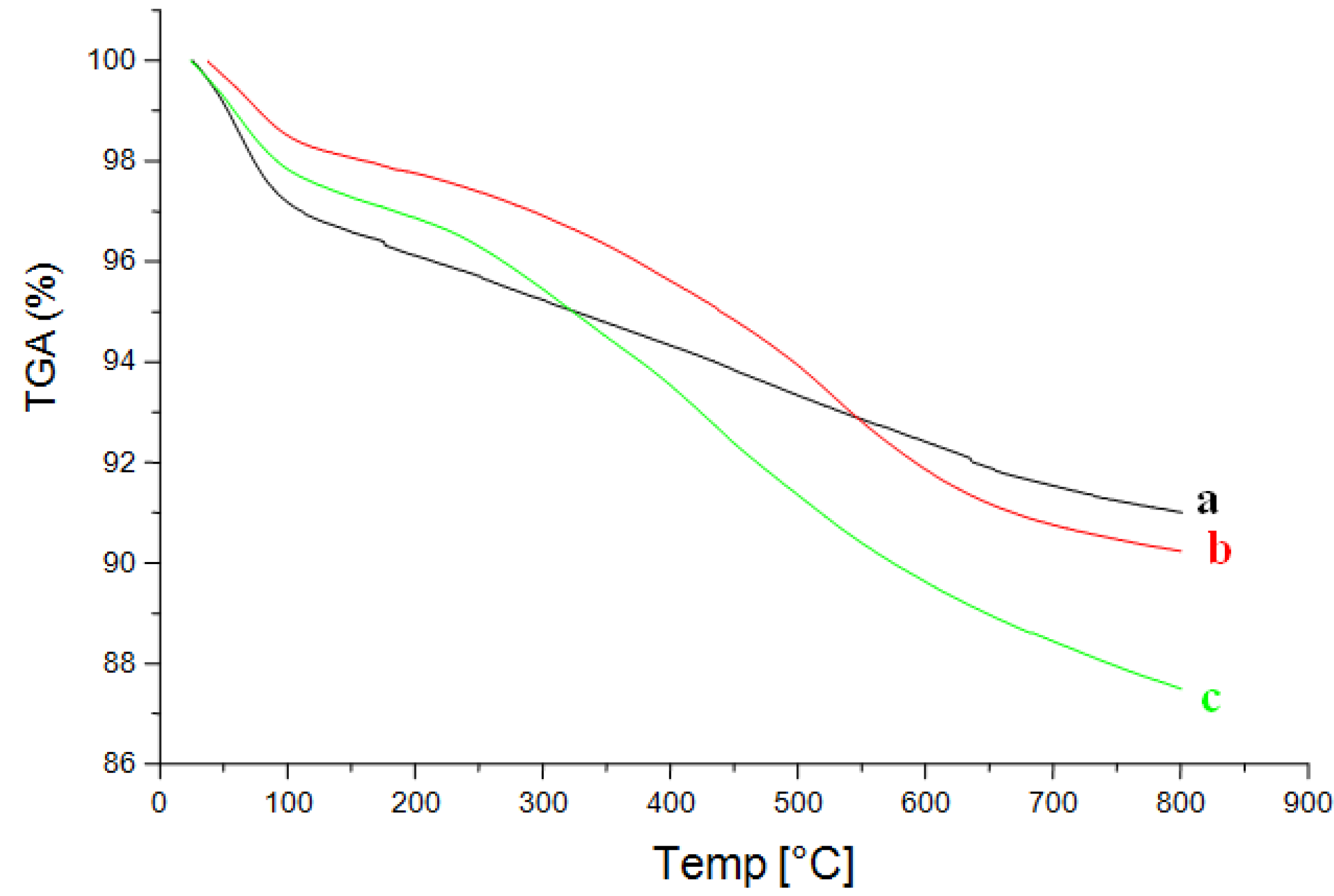
2.2.4.TGA Analysis and Thermal Stability

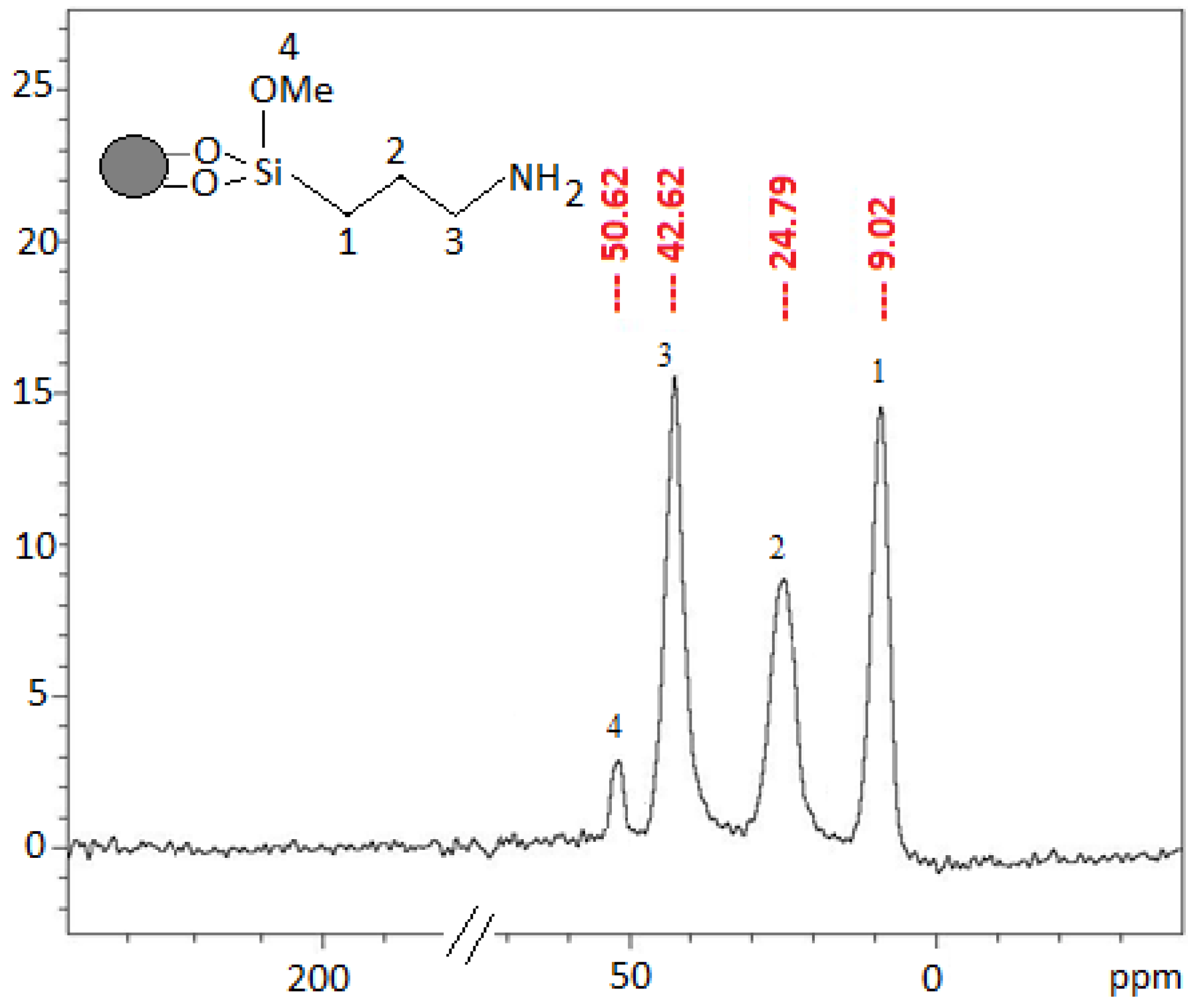
2.2.5. 13C-NMR Characterization
2.2.6. Chemical Stability
2.2.7. Surface Properties
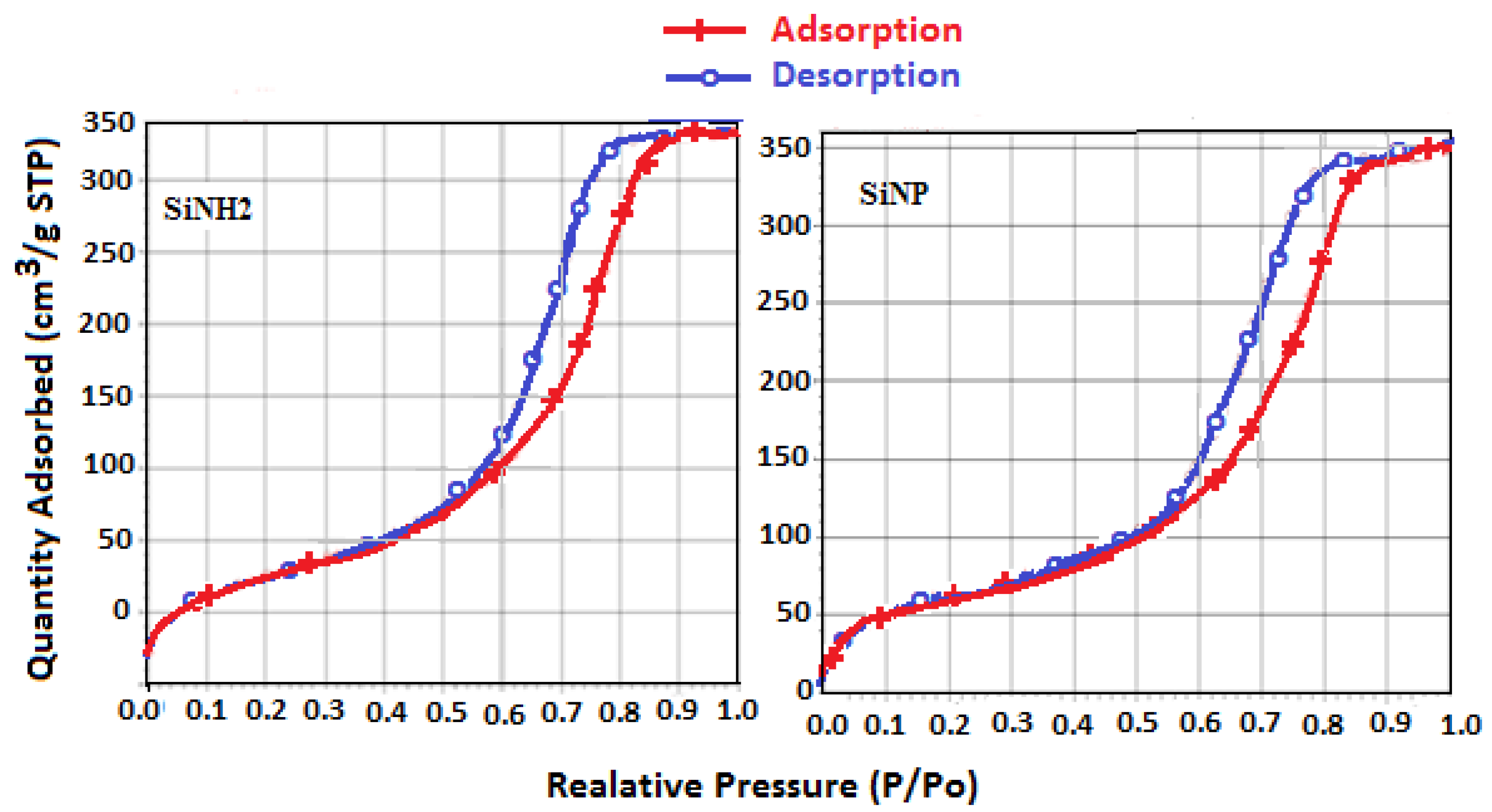
| Silica derivatives | Specific surface SBET (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|
| Free silica | 305.21 | 0.77 |
| SiNH2 | 283.08 | 0.69 |
| SiNP | 236.60 | 0.64 |
2.3. Solid–Liquid Adsorption of Metal Ions by SiNP
2.3.1. Effect of pH
| pH | Pb(II) | Cd(II) | Cu(II) | Zn(II) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 |
| 2 | 3.26 | 5.99 | 0.96 | 0 |
| 3 | 13.92 | 10.43 | 17.08 | 12.24 |
| 4 | 52.83 | 12.66 | 18.68 | 12.25 |
| 5 | 60.38 | 14.34 | 22.04 | 16.55 |
| 6 | 66.14 | 25.29 | 22.1 | 19.13 |
| 7 | 74.86 | 26.89 | 22.08 | 20.43 |
| 8 | 74.89 | 26.93 | 22.06 | 20.43 |
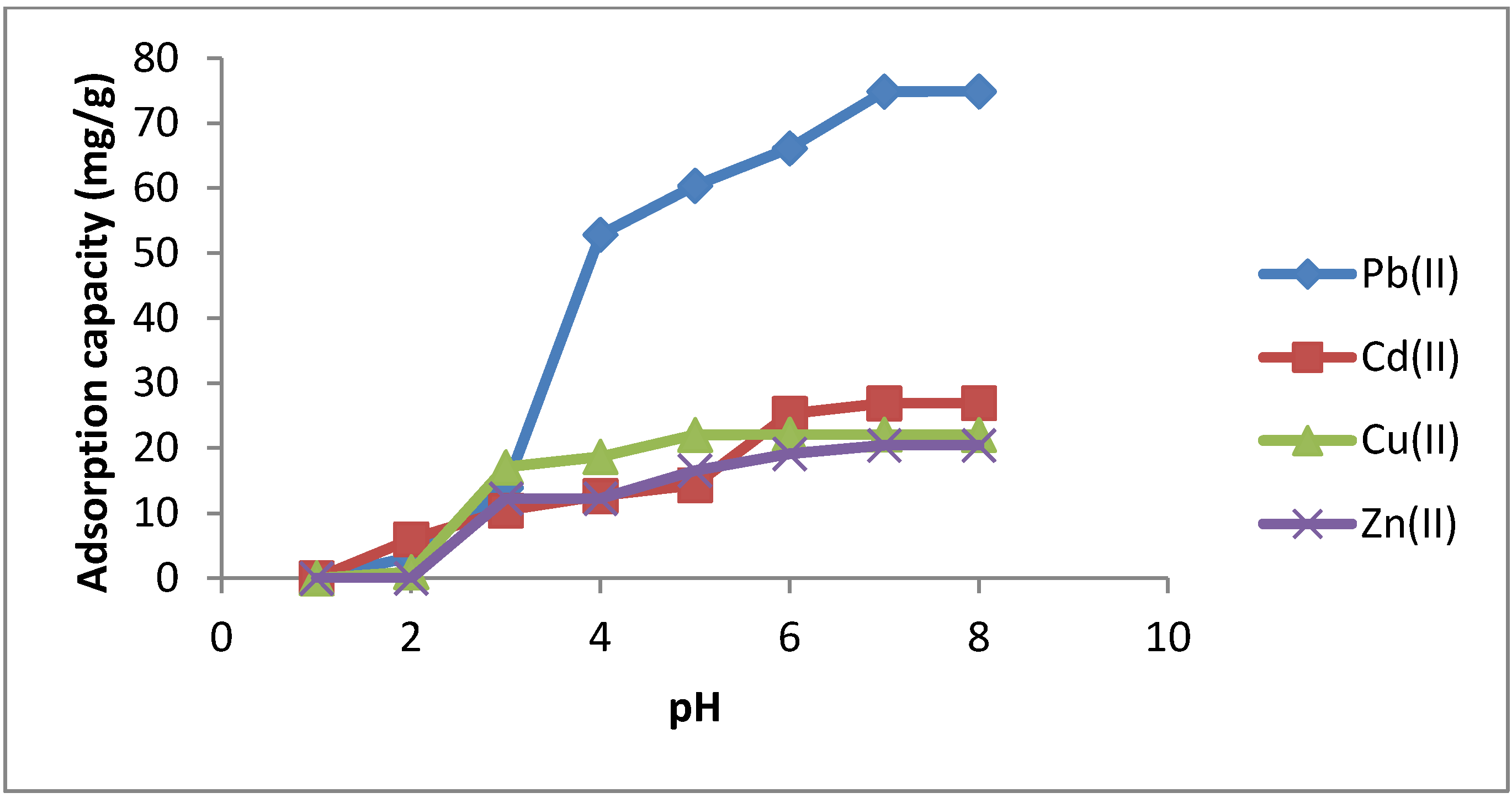
2.3.2. Effect of Stirring Time

2.3.3. Effect of Coexisting Ions
2.3.4. Comparison with Alternative Materials
| Support: silica gel/ligand | Reference | Capacity (mg of Pb2+/g of silica) |
|---|---|---|
| Pyrazol-3-ylimine (this work) | - | 74.89 |
| Gallic acid | [54] | 12.63 |
| Ethylediamine derivatives | [55] | 38.12 |
| C,N-pyridylpyrazole | [56] | 09.5 |
| Thiophene | [57] | 11.3 |
| Acid red 88 | [58] | 03.35 |
| Dithizone | [59] | 08.28 |
| Resacetophenone | [6] | 13.79 |
| Tris(2-aminoethyl) amine | [60] | 64.61 |
| 3-Aminopropytriethoxysilane (SiNH2) | [61] | 23.70 |
3. Experimental
3.1. General Information
3.2. Synthesis of 3-Aminopropylsilica (SiNH2)
3.3. Synthesis of 1.5-Dimethyl-1H-pyrazole-3-carbaldehyde
3.4. Synthesis of ((1,5-Dimethyl-1H-pyrazol-3-yl)methylene)imine-Substituted Silica (SiNP)
3.5. Batch Experiments
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metal uptake from contaminated water: A review. J. Haz. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Saeed, M.M.; Ahmed, M. Retention, kinetics and thermodynamics pro-file of cadmium adsorption from iodide medium onto polyurethane foam and its separation from zinc bulk. Anal. Chem. Acta 2004, 525, 289–297. [Google Scholar] [CrossRef]
- Sanchez, L.R.B.; de La Riva, B.S.V.; Fernandez, J.M.C.; Pereiro, R.; Medel, A.S. Determination of lead and mercury in sea water by preconcentration in a flow injection system followed by atomic adsorption spectrometry detection. Talanta 2001, 55, 1071–1078. [Google Scholar] [CrossRef]
- Silbergeld, E. The Elimination of Lead from Gasoline: Impacts of Lead in Gasoline on Human Health, and the Costs and Benefits of Elimination Lead Additives, Draft Paper; The World Bank: Washington, DC, USA, 1996; p. 3. [Google Scholar]
- Solé, E.; Ballbriga, A.; Dominguez, C. Lead exposure in children: Levels in blood, prevalence of intoxication and related factors. BioMetals 1998, 11, 189–197. [Google Scholar] [CrossRef]
- Goswami, A.; Singh, A.K. Silica gel functionalized with resacetophenone: Synthesis of a new chelating matrix and its application as metal ion collector for their flame atomic absorption spectrometric determination. Anal. Chim. Acta 2002, 454, 229–240. [Google Scholar] [CrossRef]
- Melek, E.; Tuzen, M.; Soylak, M. Flame atomic absorption spectrometric determination of cadmium(II) and lead(II) after their solid phase extraction as dibenzyldithiocarbamate chelates on Dowex Optipore V-493. Anal. Chim. Acta 2006, 578, 213–219. [Google Scholar] [CrossRef]
- Jiang, N.; Chang, X.J.; Zheng, H.; He, Q.; Hu, Z. Selective solid-phase extraction of nickel (II) using a surface-imprinted silica gel sorbent. Anal. Chim. Acta 2006, 577, 225–231. [Google Scholar] [CrossRef]
- Duan, T.C.; Song, X.J.; Jin, D.; Li, H.F.; Xu, J.W.; Chen, H.T. Preliminary results on the determination of ultratrace amounts of cadmium in tea samples using a flow injection on-line solid phase extraction separation and preconcentration technique to couple with a sequential injection hydride generation atomic fluorescence spectrometry. Talanta 2005, 67, 968–974. [Google Scholar] [CrossRef]
- Suvardhan, K.; Suresh Kumar, K.; Rekha, D.; Jayaraj, B.; Krishnamurthy, N.G.; Chiranjeevi, P. Preconcentration and solid-phase extraction of beryllium, lead, nickel, and bismuth from various water samples using 2-propylpiperidine-1-carbodithioate with flame atomic absorption spectrometry (FAAS). Talanta 2006, 68, 735–740. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, Z.R.; Wang, J.H. Flow injection on-line solid phase extraction for ultra-trace lead screening with hydride generation atomic fluorescence spectrometry. Analyst 2006, 131, 141–147. [Google Scholar] [CrossRef]
- Fang, J.; Iang, Y.J.; Yan, X.P.; Ni, Z.M. Selective quantification of trace palladium in road dusts and roadside soils by displacement solid-phase extraction online coupled with electrothermal atomic absorption spectrometry. Environ. Sci. Technol. 2005, 39, 288–292. [Google Scholar] [CrossRef]
- Haginaka, J. Selectivty of affinity media in solide-phase extraction of analytes. Trends Anal. Chem. 2005, 24, 407–415. [Google Scholar] [CrossRef]
- Sarkar, A.R.; Datta, P.K.; Sarkar, M. Sorption recovery of metal ions using silica gel modified with salicyladoxime. Talanta 1996, 43, 1857–1862. [Google Scholar] [CrossRef]
- Price, P.M.; Clark, J.H.; Macquarrie, D.J. Modified silicas for clean technology. J. Chem. Soc. Dalton Trans. 2000, 101–110. [Google Scholar] [CrossRef]
- Grigoropoulou, G.; Stathi, P.; Karakassides, M.A.; Louloudi, M.; Deligibiannakis, Y. Functionalized SiO2 with N-,S-containing ligands for Pb(II) and Cd(II) adsorption. Colloids Surf. 2008, 320, 25–35. [Google Scholar] [CrossRef]
- Fan, J.; Wu, C.; Peng, C.; Peng, P. Preparation of xylenol orange functionalized silica gel as a selective solid phase extractor and application for preconcentration-separtion of mercury from waters. J. Haz. Mater. 2007, 145, 323–330. [Google Scholar] [CrossRef]
- Jamali, M.R.; Assadi, Y.; Shemirani, F.; Salavati-Niasari, M. Application of thiophene-2-carbaldehyde-modified mesoporous silica as a new sorbent for separation and preconcentration of palladium prior to inductively coupled plasma atomic emission spectrometric determination. Talanta 2007, 71, 1524–1529. [Google Scholar] [CrossRef]
- Akhond, M.; Absalan, G.; Sheikhian, L.; Eskandari, M.M.; Sharghi, H. Di (n-propyl) thiuram disulfide bonded on silica gel as a new sorbent for separation, preconcentration, and measurement of silver ion from aqueous samples. Sep. Purif. Technol. 2006, 52, 53–59. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Owereh, O.S.; Aghara, S.K. Synthesis of 4-acylpyrazolone Schiff base ligand grafted silica and selectivity in adsorption of lanthanides from aqueous solutions. J. Rare Eart. 2009, 27, 870–874. [Google Scholar] [CrossRef]
- Ngeontae, W.; Aeungmaitrepirom, W.; Tuntulani, T. Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb(II), Cu(II), Ni(II), Co(II) and Cd(II). Talanta 2007, 71, 1075–1082. [Google Scholar] [CrossRef]
- Goswami, A.; Singh, A.K. 1,8-Dihydroxyanthraquinone anchored on silica gel: Synthesis and application as solid phase extraction for lead(II), Zinc(II) and cadmium(II) prior to determination by flame atomic adsorption spectrometry. Talanta 2002, 58, 669–678. [Google Scholar]
- Sadeghi, S.; Sheikhzadeh, E. Solid phase extraction using silica gel modified with murexide for preconcentration of uranium (VI) ions from water samples. J. Haz. Mater. 2009, 163, 861–868. [Google Scholar] [CrossRef]
- Gubbuk, I.H.; Hatay, I.; Coskun, A.; Ersoz, M. Immobilization of oxime derivative on silica for the preparation of new adsorbent. J. Haz. Mater. 2009, 172, 1532–1537. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pandey, A.; Gulati, S.; Adholeya, A. An optimized procedure for preconcentration, determination and on-line recovery of palladium using highly selective diphenyldiketone-monothiosemicarbazone modified silica gel. J. Haz. Mater. 2012, 209, 285–292. [Google Scholar]
- Mukherjee, R. Coordination chemistry with pyrazol-based chelating ligands: Molecular structural aspects. Coord. Chem. Rev. 2000, 203, 151–218. [Google Scholar] [CrossRef]
- Trofimenko, S. Recent advances in poly(pyrazolyl)borate (scorpionate) chemistry. Chem. Rev. 1993, 93, 943–980. [Google Scholar] [CrossRef]
- Trofimenho, S. The coordination chemistry of pyrazole-derived ligands. Prog. Inorg. Chem. 1986, 34, 115–210. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Tighadouini, S.; Baquet, M. Solid-phase extraction of Hg(II), Zn(II) and Cd(II) from water using silica gel modified with bipyrazolic tripodal receptor. Ind. J. Chem. Tech. 2013, 20, 423–428. [Google Scholar]
- Radi, S.; Basbas, N.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F. New amine-modified silica: Synthesis, characterisation and its use in the Cu(II)-Removal from aqueous solutions. Prog. Nanotech. Nanomat. 2013, 2, 108–116. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, T.; Bacquet, M.; Degoutin, S.; Cazier, F. 1-(Pyridin-2-yl) Imine functionalized silica gel: Synthesis, Characterization and preliminary use in metal ion extraction. Sep. Sci. Tech. 2013, 48, 1349–1355. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Bacquet, M. Synthesis of pyridin-3-yl-functionalized silica as a chelating sorbent for solid-phase adsorption of Hg(II), Pb(II), Zn(II) and Cd(II) from water. Chem. Res. Intermed. 2013, 39, 3791–3802. [Google Scholar] [CrossRef]
- Montoya, V.; Pons, J.; Garcia-Anton, J.; Solans, X.; Font-Bardia, M.; Ros, J. New (η3-Allyl) palladium complexes with pyridylpyrazole ligands: Synthesis, characterization, and study of the influence of N1 substituents on the apparent allyl rotation. Organometallics 2007, 26, 3183–3190. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chen, J.-L.; Chi, Y.; Cheng, Y.-M.; Lee, G.-H.; Jiang, C.-M.; Chou, P.-T. Blue-emitting platinum(II) complexes bearing both pyridylpyrazolate chelate and bridging pyrazolate ligands: Synthesis, structures and photophysical properties. Inorg. Chem. 2007, 46, 11202–11212. [Google Scholar] [CrossRef]
- Qu, R.; Wang, M.; Sun, C.; Zhang, Y.; Ji, C.; Chen, H.; Meng, Y.; Yin, P. Chemical modification of silica-gel with hydroxyl- or amino-terminated polyamine for adsorption of Au(III). Appl. Surf. Sci. 2008, 255, 3361–3370. [Google Scholar]
- Han, D.M.; Fang, G.Z.; Yan, X.P. Preparation and evaluation of a molecularly imprinted sol–gel material for on-line solid-phase extraction coupled with high performance liquid chromatography for the determination of trace pentachlorophenol in water samples. J. Chromatogr. A 2005, 110, 131–136. [Google Scholar]
- Jiang, Y.J.; Gao, Q.M.; Yu, H.G.; Chen, Y.R.; Deng, F. Intensively competitive adsorption for heavy metal ions by PAMAM-SBA-15 and EDTA-PAMAM-SBA-15 inorganic-organic hybrid materials. Micropor. Mesopor. Mater. 2007, 103, 316–324. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.; Kenawy, I.; Hamed, M.; Issa, R.; Elmorsi, R. Separation and preconcentration in a batch mode of Cd(II), Cr(III, VI), Cu(II), Mn(II, VII) and Pb(II) by solid-phase extraction by using of silica modified with N-propylsalicylaldimine. Talanta 2002, 58, 289–300. [Google Scholar] [CrossRef]
- Sales, J.A.A.; Airoldi, C. Calorimetric investigation of metal ion adsorption on 3-glycidoxypropyltrimethylsiloxane + propane-1,3-diamine immobilized on silica gel. Thermochim. Acta 2005, 427, 77–83. [Google Scholar] [CrossRef]
- Sales, J.A.A.; Faria, F.P.; Prado, A.G.S.; Airoldi, C. Attachment of 2-aminomethyl-pyridine molecule onto grafted silica gel surface and its ability in chelating cations. Polyhedron 2004, 23, 719–725. [Google Scholar] [CrossRef]
- Roumeliotis, P.; Kurganov, A.A.; Davankov, V.A. Effect of the hydrophobic spacer in bonded (Cu(l-hydroxyprolyl)alkyl)+ silicas on retention and enantioselectivity of α-amino acids in high-performance liquid chromatography. J. Chromatogr. A 1983, 266, 439–450. [Google Scholar] [CrossRef]
- Kudryavtsev, G.V.; Milchenko, D.V.; Bernadyuk, S.Z.; Vertinskaya, T.E.; Lisichkin, G.V. Synthesis and properties of phosphate cation-exchangers based on silica. Theor. Exp. Chem. USSR 1988, 23, 658–663. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Banerjee, I.A.; Yu, L.; Matsui, H. Cu nanocrystal growth on peptide nanotubes by biomineralization: Size control of Cu nanocrystals by tuning peptide conformation. Proc. Natl. Acad. Sci. USA 2003, 100, 14678–14682. [Google Scholar] [CrossRef]
- Xue, X.; Li, F. Removal of Cu (II) from aqueous solution by adsorption onto functionalized SBA-16 mesoporous silica. Micropor. Mesopor. Mater. 2008, 116, 116–122. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Myers, R.T. Thermodynamics of chelation. Inorg. Chem. 1978, 17, 952–958. [Google Scholar]
- Mahmoud, R.E. Silica gel-immobilized Eriochrome blach-T as a potential solid phase extractor for zinc (II) and magnesium (II) from calcium (II). Talanta 1997, 45, 309–315. [Google Scholar] [CrossRef]
- Maquieira, A.; Elmahadi, H.A.M.; Puchades, R. Immobilized cyanobacteria for online trace metal enrichment by flow injection atomic absorption spectrometry. Anal. Chem. 1994, 66, 3632–3638. [Google Scholar] [CrossRef]
- Lehn, J.M.; Sauvage, J.P. (2)-Cryptates: Stability and selectivity of alkali and alkaline-earth macrobicyclic complexes. J. Am. Chem. Soc. 1975, 97, 6700–6707. [Google Scholar] [CrossRef]
- Radi, S.; Yahyi, A.; Ramdani, A.; Zidane, I.; Hacht, B. A new tetrapyrazolic macrocycle. Synthesis and its use in extraction and transport of Na+, Li+, and K+. Tetrahedron 2006, 62, 9153–9155. [Google Scholar]
- Radi, S.; Ramdani, A.; Lekchiri, Y.; Morcellet, M.; Crini, G.; Morcellet, J.; Janus, L. New tetrapyrazolic macrocycle. Synthesis and cation binding properties. J. Chem. Res. 2003, 11, 712–714. [Google Scholar]
- Radi, S.; Ramdani, A.; Lekchiri, Y.; Morcellet., M.; Crini, G.; Morcellet, J.; Janus, L. New tetrapyrazolic macrocycle. Synthesis and preliminary use in metal ion extraction. Tetrahedron 2004, 60, 939–942. [Google Scholar] [CrossRef]
- Xie, F.; Lin, X.; Wu, W.; Xie, Z. Solid phase extraction of lead (II), copper (II), cadmium (II), and nickel (II) using gallic acid modified silica gel prior to determination by flame atomic adsorption spectrometry. Talanta 2008, 74, 836–843. [Google Scholar] [CrossRef]
- Chiron, N.; Guilet, R. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinrtic models. Water Res. 2003, 37, 3079–3086. [Google Scholar] [CrossRef]
- Radi, S.; Ramdani, A.; Lekchiri, Y.; Morcellet, M.; Crini, G.; Janus, L.; Bacquet, M. Immobilization of pyrazole compounds on silica gels and their preliminary use in metal ion extraction. New J. Chem. 2003, 27, 1224–1227. [Google Scholar] [CrossRef]
- Radi, S.; Attayibat, A. Functionalized SiO2 with S-donor thiophene. Synthesis, Characterisation and its heavy metals adsorption. Phos. Sul. Sil. Relat. Elem. 2010, 185, 2003–2013. [Google Scholar]
- Kocjan, R. Retention of some metal ions and their separation on silica gel modified with Acid Red 88. Mikrochim. Acta 1999, 131, 153–158. [Google Scholar] [CrossRef]
- Zaporozhets, O.; Petruniock, N.; Sukhan, V. Determination of Ag(I), Hg(II) and Pb(II) by using silica gel loeded with dithizone and zinc dithizonate. Talanta 1999, 50, 865–873. [Google Scholar] [CrossRef]
- Huang, X.; Chang, X.; He, Q.; Cui, Y.; Zhai, Y.; Jiang, N. Tris(2-aminoethyl) amine functionalized silica gel for solid-phase extraction and preconcentration of Cr(II), Cd(II), and Pb(II) from waters. J. Haz. Mater. 2008, 157, 154–160. [Google Scholar] [CrossRef]
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu(II) and Cd(II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Fifani, J.; Ramdani, A.; Tarrago, G. 1,6,11,16-Tetraazaporphyrinogen, Synthesis and behavior. New J. Chem. 1977, 1, 521–528. [Google Scholar]
- Tarrago, G.; Ramdani, A.; Elguero, J.; Espada, M. Orientation de la réaction d'alkylation des pyrazoles dans des conditions neutres et en catalyse par transfert de phase. J. Heterocycl. Chem. 1980, 17, 137–142. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the adsorbents are available from the authors.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F.; Zaghrioui, M.; Mabkhot, Y.N. Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals. Molecules 2014, 19, 247-262. https://doi.org/10.3390/molecules19010247
Radi S, Tighadouini S, Bacquet M, Degoutin S, Cazier F, Zaghrioui M, Mabkhot YN. Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals. Molecules. 2014; 19(1):247-262. https://doi.org/10.3390/molecules19010247
Chicago/Turabian StyleRadi, Smaail, Said Tighadouini, Maryse Bacquet, Stéphanie Degoutin, Francine Cazier, Mustapha Zaghrioui, and Yahia N. Mabkhot. 2014. "Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals" Molecules 19, no. 1: 247-262. https://doi.org/10.3390/molecules19010247
APA StyleRadi, S., Tighadouini, S., Bacquet, M., Degoutin, S., Cazier, F., Zaghrioui, M., & Mabkhot, Y. N. (2014). Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals. Molecules, 19(1), 247-262. https://doi.org/10.3390/molecules19010247





