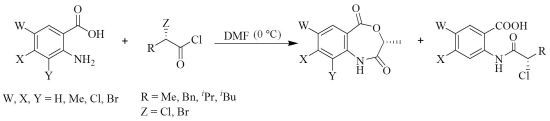
Asymmetric Synthesis of 4,1-Benzoxazepine-2,5-Diones — Effect of the Halogen of (2S)-α-Haloacids
Abstract
:1. Introduction
2. Results and Discussion
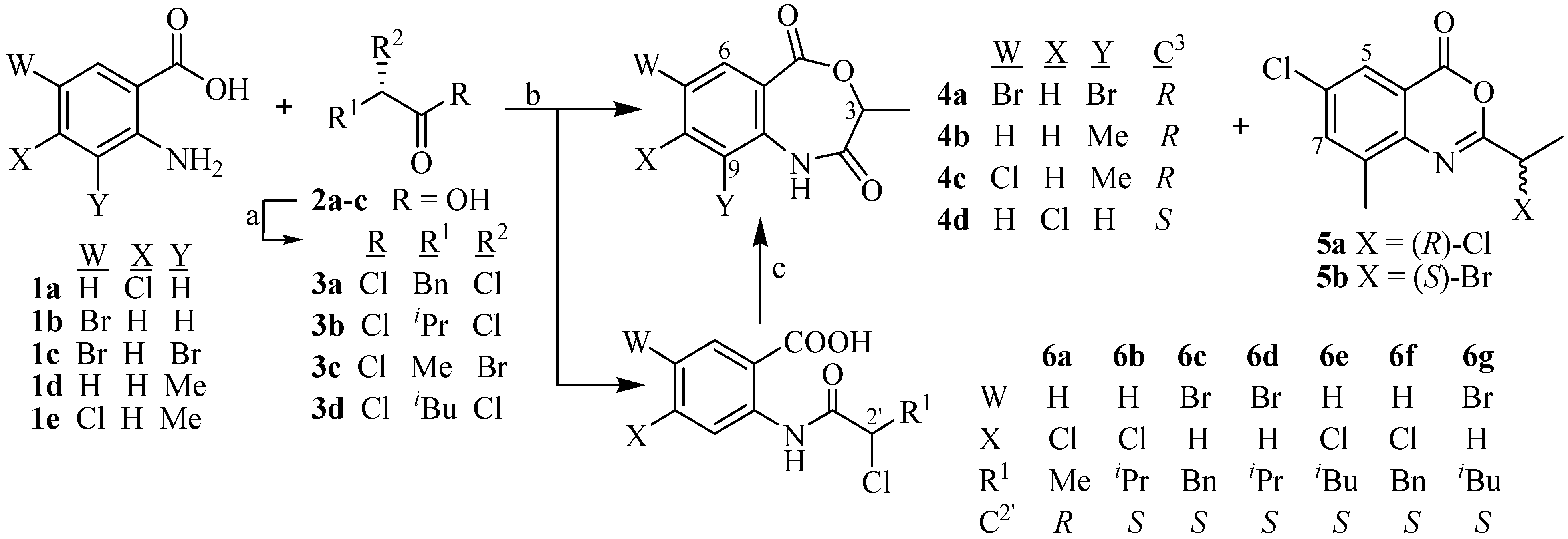
| 4a | 4b | 4c | 4d | 5 § | 6a | 6b | 6c | 6d | 6e | 6f | 6g | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Yield | 50 | 78 | 66 | 57 | 32 | 67 | 86 | 70 | 71 | 68 | 67 | 46 |
| c * | 0.5 | 0.2 | 0.2 | 0.2 | 0.3 | 0.5 | 0.6 | 1.0 | 1.0 | 1.0 | 1.0 | 0.2 |
| [α]D (°C) | +12.0 (30) | +80.0 (30) | +67.9 (30) | +54.0 (30) | –19.0 (23) | +80.0 (30) | +16.9 (30) | +17.0 (30) | +32.0 (30) | +16.2 (25) | +35.2 (25) | +23.3 (26) |
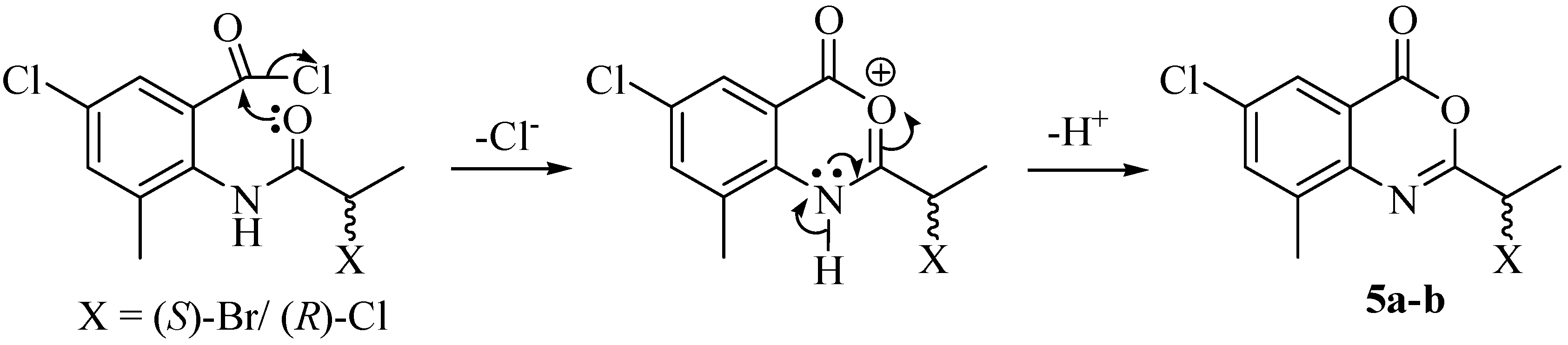
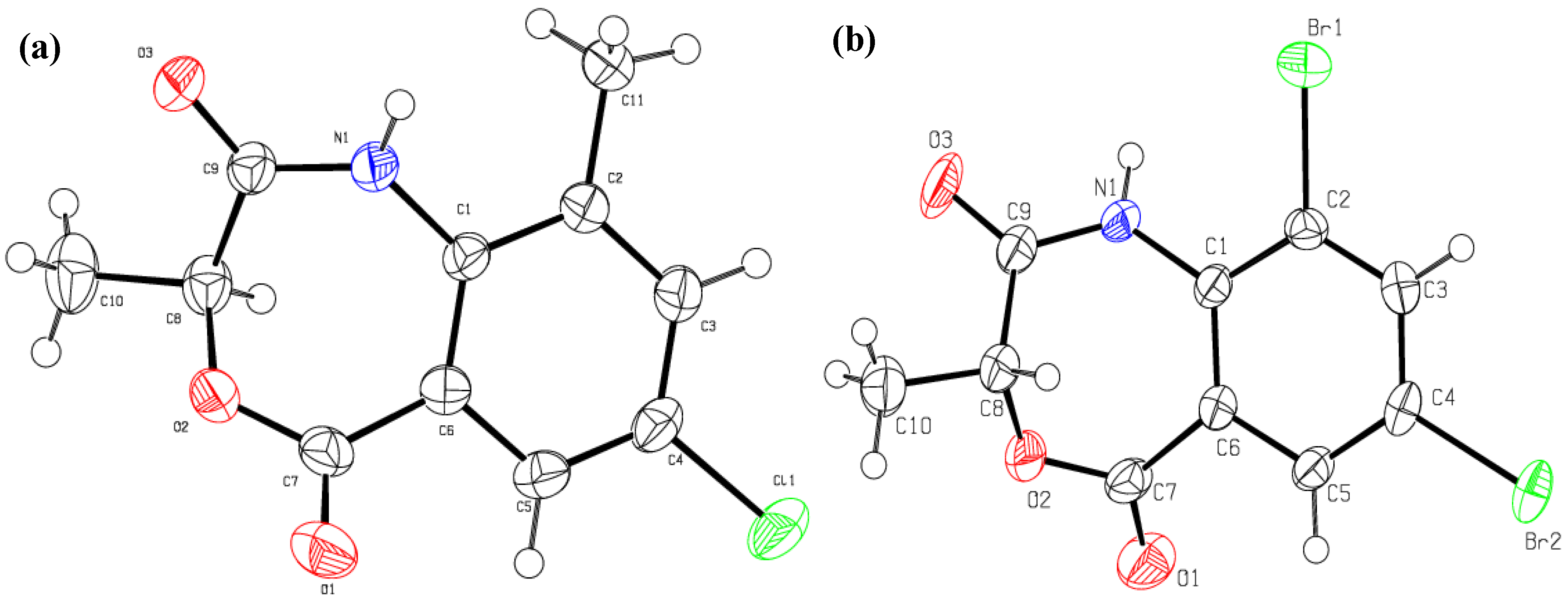
3. Experimental
General Information
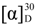 = +12.0 (c 0.5, MeOH); MP: 165 °C; 1H-NMR (300 MHz, CD3OD): δ (ppm) 2.60 (3H, d, J = 6.9 Hz, CH3), 4.35 (1H, q, J = 6.9 Hz, H3), 7.70 (1H, d, J = 1.5 Hz, H8), 7.94 (1H, d, J = 1.8 Hz, H6); IR (KBr): ύmax (cm−1) 1697 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 304 nm (log ε = 3.21670 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 351, 349, 347 (6, 12, 6 in 1:2:1 ratio) [M]+•, 279, 277 and 275 (39, 77 and 40 in 1:2:1 ratio) [M-C3H4O2, A]+•, 251, 249 and 247 (8, 17 and 9 in 1:2:1 ratio) [A-CO]+•, ESI MS (m/z) for C10H7Br2NO3: 373.8649, 371.8669 and 369.8690 found for 373.8645 [M+4+Na], 371.8665 [M+2+Na] and 369.8686 [M+Na] in 1:2:1.
= +12.0 (c 0.5, MeOH); MP: 165 °C; 1H-NMR (300 MHz, CD3OD): δ (ppm) 2.60 (3H, d, J = 6.9 Hz, CH3), 4.35 (1H, q, J = 6.9 Hz, H3), 7.70 (1H, d, J = 1.5 Hz, H8), 7.94 (1H, d, J = 1.8 Hz, H6); IR (KBr): ύmax (cm−1) 1697 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 304 nm (log ε = 3.21670 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 351, 349, 347 (6, 12, 6 in 1:2:1 ratio) [M]+•, 279, 277 and 275 (39, 77 and 40 in 1:2:1 ratio) [M-C3H4O2, A]+•, 251, 249 and 247 (8, 17 and 9 in 1:2:1 ratio) [A-CO]+•, ESI MS (m/z) for C10H7Br2NO3: 373.8649, 371.8669 and 369.8690 found for 373.8645 [M+4+Na], 371.8665 [M+2+Na] and 369.8686 [M+Na] in 1:2:1.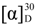 = +80.0 (c 0.2, MeOH); MP: 190 °C; 1H-NMR (300 MHz, CDCl3): δ (ppm) 1.83 (3H, d, J = 6.9 Hz, CH3), 2.27 (3H, s, Ar-CH3), 4.55 (1H, q, J = 6.9 Hz, H3), 7.24 (1H, t, J = 7.5 Hz, H7), 7.49 (1H, d, J = 7.5 Hz, H8), 7.90 (1H, d, J = 7.8 Hz, H6), 9.56 (1H, s, NH); 13C-NMR (75 MHz, CDCl3): δ (ppm) 18.8 (Ar-CH3), 22.8 (C1'), 55.9 (C3), 126.2 (C9), 126.3 (C7), 129.3 (C8), 129.4 (C5a), 136.5 (C6), 136.6 (C9a), 168.0, 170.6 (C2 and C5); IR (KBr): ύmax (cm−1) 1697 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 294 nm (log ε = 3.32135 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 205 (72) [M]+•, 133 (100) [M-C3H4O2, A]+•, 105 (100) [A-CO]+•, ESI MS (m/z) for C11H11NO3: 228.0636 found for 228.0631 [M+Na].
= +80.0 (c 0.2, MeOH); MP: 190 °C; 1H-NMR (300 MHz, CDCl3): δ (ppm) 1.83 (3H, d, J = 6.9 Hz, CH3), 2.27 (3H, s, Ar-CH3), 4.55 (1H, q, J = 6.9 Hz, H3), 7.24 (1H, t, J = 7.5 Hz, H7), 7.49 (1H, d, J = 7.5 Hz, H8), 7.90 (1H, d, J = 7.8 Hz, H6), 9.56 (1H, s, NH); 13C-NMR (75 MHz, CDCl3): δ (ppm) 18.8 (Ar-CH3), 22.8 (C1'), 55.9 (C3), 126.2 (C9), 126.3 (C7), 129.3 (C8), 129.4 (C5a), 136.5 (C6), 136.6 (C9a), 168.0, 170.6 (C2 and C5); IR (KBr): ύmax (cm−1) 1697 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 294 nm (log ε = 3.32135 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 205 (72) [M]+•, 133 (100) [M-C3H4O2, A]+•, 105 (100) [A-CO]+•, ESI MS (m/z) for C11H11NO3: 228.0636 found for 228.0631 [M+Na].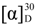 = +67.9 (c 0.2, EtOAc); MP: 187 °C; 1H-NMR (300 MHz, CD3OD): δ (ppm) 1.73 (3H, d, J = 6.9 Hz, H1'), 2.26 (3H, s, Ar-CH3), 4.67 (1H, q, J = 6.9 Hz, H3), 7.50 (1H, broad s, H8), 7.76 (1H, d, J = 2.4 Hz, H6); 13C-NMR (75 MHz, CD3OD): δ (ppm) 18.2 (Ar-CH3), 22.1 (C1'), 55.7 (C3), 129.4 (C8), 129.8 (C5a), 135.0 (C6), 135.6 (C7), 168.0, 170.6 (C2 and C5); IR (KBr): ύmax (cm−1) 3362 (N-H), 1693 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 306 nm (log ε = 3.25701 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 241, 239 (13, 37 in 1:3 ratio) [M]+•, 169, 167 (29, 100 in 1:3 ratio) [M-(3-methyloxirane-2-one), A]+•, 141, 139 (31, 90 in 1:3 ratio) [A-CO]+•; ESI MS (m/z) for C11H10ClNO3: 264.0217 and 262.0246 found for 264.0214 [M+2+Na] and 262.0242 [M+Na] in 1:3 ratio.
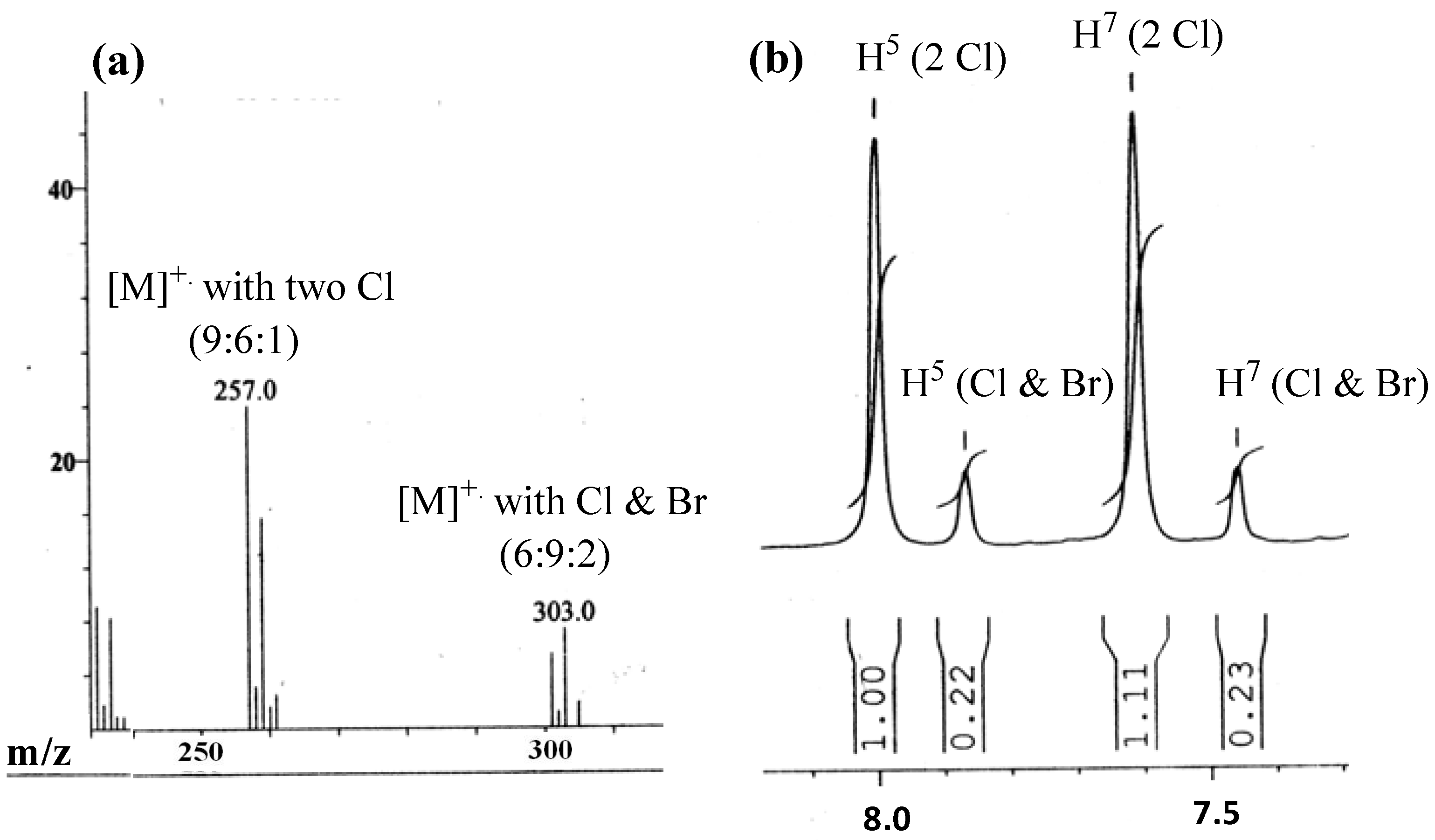
= +67.9 (c 0.2, EtOAc); MP: 187 °C; 1H-NMR (300 MHz, CD3OD): δ (ppm) 1.73 (3H, d, J = 6.9 Hz, H1'), 2.26 (3H, s, Ar-CH3), 4.67 (1H, q, J = 6.9 Hz, H3), 7.50 (1H, broad s, H8), 7.76 (1H, d, J = 2.4 Hz, H6); 13C-NMR (75 MHz, CD3OD): δ (ppm) 18.2 (Ar-CH3), 22.1 (C1'), 55.7 (C3), 129.4 (C8), 129.8 (C5a), 135.0 (C6), 135.6 (C7), 168.0, 170.6 (C2 and C5); IR (KBr): ύmax (cm−1) 3362 (N-H), 1693 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 306 nm (log ε = 3.25701 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 241, 239 (13, 37 in 1:3 ratio) [M]+•, 169, 167 (29, 100 in 1:3 ratio) [M-(3-methyloxirane-2-one), A]+•, 141, 139 (31, 90 in 1:3 ratio) [A-CO]+•; ESI MS (m/z) for C11H10ClNO3: 264.0217 and 262.0246 found for 264.0214 [M+2+Na] and 262.0242 [M+Na] in 1:3 ratio.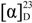 = −19.0 (c 0.3, EtOAc); MP: 129 °C; 1H-NMR (300 MHz, CDCl3): δ (ppm) 2.04 (3H, d, J = 6.9 Hz, H2'), 2.53 (3H, s, Ar-CH3), 4.82 (1H, q, J = 6.9 Hz, H1'), 7.61 (1H, broad s, H7), 8.00 (1H, broad s, H5), IR (KBr): ύmax (cm−1) 1764 (lactonic OC=O), 1528 (C=N); UV-Vis (MeOH): λmax 326 nm (log ε = 3.96534 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 261, 259 and 257 (2.5, 16 and 24 in 1:6:9 ratio) [M with 2 Cl]+•, 224, 222 (22, 63 in 1:3 ratio) [M-•Cl]+, 196, 194 (31, 100 in 1:3 ratio) [M-H3CC∙(H)Cl]+; ESI MS (m/z) for C11H9Cl2NO2: 283.9849, 281.9878 and 279.9908 found for 283.9846 [M+4+Na], 281.9875 [M+2+Na] and 279.9905 [M+Na] in 1:6:9 ratio.
= −19.0 (c 0.3, EtOAc); MP: 129 °C; 1H-NMR (300 MHz, CDCl3): δ (ppm) 2.04 (3H, d, J = 6.9 Hz, H2'), 2.53 (3H, s, Ar-CH3), 4.82 (1H, q, J = 6.9 Hz, H1'), 7.61 (1H, broad s, H7), 8.00 (1H, broad s, H5), IR (KBr): ύmax (cm−1) 1764 (lactonic OC=O), 1528 (C=N); UV-Vis (MeOH): λmax 326 nm (log ε = 3.96534 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 261, 259 and 257 (2.5, 16 and 24 in 1:6:9 ratio) [M with 2 Cl]+•, 224, 222 (22, 63 in 1:3 ratio) [M-•Cl]+, 196, 194 (31, 100 in 1:3 ratio) [M-H3CC∙(H)Cl]+; ESI MS (m/z) for C11H9Cl2NO2: 283.9849, 281.9878 and 279.9908 found for 283.9846 [M+4+Na], 281.9875 [M+2+Na] and 279.9905 [M+Na] in 1:6:9 ratio.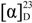 = −19.0 (c 0.3, EtOAc); MP: 129 °C; 1H-NMR (300 MHz, CDCl3): δ (ppm) 1.95 (3H, d, J = 6.6 Hz, H2'), 2.24 (3H, s, Ar-CH3), 4.51 (1H, q, J = 6.9 Hz, H1'), 7.45 (1H, broad s, H7), 7.86 (1H, broad s, H5), IR (KBr): ύmax (cm−1) 1761 (lactonic OC=O), 1528 (C=N); UV-Vis (MeOH): λmax 326 nm (log ε = 3.96534 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 305, 303 and 301 (1.9, 7.4 and 5.5 in 2:9:6 ratio) [M with Br and Cl]+•, 224, 222 (22, 63 in 1:3) [M-•Br]+, 196, 194 (31, 100 in 1:3 ratio) [M-H3CC•(H)Br]+; ESI MS (m/z) for C11H9BrClNO2: 327.9352, 325.9373 and 323.9402 found for 327.9350 [M+4+Na], 325.9370 [M+2+Na] and 323.9400 [M+Na] in 2:9:6 ratio.
= −19.0 (c 0.3, EtOAc); MP: 129 °C; 1H-NMR (300 MHz, CDCl3): δ (ppm) 1.95 (3H, d, J = 6.6 Hz, H2'), 2.24 (3H, s, Ar-CH3), 4.51 (1H, q, J = 6.9 Hz, H1'), 7.45 (1H, broad s, H7), 7.86 (1H, broad s, H5), IR (KBr): ύmax (cm−1) 1761 (lactonic OC=O), 1528 (C=N); UV-Vis (MeOH): λmax 326 nm (log ε = 3.96534 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 305, 303 and 301 (1.9, 7.4 and 5.5 in 2:9:6 ratio) [M with Br and Cl]+•, 224, 222 (22, 63 in 1:3) [M-•Br]+, 196, 194 (31, 100 in 1:3 ratio) [M-H3CC•(H)Br]+; ESI MS (m/z) for C11H9BrClNO2: 327.9352, 325.9373 and 323.9402 found for 327.9350 [M+4+Na], 325.9370 [M+2+Na] and 323.9400 [M+Na] in 2:9:6 ratio.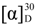 = +80.0 (c 0.5, EtOAc); MP: 187 °C; 1H-NMR (400 MHz, CDCl3): δ (ppm) 1.82 (3H, d, J = 7.2 Hz, H3'), 4.54 (1H, q, J = 7.2 Hz, H2'), 7.15 (1H, dd, J = 8.4, 1.6 Hz, H5), 8.07 (1H, d, J = 8.8 Hz, H6), 8.82 (1H, d, J = 1.5 Hz, H3), 11.7 (1H, s, NH); IR (KBr): ύmax (cm−1) 1678 (OC=O), 1583 (NC=O); UV-Vis (MeOH): λmax 306 nm (log ε = 3.950121 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 265, 263 and 261 (2, 11 and 21 in 1:6:9 ratio) [M]+•, 200, 198 (15, 45 in 1:3 ratio) [M-H3CC•(H)Cl, A]+, 182, 180 (45, 100) [A-H2O]+.
= +80.0 (c 0.5, EtOAc); MP: 187 °C; 1H-NMR (400 MHz, CDCl3): δ (ppm) 1.82 (3H, d, J = 7.2 Hz, H3'), 4.54 (1H, q, J = 7.2 Hz, H2'), 7.15 (1H, dd, J = 8.4, 1.6 Hz, H5), 8.07 (1H, d, J = 8.8 Hz, H6), 8.82 (1H, d, J = 1.5 Hz, H3), 11.7 (1H, s, NH); IR (KBr): ύmax (cm−1) 1678 (OC=O), 1583 (NC=O); UV-Vis (MeOH): λmax 306 nm (log ε = 3.950121 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 265, 263 and 261 (2, 11 and 21 in 1:6:9 ratio) [M]+•, 200, 198 (15, 45 in 1:3 ratio) [M-H3CC•(H)Cl, A]+, 182, 180 (45, 100) [A-H2O]+.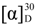 = +16.9 (c 0.6, MeOH); MP: 178 °C; 1H-NMR (300 MHz, CDCl3): 1.00 (3H, d, J = 6.3 Hz, CH3), 1.09 (3H, d, J = 6.6 Hz, H4'), 2.55 (1H, m, H3'), 4.35 (1H, d, 4.5 Hz, H2'), 7.13 (1H, dd, J = 8.7, 1.5 Hz, H5), 8.06 (1H, d, J = 8.6 Hz, H6), 8.83 (1H, d, J = 1.5 Hz, H3), 11.78 (1H, s, NH); IR (KBr): ύmax (cm−1) 3500 (O-H), 3383 (N-H), 1666 (OC=O), 1595 (NC=O); UV-Vis (MeOH): λmax 336 nm (log ε = 3.01872 L cm−1 M−1). ESI MS (m/z) for C12H13Cl2NO3: 316.0111, 314.0140 and 312.0170 found for 316.0108 [M+4+Na], 314.0136 [M+2+Na] and 312.0167 [M+Na] in 1:6:9 ratio.
= +16.9 (c 0.6, MeOH); MP: 178 °C; 1H-NMR (300 MHz, CDCl3): 1.00 (3H, d, J = 6.3 Hz, CH3), 1.09 (3H, d, J = 6.6 Hz, H4'), 2.55 (1H, m, H3'), 4.35 (1H, d, 4.5 Hz, H2'), 7.13 (1H, dd, J = 8.7, 1.5 Hz, H5), 8.06 (1H, d, J = 8.6 Hz, H6), 8.83 (1H, d, J = 1.5 Hz, H3), 11.78 (1H, s, NH); IR (KBr): ύmax (cm−1) 3500 (O-H), 3383 (N-H), 1666 (OC=O), 1595 (NC=O); UV-Vis (MeOH): λmax 336 nm (log ε = 3.01872 L cm−1 M−1). ESI MS (m/z) for C12H13Cl2NO3: 316.0111, 314.0140 and 312.0170 found for 316.0108 [M+4+Na], 314.0136 [M+2+Na] and 312.0167 [M+Na] in 1:6:9 ratio.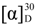 = +17.0 (c 1.0, MeOH); MP: 110 °C; 1H-NMR (500 MHz, CD3OD): 3.21 (1H, dd, J = −14.0, 8.0 Hz, Hα3'), 3.43 (1H, dd, J = −14.0, 8.0 Hz, Hβ3'), 4.69 (1H, dd, J = 8.0, 6.0 Hz, H2'), 7.17-7.24 (5H, m, Ph), 7.63 (1H, dd, J = 9.0, 2.5 Hz, H4), 8.11 (1H, d, J = 2.5 Hz, H6), 8.48 (1H, d, J = 9.0, H3); 13C-NMR (125 MHz, CD3OD) δ (ppm): 42.46 (C3'), 62.29 (C2'), 116.76 (C1''), 119.97 (C1), 123.08 (C4''), 128.18 (C3), 129.45, 130.54 (C3'' and C2''), 134.92 (C4), 137.47 (C5), 137.71 (C6), 140.58 (C2), 169.23 (NC=O), 169.57 (OC=O); IR (KBr): ύmax (cm−1) 3028 (O-H), 2916 (N-H), 1709 (OC=O), 1531 (NC=O); UV-Vis (MeOH): λmax 317 nm (log ε = 3.56741 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 385, 383, 381 (1, 5, 3 in 2:9:6 ratio) [M]+•, 226, 224 (16, 15 in 1:1 ratio) [M-•CH2(Cl)Bn and H2O, A]+, 217, 215 (53, 52 in 1:1) [M-•CH2(Cl)Bn and CO]+, 198, 196 (56, 56) [A-CO]+.
= +17.0 (c 1.0, MeOH); MP: 110 °C; 1H-NMR (500 MHz, CD3OD): 3.21 (1H, dd, J = −14.0, 8.0 Hz, Hα3'), 3.43 (1H, dd, J = −14.0, 8.0 Hz, Hβ3'), 4.69 (1H, dd, J = 8.0, 6.0 Hz, H2'), 7.17-7.24 (5H, m, Ph), 7.63 (1H, dd, J = 9.0, 2.5 Hz, H4), 8.11 (1H, d, J = 2.5 Hz, H6), 8.48 (1H, d, J = 9.0, H3); 13C-NMR (125 MHz, CD3OD) δ (ppm): 42.46 (C3'), 62.29 (C2'), 116.76 (C1''), 119.97 (C1), 123.08 (C4''), 128.18 (C3), 129.45, 130.54 (C3'' and C2''), 134.92 (C4), 137.47 (C5), 137.71 (C6), 140.58 (C2), 169.23 (NC=O), 169.57 (OC=O); IR (KBr): ύmax (cm−1) 3028 (O-H), 2916 (N-H), 1709 (OC=O), 1531 (NC=O); UV-Vis (MeOH): λmax 317 nm (log ε = 3.56741 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 385, 383, 381 (1, 5, 3 in 2:9:6 ratio) [M]+•, 226, 224 (16, 15 in 1:1 ratio) [M-•CH2(Cl)Bn and H2O, A]+, 217, 215 (53, 52 in 1:1) [M-•CH2(Cl)Bn and CO]+, 198, 196 (56, 56) [A-CO]+.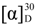 = +32.0 (c 1.0, MeOH); MP: 178 °C; 1H-NMR (300 MHz, CDCl3): 1.00 (3H, d, J = 6.3 Hz, CH3), 1.09 (3H, d, J = 6.6 Hz, H4'), 2.55 (1H, m, H3'), 4.35 (1H, d, 4.5 Hz, H2'), 7.13 (1H, dd, J = 8.7, 1.5 Hz, H4), 8.06 (1H, d, J = 8.6 Hz, H3), 8.83 (1H, d, J = 1.5 Hz, H6), 11.78 (1H, s, NH); IR (KBr): ύmax (cm−1) 3500 (O-H), 3383 (N-H), 1666 (OC=O), 1595 (NC=O); UV-Vis (MeOH): λmax 336 nm (log ε = 3.01872 L cm−1 M−1); ESI MS (m/z) for C12H13BrClNO3: 359.9615, 357.9644 and 355.9665 found for 359.9612 [M+4+Na], 357.9642 [M+2+Na] and 355.9663 [M+Na] in 2:9:6 ratio.
= +32.0 (c 1.0, MeOH); MP: 178 °C; 1H-NMR (300 MHz, CDCl3): 1.00 (3H, d, J = 6.3 Hz, CH3), 1.09 (3H, d, J = 6.6 Hz, H4'), 2.55 (1H, m, H3'), 4.35 (1H, d, 4.5 Hz, H2'), 7.13 (1H, dd, J = 8.7, 1.5 Hz, H4), 8.06 (1H, d, J = 8.6 Hz, H3), 8.83 (1H, d, J = 1.5 Hz, H6), 11.78 (1H, s, NH); IR (KBr): ύmax (cm−1) 3500 (O-H), 3383 (N-H), 1666 (OC=O), 1595 (NC=O); UV-Vis (MeOH): λmax 336 nm (log ε = 3.01872 L cm−1 M−1); ESI MS (m/z) for C12H13BrClNO3: 359.9615, 357.9644 and 355.9665 found for 359.9612 [M+4+Na], 357.9642 [M+2+Na] and 355.9663 [M+Na] in 2:9:6 ratio.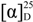 = +16.2 (c 1.0, MeOH); MP: 126 °C; 1H-NMR (600 MHz, CD3OD): 0.96 (3H, d, J = 6.6 Hz, CH3), 0.98 (3H, d, J = 6.6 Hz, H5'), 1.85–1.98 (3H, m, H3' and H4'), 4.51 (1H, dd, J = 9.6, 4.8 Hz, H2'), 7.18 (1H, dd, J = 8.4, 1.8 Hz, H5), 8.06 (1H, d, J = 8.4 Hz, H6), 8.69 (1H, d, J = 1.8, H3); 13C-NMR (125 MHz, CD3OD) δ (ppm): 21.56 (Me), 23.06 (C5'), 26.52 (C4'), 45.44 (C3'), 60.48 (C2'), 116.42 (C1), 120.97 (C5), 124.53 (C3), 133.99 (C6), 141.13 and 142.81 (C2 and C4), 170.39 (NC=O), 170.44 (OC=O); IR (KBr): ύmax (cm−1) 3221 (O-H), 3120 (N-H), 1640 (OC=O), 1550 (NC=O); UV-Vis (EtOAc): λmax 307 nm (log ε = 3.44321 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 307, 305 and 303 (0.1, 1.2 and 2.1 in 1:6:9 ratio) [M]+•, 251, 249 and 247 (6, 45 and 77 in 1:6:9 ratio) [M-C4H8, A]+•, 200, 198 (3, 7 in 1:3) [A-•CH2Cl, B]+, 182, 180 (37, 80) [B-H2O, C]+, 173, 171 (29, 100) [B-CO]+, 155, 153 (17, 52) [C-CO]+.
= +16.2 (c 1.0, MeOH); MP: 126 °C; 1H-NMR (600 MHz, CD3OD): 0.96 (3H, d, J = 6.6 Hz, CH3), 0.98 (3H, d, J = 6.6 Hz, H5'), 1.85–1.98 (3H, m, H3' and H4'), 4.51 (1H, dd, J = 9.6, 4.8 Hz, H2'), 7.18 (1H, dd, J = 8.4, 1.8 Hz, H5), 8.06 (1H, d, J = 8.4 Hz, H6), 8.69 (1H, d, J = 1.8, H3); 13C-NMR (125 MHz, CD3OD) δ (ppm): 21.56 (Me), 23.06 (C5'), 26.52 (C4'), 45.44 (C3'), 60.48 (C2'), 116.42 (C1), 120.97 (C5), 124.53 (C3), 133.99 (C6), 141.13 and 142.81 (C2 and C4), 170.39 (NC=O), 170.44 (OC=O); IR (KBr): ύmax (cm−1) 3221 (O-H), 3120 (N-H), 1640 (OC=O), 1550 (NC=O); UV-Vis (EtOAc): λmax 307 nm (log ε = 3.44321 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 307, 305 and 303 (0.1, 1.2 and 2.1 in 1:6:9 ratio) [M]+•, 251, 249 and 247 (6, 45 and 77 in 1:6:9 ratio) [M-C4H8, A]+•, 200, 198 (3, 7 in 1:3) [A-•CH2Cl, B]+, 182, 180 (37, 80) [B-H2O, C]+, 173, 171 (29, 100) [B-CO]+, 155, 153 (17, 52) [C-CO]+.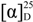 = +35.2 (c 1.0, MeOH); MP: 121 °C; 1H-NMR (500 MHz, CD3OD): 3.23 (1H, dd, J = −14.0, 7.5 Hz, Hα3'), 3.44 (1H, dd, J = −14.0, 6.0 Hz, Hβ3'), 4.71 (1H, dd, J = 7.5, 6.0 Hz, H2'), 7.15 (1H, dd, J = 8.5, 2.0 Hz, H5), 7.19-7.26 (5H, m, Ph), 8.01 (1H, d, J = 8.5 Hz, H6), 8.66 (1H, d, J = 2.0, H3); 13C-NMR (125 MHz, CD3OD) δ (ppm): 42.43 (C3'), 62.24 (C2'), 116.35 (C1''), 120.91 (C5), 124.55 (C3), 128.12 (C4''), 129.47, 130.57 (C3'' and C2''), 133.91 (C6), 137.45 (C1), 141.04 (C4), 142.56 (C2), 169.46 (NC=O), 170.18 (OC=O); IR (KBr): ύmax (cm−1) 3221 (O-H), 3001 (N-H), 1670 (OC=O), 1543 (NC=O); UV-Vis (MeOH): λmax 306 nm (log ε = 3.09876 L cm−1 M−1). LR EIMS: m/z in amu (% abundance) 304, 302 (9, 31 in 1:3 ratio) [M-•Cl]+, 182, 180 (25, 8) [M-•CH2(Cl)Bn and H2O, A]+.
= +35.2 (c 1.0, MeOH); MP: 121 °C; 1H-NMR (500 MHz, CD3OD): 3.23 (1H, dd, J = −14.0, 7.5 Hz, Hα3'), 3.44 (1H, dd, J = −14.0, 6.0 Hz, Hβ3'), 4.71 (1H, dd, J = 7.5, 6.0 Hz, H2'), 7.15 (1H, dd, J = 8.5, 2.0 Hz, H5), 7.19-7.26 (5H, m, Ph), 8.01 (1H, d, J = 8.5 Hz, H6), 8.66 (1H, d, J = 2.0, H3); 13C-NMR (125 MHz, CD3OD) δ (ppm): 42.43 (C3'), 62.24 (C2'), 116.35 (C1''), 120.91 (C5), 124.55 (C3), 128.12 (C4''), 129.47, 130.57 (C3'' and C2''), 133.91 (C6), 137.45 (C1), 141.04 (C4), 142.56 (C2), 169.46 (NC=O), 170.18 (OC=O); IR (KBr): ύmax (cm−1) 3221 (O-H), 3001 (N-H), 1670 (OC=O), 1543 (NC=O); UV-Vis (MeOH): λmax 306 nm (log ε = 3.09876 L cm−1 M−1). LR EIMS: m/z in amu (% abundance) 304, 302 (9, 31 in 1:3 ratio) [M-•Cl]+, 182, 180 (25, 8) [M-•CH2(Cl)Bn and H2O, A]+.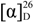 = +23.3 (c 0.2, MeOH); MP: 118 °C; 1H-NMR (500 MHz, CD3OD): 0.96 (3H, d, J = 6.5 Hz, CH3), 0.98 (3H, d, J = 6.0 Hz, H5'), 1.84–1.97 (3H, m, H3'and H4'), 4.51 (1H, dd, J = 9.0, 5.0 Hz, H2'), 7.69 (1H, dd, J = 9.0, 2.5 Hz, H4), 8.17 (1H, d, J = 2.5 Hz, H3), 8.53 (1H, d, J = 9.0, H6); 13C-NMR (125 MHz, CD3OD) δ (ppm): 21.59, 23.06 (CH3 and C5'), 25.53 (C4'), 45.49 (C3'), 60.53 (C2'), 116.75 (C1), 119.97 (C5), 123.20 (C4), 135.02 (C3), 137.87 (C6), 140.89 (C2), 169.76 (NC=O), 170.26 (OC=O); IR (KBr): ύmax (cm−1) 3259 (O-H), 3044 (N-H), 1685 (OC=O), 1531 (NC=O); UV-Vis (MeOH): λmax 310 nm (log ε = 3.23921 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 351, 349 and 347 (4, 12 and 11) [M]+•, 226, 224 (62, 43) [M-∙CH2(Cl)Bn and H2O, A]+, 217, 215 (99, 100) [M-∙CH2(Cl)Bn and CO]+, 199, 197 (61, 60) [A-CO]+.
= +23.3 (c 0.2, MeOH); MP: 118 °C; 1H-NMR (500 MHz, CD3OD): 0.96 (3H, d, J = 6.5 Hz, CH3), 0.98 (3H, d, J = 6.0 Hz, H5'), 1.84–1.97 (3H, m, H3'and H4'), 4.51 (1H, dd, J = 9.0, 5.0 Hz, H2'), 7.69 (1H, dd, J = 9.0, 2.5 Hz, H4), 8.17 (1H, d, J = 2.5 Hz, H3), 8.53 (1H, d, J = 9.0, H6); 13C-NMR (125 MHz, CD3OD) δ (ppm): 21.59, 23.06 (CH3 and C5'), 25.53 (C4'), 45.49 (C3'), 60.53 (C2'), 116.75 (C1), 119.97 (C5), 123.20 (C4), 135.02 (C3), 137.87 (C6), 140.89 (C2), 169.76 (NC=O), 170.26 (OC=O); IR (KBr): ύmax (cm−1) 3259 (O-H), 3044 (N-H), 1685 (OC=O), 1531 (NC=O); UV-Vis (MeOH): λmax 310 nm (log ε = 3.23921 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 351, 349 and 347 (4, 12 and 11) [M]+•, 226, 224 (62, 43) [M-∙CH2(Cl)Bn and H2O, A]+, 217, 215 (99, 100) [M-∙CH2(Cl)Bn and CO]+, 199, 197 (61, 60) [A-CO]+.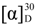 = +54.0 (c 0.2, MeOH); MP: 134 °C; 1H-NMR (400 MHz, CDCl3): δ (ppm) 1.61 (3H, d, J = 6.8 Hz, H1'), 4.79 (1H, q, J = 4.8 Hz, H3), 7.00 (1H, d, J = 1.6 Hz, H9), 7.26 (1H, dd, J = 8.4, 1.6 Hz, H7), 7.92 (1H, d, J = 8.4 Hz, H6), 7.94 (1H, s NH); IR (KBr): ύmax (cm−1) 3262 (N-H), 1707 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 302 nm (log ε = 3.98631 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 227 and 225 (10 and 30 in 1:3) [M]+•, 155 and 153 (30 and 100 in 1:3 ratio) [M-(3-methyloxirane-2-one)]+•, 183 and 181 (3 and 10 in 1:3 ratio) [M-CO]+•.
= +54.0 (c 0.2, MeOH); MP: 134 °C; 1H-NMR (400 MHz, CDCl3): δ (ppm) 1.61 (3H, d, J = 6.8 Hz, H1'), 4.79 (1H, q, J = 4.8 Hz, H3), 7.00 (1H, d, J = 1.6 Hz, H9), 7.26 (1H, dd, J = 8.4, 1.6 Hz, H7), 7.92 (1H, d, J = 8.4 Hz, H6), 7.94 (1H, s NH); IR (KBr): ύmax (cm−1) 3262 (N-H), 1707 (a broad signal of OC=O and NC=O); UV-Vis (MeOH): λmax 302 nm (log ε = 3.98631 L cm−1 M−1); LR EIMS: m/z in amu (% abundance) 227 and 225 (10 and 30 in 1:3) [M]+•, 155 and 153 (30 and 100 in 1:3 ratio) [M-(3-methyloxirane-2-one)]+•, 183 and 181 (3 and 10 in 1:3 ratio) [M-CO]+•.4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- López-Cara, L.C.; Conejo-García, A.; Marchal, J.A.; Macchione, G.; Cruz-López, O.; Boulaiz, H.; García, M.A.; Rodríguez-Serrano, F.; Ramírez, A.; Cativiela, C.; et al. New (RS)-benzoxazepin-purines with antitumour activity: The chiral switch from (RS)-2,6-dichloro-9-[1-(p-nitrobenz enesulfonyl)-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]-9H-purine. Eur. J. Med. Chem. 2011, 46, 249–258. [Google Scholar] [CrossRef]
- Hargrave, K.D.; Deceased, S.; Gunther, E. Pyrido[2,3-b][1,5]benzoxazepin (and Thiazepin)-5(6H)-ones and Thiones and Their Use on the Treatment of HIV Infection. U.S. Patent 5,550,122, 27 August 1996. [Google Scholar]
- Mc Gee, M.M.; Campiani, G.; Zisterer, D.M. Pyrrolo-1,5-benzoxazepines induce apoptosis in chronic myelogenous leukemia (CML) cells by bypassing the apoptotic suppressor bcr-abl. J. Pharm. Exp. Ther. 2001, 296, 31–40. [Google Scholar]
- Richard, C.E.; Larry, D.; Wolfgang, S. Process for Preparing Aminoalkylpyrrolobenzoxaz Alkanes. U.S. Patent 4,169,095, 25 September 1979. [Google Scholar]
- Emilio, T.; Luigi, F. 1,2,3,5-Tetrahydro-4,1-benzoxazepines and 3,5-Dihydro-5-phenyl-4,1-benzoazepin-2-one. U.S. Patent 3,346,565 A, 10 October 1967. [Google Scholar]
- Wiklund, P.; Bergman, J. Ring forming reactions of imines of 2-aminobenzaldehyde and related compounds. Org. Biomol. Chem. 2003, 1, 367–372. [Google Scholar] [CrossRef]
- Yar, M.; McGarrigle, E.M.; Aggarwal, V.K. Bromoethylsulfonium salt—A more effective annulation agent for the synthesis of 6- and 7-membered 1,4-Heterocyclic compounds. Org. Lett. 2009, 11, 257–260. [Google Scholar] [CrossRef]
- Winter, A. Organic Chemistry: For Dummies; John Wiley & Sons: New York, NY, USA, 2005; p. 109. [Google Scholar]
- Smith, J.G. Organic Chemistry; McGraw-Hill: New Delhi, India, 2008; Volume 2, p. 188. [Google Scholar]
- Ma, D.; Zhang, Y.; Yao, J.; Wu, S.; Tao, F. Accelerating effect induced structure of (R)-Amino acid in the copper-catalyzed coupling reaction of aryl halides with (R)-Amino acids. Synthesis of Benzolactam-V8. J. Am. Chem. Soc. 1998, 120, 12459–12467. [Google Scholar] [CrossRef]
- Miki, T.; Kori, M.; Mabuchi, H.; Tozawa, R.; Nishimoto, T.; Sugiyama, Y.; Teshima, K.; Yukimasa, H. Synthesis of novel 4,1-Benzoxazepine derivatives as squalene synthase inhibitors and their inhibition of cholesterol synthesis. J. Med. Chem. 2002, 45, 4571–4580. [Google Scholar] [CrossRef]
- Nisar, B.; Raza, A.R.; Black, D.; Kumar, N.; Tahir, M.N. Stereoselective synthesis of (3R)-3-alkyl-4,1-benzoxazepine-2,5-diones. Chirality 2013, 25, 865–870. [Google Scholar] [CrossRef]
- Koppenhoefer, B.; Schurig, V. (S)-2-chloroalkanoic acids of high enantiomeric purity from (S)-2-amino acids: (S)-2-chloropropanoic acid. Org. Synth. 1993, 8, 119–123. [Google Scholar]
- Crystallographic data in this article have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication No. 946961 and 946962 for 4a and 4c respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). The X-ray structure was obtained by Prof. Dr. Muhammad Nawaz Tahir, Department of Physics, University of Sargodha, Sargodha, Pakistan.
- Sample Availability: Samples of the compounds 4a–d, 5 and 6a–g are available from the authors.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rubab, S.L.; Nisar, B.; Raza, A.R.; Ullah, N.; Tahir, M.N. Asymmetric Synthesis of 4,1-Benzoxazepine-2,5-Diones — Effect of the Halogen of (2S)-α-Haloacids. Molecules 2014, 19, 139-148. https://doi.org/10.3390/molecules19010139
Rubab SL, Nisar B, Raza AR, Ullah N, Tahir MN. Asymmetric Synthesis of 4,1-Benzoxazepine-2,5-Diones — Effect of the Halogen of (2S)-α-Haloacids. Molecules. 2014; 19(1):139-148. https://doi.org/10.3390/molecules19010139
Chicago/Turabian StyleRubab, Syeda Laila, Bushra Nisar, Abdul Rauf Raza, Nisar Ullah, and Muhammad Nawaz Tahir. 2014. "Asymmetric Synthesis of 4,1-Benzoxazepine-2,5-Diones — Effect of the Halogen of (2S)-α-Haloacids" Molecules 19, no. 1: 139-148. https://doi.org/10.3390/molecules19010139
APA StyleRubab, S. L., Nisar, B., Raza, A. R., Ullah, N., & Tahir, M. N. (2014). Asymmetric Synthesis of 4,1-Benzoxazepine-2,5-Diones — Effect of the Halogen of (2S)-α-Haloacids. Molecules, 19(1), 139-148. https://doi.org/10.3390/molecules19010139