Abstract
Herein, the preparation of neoglycoconjugates bearing mannose-6-phosphate analogues is described by: (a) synthesis of a cyclic sulfate precursor to access the carbohydrate head-group by nucleophilic displacement with an appropriate nucleophile; (b) introduction of spacers on the mannose-6-phosphate analogues via Huisgen’s cycloaddition, the Julia reaction, or the thiol-ene reaction under ultrasound activation. With the resulting compounds in hand, gold nanoparticles could be functionalized with various carbohydrate derivatives (glycoconjugates) and then tested for angiogenic activity. It was observed that the length and flexibility of the spacer separating the sugar analogue from the nanoparticle have little influence on the biological response. One particular nanoparticle system substantially inhibits blood vessel growth in contrast to activation by the corresponding monomeric glycoconjugate, thereby demonstrating the importance of multivalency in angiogenic activity.
1. Introduction
The ensuing article was initially motivated by the biomedical importance of mannose 6-phosphate (M6P) [1]. While two different mannose 6-phosphate receptors (M6PR) recognize the M6P residues and mediate the endocytosis of extracellular M6P-containing ligands, only the larger of these (CI-M6PR, 275 kDa) has been reported to also bind retinoic acid and IGF-II [2]. The biological importance of this receptor is found in numerous processes and it has been reported that the angiogenic action of proliferin was mediated by this receptor [3]. We have recently described the synthesis of a series of mannose-6-phosphate (M6P) analogues, showing for the first time that these monosaccharides play a role in angiogenesis [4,5,6,7]. The replacement of the phosphate head-group by analogues, mostly bioisosteres, was intended to provide a better understanding of the chemical factors involved in the modulation of angiogenic activities. It is known, however, that a monovalent carbohydrate ligand possesses only a weak binding affinity toward its associated receptor protein [8,9,10]. To impart biological relevance to such interactions Nature often utilizes multivalency [11,12]. Therefore, interest in designing multivalent carbohydrate systems has been growing [13]. In particular, glyconanoparticles (GNPs), that offer useful tools for investigating carbohydrate-mediated interactions, have been developed [14]. The purpose of the present study was: (a) to synthesize new glycoconjugates bearing M6P-like groups and (b) to insert these compounds onto the surfaces of gold particles via a spacer for angiogenic testing. Our objective, therefore, is to investigate the effect of clustered sugar derivatives on angiogenesis and to determine whether or not the spacer has an influence on the biological response. The choice of the M6P analogues has been guided by previous results conducted in our laboratory including the synthesis of carboxylate and azido analogues (with 123% and 125% relative angiogenic activity, respectively, compared to phosphate buffer saline (PBS) as control in an egg membrane assay) [4]. Additional considerations include varying the length, hydrophilic or hydrophobic nature, and flexibility of the spacer between the sugar headgroups and the nanoparticle core. In this manner we could modify the presentation of the carbohydrates and, consequently, affect their accessibility during the molecular recognition events. Many of the mannose derivatives with their different spacers were assembled using the “click chemistry” strategy introduced by Huisgen and improved by Sharpless and co-workers in 2001 [15,16]. Within a short time-frame, the click chemistry reaction has proven to be of remarkable utility and broad scope, not only in organic synthesis but in chemical biology and drug discovery [17,18]. Although 1,3-dipo lar cycloaddition reaction is central to click chemistry, the resulting creation of a triazole moiety may have an adverse influence on a biological response. For this reason two other reactions were used for chain elongation or for conjugation of two synthons: the Julia reaction and the thiol-ene reaction that was run under unprecedented ultrasound activation.
2. Results and Discussion
The preparation of the neoglycoconjugates we describe herein took place in three major steps: (a) the synthesis of a cyclic sulfate precursor to access the ligand head-group by nucleophilic displacement with the appropriate nucleophile; (b) the introduction of the spacers on the M6P via either Huisgen’s cycloaddition, the Julia reaction, or the thiol-ene reaction under ultrasound activation; (c) the coupling between the spacer and the sugar moiety. By this means gold nanoparticles as functionalized by various carbohydrates could be compared for their effect on angiogenic processes. Although preliminary biological data are presented at the end of the paper, the emphasis here will be on the synthetic challenges involved in obtaining the necessary neoglycoconjugates.
2.1. 1,3Dipolar Cycloaddition
Huisgen’s 1,3-dipolar cycloaddition is the primary example of a “click reaction”. It is the reaction between a 1,3-dipole (an azide) and a dipolarophile (an acetylene) to form a five-membered heterocycle. The classical reaction proceeds by a concerted mechanism under thermal conditions to afford a mixture of 1,4- and 1,5-disubstituted [1,2,3]-triazole regioisomers [19], but when the reaction is catalyzed by Cu(I), only the 1,4-substituted-triazole is obtained [20]. We selected this reaction as one means for securing our mannose-6-phosphate analogues. Thus to prepare the nanoparticles, the carbohydrate moiety had to bear either an azide or alkyne function. The linker chain, in turn, would provide the complementary group. The cyclic sulfate strategy, utilized in our laboratory to prepare M6P analogues, demanded that the carbohydrate possess the azide group because an alkyne function would become oxidized during the preparation of the sulfate. Thus, peracetylated mannose has been coupled in very good yield to 2-bromoethanol, under classical conditions [21], in the presence of boron trifluoride etherate (Scheme 1). The azide group was then introduced with sodium azide, and the acetate protecting groups were removed under Zemplen conditions [22] to give the 2-azidoethyl-α-d-mannopyranoside 3. After selectively introducing isopropylidene protection at the 2 and 3 positions of the mannose, the cyclic sulfate 5 was prepared according to a modified published procedure [23,24]. Compound 4 was converted via thionyl chloride into the cyclic sulfite which was then oxidized by ruthenium oxide (prepared in situ) into the corresponding cyclic sulfate 5.
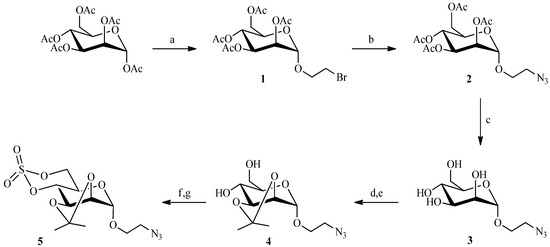
Scheme 1.
Preparation of the cyclic sulfate intermediate 5.
A “spacer” refers to a chain that can be used to join our sugar derivatives to the gold particles. One of the spacers, possessing an alkyne unit for reaction with a sugar-azide, was designed on the basis of its flexibility and aqueous solubility (Scheme 2). Thus, the reaction of 5-bromopentene with a slight excess of 50% sodium hydroxide and hexaethylene glycol provided the monoether 6 [25]. Photochemical addition of thioacetic acid to the double bond gave the thioacetate in good yield [26]. The next step was to introduce the alkyne function on the spacer in the presence of NaH, but the acetate protecting groups, being sensitive to hydrides, were first replaced by 4-methoxytrityl. Thus, compound 7 was deacetylated by concentrated hydrochloric acid in ethanol to avoid the formation of disulfide under basic conditions. The thiol was then protected by reaction with 4-methoxytrityl chloride. Finally, the free hydroxyl of 8 was reacted with 3-bromopropyne in the presence of sodium hydride in anhydrous THF to introduce the alkyne function required for the click reaction.
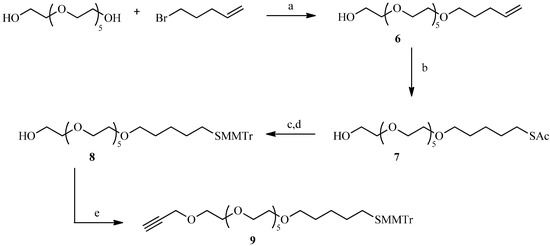
Scheme 2.
Preparation of the alkyne 9 for the click reaction.
The literature describes a variety of ways in which the Huisgen cycloaddition can be performed to join two entities. Sources of copper (I) catalyst can be produced in situ by reduction of copper (II) salts [20] or obtained through disproportionation of Cu (0) and Cu (II) salts [27]. Cu (I) can also be introduced as copper (I) salts such as CuI or obtained from oxidation of Cu (0) salt [28,29,30,31]. In search for the optimal reaction conditions, we initially tested the most commonly employed system, namely CuSO4·5H2O and sodium ascorbate as source of copper (I) in tert-BuOH/H2O [32]. Interestingly, no reaction was observed after 24 h. In addition, several parameters were altered without success: increasing the concentration of reactants, changing the ratio copper/sodium ascorbate, using a co-solvent (acetonitrile), or substituting tBuOH with pyridine. The click reaction was also attempted using cuprous iodide in pyridine as catalyst [33]. Despite many modifications to the original protocol the desired product was never obtained in good yield. Thus, another copper catalyst system consisting of formation of Cu(I) by oxidation of copper metal was investigated. The oxidative cycloaddition of Cu(0) with ammonium chloride [34] in a mixture of tert-BuOH/H2O was also unsuccessful. It should be noted that heating to 40–60 °C, and increasing the reagents’concentration, failed to improve the performance, as they often do in many examples of click chemistry reactions, but led only to degradation. Ultrasound in place of classical activation was carried out again without success. Only a system using copper powder, rarely encountered in the literature, gave positive results, giving compound 10 in 60% yield (Scheme 3). Starting from compound 10 two mannose-6-phosphate analogues were prepared with only slight modification to the previously reported protocols [4]. First, the azide function was easily introduced on the cyclic sulfate 10 by reaction with sodium azide to afford compound 11. Although isopropylidene and trityl are usually deprotected under acid conditions, our assays did not allow simultaneous cleavage of the two functions. The final ligand 12 was therefore obtained in two separate steps. The trityl group was first cleaved by ceric ammonium nitrate (a redox reaction) [35,36] prior to deprotection of the isopropylidene and the sulfate groups via acidic ion exchange resins. To afford the carboxylic acid analogue of M6P 14, sodium cyanide was first reacted with the cyclic sulfate 10, and the nitrile function was then hydrolyzed with sodium hydroxide in a 30% solution of hydrogen peroxide to give the corresponding carboxylic acid. The ligand 14 was obtained using the same deprotection conditions as described for the azide analogue 12 (Scheme 3).
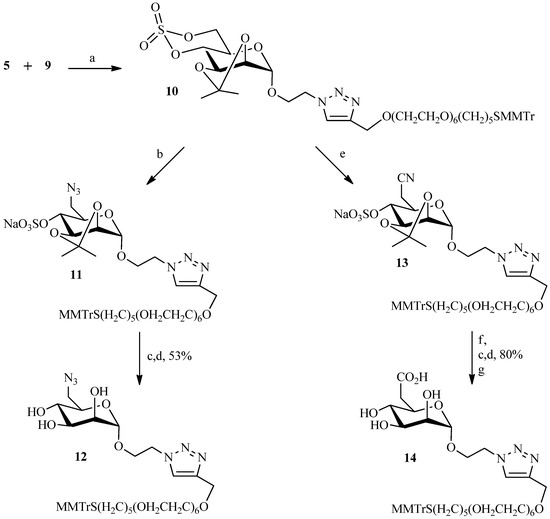
Scheme 3.
Preparation of the functionalized ligands 12 and 14.
2.2. Julia Reaction
Among the olefination reactions to form a regio- and stereoselective alkene, the Julia olefination is one of the well-known methods, along with the Wittig reaction [37,38], the Wittig-Horner reaction [39,40,41], the Horner-Wadsworth-Emmons [42,43], the Peterson reaction [44,45,46] and the Johnson reaction [47]. The classical Julia olefination, also known as the Julia-Lythgoe olefination, was developed fourty years ago and is based on a reductive elimination process of β-acyloxysulfones [48]. Since its discovery, significant improvements have been made to the methodology of this reaction, and it has become a crucial step in the synthesis of many natural products. A new variant of the classical Julia reaction, the Julia-Kocienski olefination, also called modified or one-pot Julia olefination, has recently emerged as a powerful tool for olefin synthesis [49,50,51]. The process involves the replacement of the aryl sulfone moiety, traditionally used in the classical reaction, with different heteroaryl sulfones, thus allowing a direct olefination process.
In our Julia olefination, a carbohydrate block was derivatized with an allyl bromide function (to be later joined with a sulfone-bearing linker). The initial steps in the sugar portion of the molecule followed the same strategy as described for compound 5 (Scheme 4). The methyl α-d-mannopyranoside was previously protected with two O-isopropylidene groups on the 2,3 and 4,6 positions using 2,2-dimethoxypropane and para-toluenesulfonic acid. After the selective opening of isopropylidene at the 4,6 positions with a mixture of AcOH/H2O, the cyclic sulfite was obtained by reaction with thionyl chloride and triethylamine, and subsequent oxidation afforded the cyclic sulfate 15 in good yield. In contrast to the chemistry in Scheme 1 and Scheme 3, the azide and carboxylic acid analogues of M6P were prepared prior to the coupling reaction. Therefore, sodium azide was reacted with the cyclic sulfate 15 to give compound 16. A solution of acetic acid in water led to the cleavage of the isopropylidene and the sulfate. The replacement of the anomeric methyl group by an acetyl group led to compound 17 in 83% yield. The allyl bromide unit required to perform the coupling reaction was then introduced by glycosylation with cis-1-bromo-but-2-en-4-ol. The same strategy was applied to form the carboxylic acid analogue of M6P.
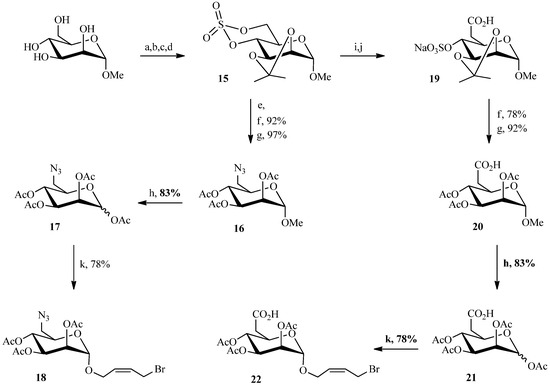
Scheme 4.
Preparation of the allyl bromides 18 and 22.
To create the Julia spacer (Scheme 5), geraniol was reacted with phosphorus tribromide to give geranyl bromide 23 in good yield followed by a reaction with sodium phenylsulfinate to provide the desired sulfone 24. Next, functionalization of the other side of the linker was carried out in one step via oxidation of a terminal methyl by selenium dioxide. The strategy described by Sharpless using tert-butyl hydroperoxide as an oxidant [52] was utilized: 24 was reacted with a catalytic amount of selenium oxide in the presence of tert-butyl hydroperoxide which enables the recycling of selenium dioxide. A 50/50 mixture of alcohol and aldehyde was obtained and, after purification, the aldehyde was reduced with sodium borohydride to give compound 26 in 63% yield. The alcohol 26 was then brominated with tetrabromomethane and triphenylphosphine after which the thiol group was introduced by reaction with potassium thioacetate. A “click-type” reaction was then performed between 18 or 22 and 27 in the presence of lithium bis(trimethylsilyl)amide (LiHMDS) in THF from which compounds 28 and 29 were obtained in 15% and 17% yield, respectively. Deprotection of the acetates and removal of the sulfone group under basic conditions gave the desired final compounds 30 and 31 in almost quantitative yield (Scheme 6). The linker in this case is polyunsaturated.
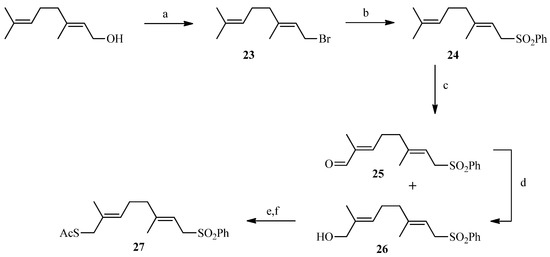
Scheme 5.
Synthesis of the sulphone 27.
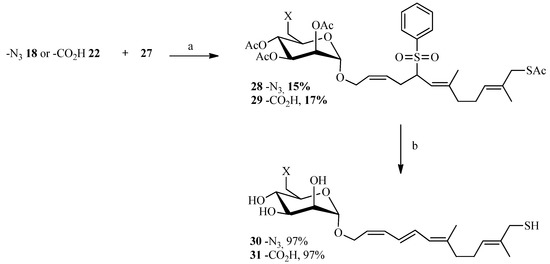
Scheme 6.
Coupling reaction between the allyl bromides and the sulphone.
2.3. Thiol-ene Reaction
One reaction that is emerging as an attractive click-type process is the century-old addition of thiols to alkenes [53], which is currently called thiol-ene coupling. In fact, the thiol-ene reaction is simply the hydrothiolation of a C=C bond, and proceeds by a radical mechanism, induced photochemical or thermally, to give an anti-Markovnikov-type thioether [54,55]. The reaction discovered in 1905 by Posner [56] has been widely used in the mid-nineteenth century, especially in polymer chemistry. However, the thiol-ene reaction has recently attracted researchers in other areas of synthesis due to recognition of its ‘‘click-type’’ characteristics: highly efficient and orthogonal to a wide range of functional groups, as well as compatible with water and oxygen. Thus, the thiol-ene reaction enables the establishment of a rapid ligation between two entities assisted by the stability of the thioether linkage in a wide range of chemical environments. To perform the reaction, the thiol function was placed on the sugar moiety while the spacer carried the vinylic group. As before, the M6P derivatives were prepared using the cyclic sulfate strategy prior to performing the click-style reaction (Scheme 7). The synthesis began by replacing the anomeric acetate with bromine on compounds 17 and 21 described previously. This was accomplished with a solution of hydrobromic acid/acetic acid in quantitative yields. The thiol function was then introduced in two steps, first via thiourea in acetone then removal of the nitrogens with sodium metabisulfide. Only thiosugars (34 and 35) having the β configuration were obtained.
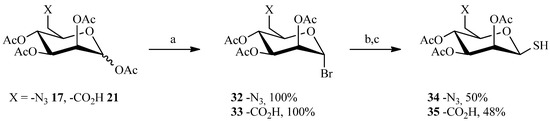
Scheme 7.
Preparation of the thiosugars 34 and 35.
Having chosen to synthesize a fully flexible spacer (Scheme 8), the triethylene glycol was coupled to allyl bromide in the presence of 50% aqueous sodium hydroxide.
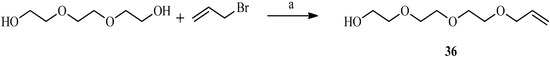
Scheme 8.
Preparation of the spacer.
Then, to facilitate the reaction, and to avoid formation of byproducts, the free hydroxyl of the linker was brominated and thioacetylated only at the end of the synthesis. Actually, coupling can be accomplished between a protected thiol group and an alkene or between a thiol and an alkene. However, the final thioether was obtained with better results when the anomeric thiol was not protected (Scheme 9). The initiation step can be triggered in several ways, by simple heating or by ultraviolet irradiation. Another method of initiation has been developed in the laboratory which is to perform the coupling under ultrasound (Table 1). When THF was replaced by dioxane, yields increased by 10%.
Scheme 9.
Preparation of the thiosugars 39 and 40 by click thiol-ene reaction.

Table 1.
Comparative results for the click thiol-ene reaction.
| Compound | Reflux, THF, 24 h | UV, THF, 5 h | Sonication, THF, 4 h | Sonication, Dioxane, 3 h |
|---|---|---|---|---|
| 39 | 76% | 50% | 72% | 79% |
| 40 | 78% | 60% | 75% | 80% |
2.4. Gold Nanoparticles
Research in developing new synthesis protocols to generate gold nanoparticles (AuNPs) with desired properties has received immense attention due to their considerable applications in biomedical field [57]. One of the primary prerequisites for using AuNPs in biomedical application is that they are non-toxic and biocompatible to both in vitro and in vivo environments. Secondly, AuNPs should be coated with a protective layer to prevent aggregation. Thirdly, AuNPs need to be labeled with biologically relevant biomolecules to impart specificity for their potential application. The two most interesting and common methods to prepare AuNPs are the Brust method [58] utilizing NaBH4 (which can’t be used in our case because NaBH4 would reduce the azide function of our derivatives) and the citrate method. This latter method includes only three starting materials, namely, auric acid, sodium citrate (the reducer), and water. Following a report by Turkevich et al. in 1951 [59], this synthetic scheme has been widely studied and often used for the preparation of AuNPs-based materials [60,61,62,63]. We have developed a protocol by adjusting the gold-to-citrate ratio to obtain 10 nm AuNPs (Table 2). Details are given in the Experimental section.

Table 2.
Size of the nanoparticles in nm.
| Nanoparticles | TEM [a] | DLS [b] |
|---|---|---|
| Azide AuNPs (Huisgen) | 10 | 18–20 |
| Azide AuNPs (Julia) | 10 | 14–16 |
| Azide AuNPs (thiol-ene) | 10 | 12–13 |
| Carboxylic acid AuNPs (Huisgen) | 10 | 19–20 |
| Carboxylic acid AuNPs (Julia) | 10 | 15–16 |
[a] Transmission electron microscopy [b] Dynamic light scattering.
2.5. Biological Assays
AuNPs functionalized with M6P analogues have been subjected to angiogenic assays using an experimental model, the avian chorioallantoic membrane assay (CAM) [64,65,66]. Paper discs were saturated with a phosphate buffer saline dispersion of coated AuNPs (60 mg/mL) in PBS or a control (phosphate buffer saline) and then deposited on chorioallantoic membranes of 7-day-old chicken embryos for 4 days in ovo at 38 °C. Sutent® (sunitinib, a non-proteic inhibitor) and endothelial cell growth supplement (ECGS) were used at 60 mg/mL as negative and positive stimuli, respectively. Quantification of the angiogenic response was carried out by measuring the area of neo-vascularization on each particular membrane (Figure 1) using Image J software. The experiments have been repeated at least four times, and the results were reproducible (see experimental part for details).
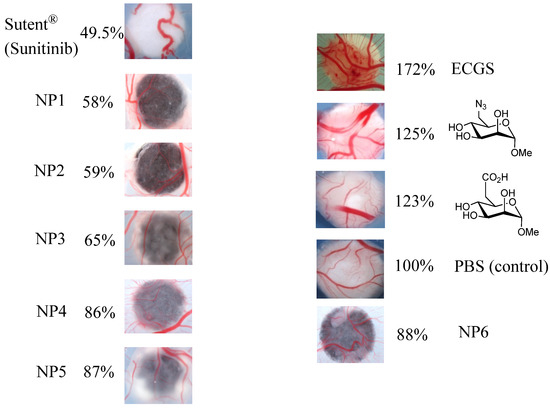
Figure 1.
CAM assays using gold nanoparticles functionalized with mannose-6-phosphate analogues compared to angiogenic inhibitor Sutent®, angiogenic activator Endothelial Cell Growth Supplement (ECGS) and Phosphate Buffer Saline (PBS) as control.
These experiments demonstrate that all our prepared AuNPs are CAM-inhibitors. Study of the three azide-AuNPs synthesized according to the coupling methods (NP1: thiol-ene 58%, NP2: Julia 59%, NP3: Huisgen 65%) revealed that the length and flexibility of spacers have little influence on the observed biological response. Interestingly, the azide sugar-monomer is a good angiogenic activator (125%), whereas the functionalized -N3 nanoparticles, representing a multi-valent collection of sugars, show a strong inhibitory effect (58%–65%). Similar results were obtained for the three carboxylic acid-AuNPs (NP4: Huisgen 86%, NP5: Julia 87%, NP6: thiol-ene 88%). Comparison of the activating effect of the carboxylic acid analogue (123% observed in previous work) [4] and the inhibitory effect of the carboxylic acid-AuNPs (86% to 88% compared to the control) indicates that multi-valency can do more than qualitatively affect the magnitude of blood vessel formation; it can convert a significant catalyzed process into an inhibition.
3. Experimental
3.1. General Information
Reactions were monitored by TLC using aluminum-backed plates coated with silica gel 60 F254 (Merck); spots were visualized with UV light (254 nm) and/or (a) by staining with p-anisaldehyde solution [anisaldehyde (25 mL), H2SO4 (25 mL), EtOH (450 mL), and CH3COOH (1 mL)], followed by heating or (c) by immersion in a 10% H2SO4/EtOH solution followed by charring. Column chromatography was performed on Carlo-Erba silica gel 60A (35–70 µm). Melting points were determined in capillary with a Büchi melting apparatus 530. Optical rotations were measured at the sodium D-line with a Perkin-Elmer-241 polarimeter. 1H-NMR spectra (400.13 MHz) and 13C-NMR spectra (100.62 MHz) were recorded on a Bruker DRX 400 instrument. Chemical shifts (δ) are given in parts per million and referenced using residual solvent signals (7.24 ppm for CHCl3 and 4.79 ppm for HOD). The following abbreviations were used to explain the signal multiplicities or characteristics: s (singlet), d (doublet), dd (double doublet), ddd (double double doublet), t (triplet), td (triplet doublet), q (quartet), and m (multiplet). Chemical shifts (δ) are given in parts per million relative to TMS as an external reference. Electron ionization mass spectra were recorded in positive or negative mode on a Waters MicroMass/ZQ 2615. Anhydrous solvents were obtained prior to use according to standard methods [67]. For transmission electron microscopy (TEM) examinations, a single drop (10 μL) of an aqueous solution (ca. 0.1 mg/mL in Milli-Qwater) of the gold glyconanoparticles (AuNPs) was placed on a coppergrid coated with a carbon film (Electron Microscopy Sciences). The grid was left to dry in air for several hours at room temperature. TEM analysis was performed with a JEOL 1200 EXII microscope, operating at 120 kV. Dynamic Light Scattering (DLS) analyses were performed on a MALVERN HPPS.
2'-Bromoethyl-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (1): To 1,2,3,4,6-penta-O-acetyl-α-d-mannopyranoside (540 mg, 1.38 mmol) dissolved in CH2Cl2 (5 mL) were added 2-bromoethanol (0.2 mL, 2.77 mmol) and BF3·Et2O (870 µL, 6.92 mmol). After 20 h stirring at room temperature, the mixture was diluted with CH2Cl2, washed with water, a saturated solution of NaHCO3 then water again. The organic layers were combined, dried over Na2SO4 and concentrated in vacuo. Purification by chromatography on silica gel (EtOAc/petroleum ether 1:1) gave the title compound as a white powder (91%). Rf = 0.86 (EtOAc/toluene 1:1); mp: 116–118 °C (lit. 115–117 °C);  = +42.1 (c = 0.5 in chloroform); 1H-NMR (CDCl3): δ = 2.00, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.52 (t, J = 6.0 Hz, 2H, CH2Br); 3.93 (m, 2H, CH2CH2Br); 4.13 (m, 2H, H5 and H6a); 4.27 (dd, 1H, J = 5.8 Hz, J = 12.6 Hz, H6b); 4.88 (d, J = 1.6 Hz, H1); 5.27 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.29 (t, 1H, J = 1.6 Hz, H4); 5.35 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3);13C-NMR (CDCl3): δ = 20.67, 20.70, 20.75, 20.87 (4CH3); 29.60 (CH2CH2Br); 62.41 (C6); 66.00 (C4); 68.48 (CH2Br); 68.93 (C5); 69.02 (C3); 69.42 (C2); 97.75 (C1); 169.76, 169.86, 170.03, 170.62 ppm (4CO); MS (ESI) m/z: 477.01, 478.95 [M+Na]+.
= +42.1 (c = 0.5 in chloroform); 1H-NMR (CDCl3): δ = 2.00, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.52 (t, J = 6.0 Hz, 2H, CH2Br); 3.93 (m, 2H, CH2CH2Br); 4.13 (m, 2H, H5 and H6a); 4.27 (dd, 1H, J = 5.8 Hz, J = 12.6 Hz, H6b); 4.88 (d, J = 1.6 Hz, H1); 5.27 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.29 (t, 1H, J = 1.6 Hz, H4); 5.35 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3);13C-NMR (CDCl3): δ = 20.67, 20.70, 20.75, 20.87 (4CH3); 29.60 (CH2CH2Br); 62.41 (C6); 66.00 (C4); 68.48 (CH2Br); 68.93 (C5); 69.02 (C3); 69.42 (C2); 97.75 (C1); 169.76, 169.86, 170.03, 170.62 ppm (4CO); MS (ESI) m/z: 477.01, 478.95 [M+Na]+.
 = +42.1 (c = 0.5 in chloroform); 1H-NMR (CDCl3): δ = 2.00, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.52 (t, J = 6.0 Hz, 2H, CH2Br); 3.93 (m, 2H, CH2CH2Br); 4.13 (m, 2H, H5 and H6a); 4.27 (dd, 1H, J = 5.8 Hz, J = 12.6 Hz, H6b); 4.88 (d, J = 1.6 Hz, H1); 5.27 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.29 (t, 1H, J = 1.6 Hz, H4); 5.35 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3);13C-NMR (CDCl3): δ = 20.67, 20.70, 20.75, 20.87 (4CH3); 29.60 (CH2CH2Br); 62.41 (C6); 66.00 (C4); 68.48 (CH2Br); 68.93 (C5); 69.02 (C3); 69.42 (C2); 97.75 (C1); 169.76, 169.86, 170.03, 170.62 ppm (4CO); MS (ESI) m/z: 477.01, 478.95 [M+Na]+.
= +42.1 (c = 0.5 in chloroform); 1H-NMR (CDCl3): δ = 2.00, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.52 (t, J = 6.0 Hz, 2H, CH2Br); 3.93 (m, 2H, CH2CH2Br); 4.13 (m, 2H, H5 and H6a); 4.27 (dd, 1H, J = 5.8 Hz, J = 12.6 Hz, H6b); 4.88 (d, J = 1.6 Hz, H1); 5.27 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.29 (t, 1H, J = 1.6 Hz, H4); 5.35 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3);13C-NMR (CDCl3): δ = 20.67, 20.70, 20.75, 20.87 (4CH3); 29.60 (CH2CH2Br); 62.41 (C6); 66.00 (C4); 68.48 (CH2Br); 68.93 (C5); 69.02 (C3); 69.42 (C2); 97.75 (C1); 169.76, 169.86, 170.03, 170.62 ppm (4CO); MS (ESI) m/z: 477.01, 478.95 [M+Na]+.2'-Azidoethyl-2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (2): Sodium azide (1.64 g, 25.05 mmol) was added to a suspension of compound 1 (5.7 g, 12.53 mmol) in DMF (50 mL). After 4 h at 65 °C, the mixture was poured into brine and extracted with CH2Cl2. The organic extracts were dried (Na2SO4) and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (petroleum ether/EtOAc 4:1) to give the appropriated intermediate as a white solid (96%). Rf = 0.86 (EtOAc/petroleum ether 1:1); mp: 80–82 °C (lit. 81.8–82.1 °C);  = +39.0 (c = 0.6 in chloroform); 1H-NMR (CDCl3): δ = 2.0, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.47 (m, 2H, CH2N3); 3.67 (m, 1H, CH2CH2N3); 3.87 (m, 1H, CH2CH2N3); 4.05 (ddd, 1H, J = 2.4 Hz, J = 5.2 Hz, J = 9.7 Hz, H5); 4.13 (dd, 1H, J = 2.6 Hz, J = 12.2 Hz, H6a); 4.29 (dd, 1H, J = 5.2 Hz, J = 12.4 Hz, H6b); 4.87 (d, 1H, J = 1.6 Hz, H1); 5.30 (t, 1H, J = 10.0 Hz, H4); 5.28 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.36 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3) : δ = 20.63, 20.68, 20.71, 20.84 (4CH3); 50.32 (CH2N3); 62.42 (C6); 65.96 (C4); 67.02 (CH2CH2N3); 68.82 (C5 and C3); 69.36 (C2); 97.71 (C1); 169.73, 169.78, 169.98, 170.59 ppm (4CO); MS (ESI) m/z: 440.12 [M+Na]+.
= +39.0 (c = 0.6 in chloroform); 1H-NMR (CDCl3): δ = 2.0, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.47 (m, 2H, CH2N3); 3.67 (m, 1H, CH2CH2N3); 3.87 (m, 1H, CH2CH2N3); 4.05 (ddd, 1H, J = 2.4 Hz, J = 5.2 Hz, J = 9.7 Hz, H5); 4.13 (dd, 1H, J = 2.6 Hz, J = 12.2 Hz, H6a); 4.29 (dd, 1H, J = 5.2 Hz, J = 12.4 Hz, H6b); 4.87 (d, 1H, J = 1.6 Hz, H1); 5.30 (t, 1H, J = 10.0 Hz, H4); 5.28 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.36 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3) : δ = 20.63, 20.68, 20.71, 20.84 (4CH3); 50.32 (CH2N3); 62.42 (C6); 65.96 (C4); 67.02 (CH2CH2N3); 68.82 (C5 and C3); 69.36 (C2); 97.71 (C1); 169.73, 169.78, 169.98, 170.59 ppm (4CO); MS (ESI) m/z: 440.12 [M+Na]+.
 = +39.0 (c = 0.6 in chloroform); 1H-NMR (CDCl3): δ = 2.0, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.47 (m, 2H, CH2N3); 3.67 (m, 1H, CH2CH2N3); 3.87 (m, 1H, CH2CH2N3); 4.05 (ddd, 1H, J = 2.4 Hz, J = 5.2 Hz, J = 9.7 Hz, H5); 4.13 (dd, 1H, J = 2.6 Hz, J = 12.2 Hz, H6a); 4.29 (dd, 1H, J = 5.2 Hz, J = 12.4 Hz, H6b); 4.87 (d, 1H, J = 1.6 Hz, H1); 5.30 (t, 1H, J = 10.0 Hz, H4); 5.28 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.36 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3) : δ = 20.63, 20.68, 20.71, 20.84 (4CH3); 50.32 (CH2N3); 62.42 (C6); 65.96 (C4); 67.02 (CH2CH2N3); 68.82 (C5 and C3); 69.36 (C2); 97.71 (C1); 169.73, 169.78, 169.98, 170.59 ppm (4CO); MS (ESI) m/z: 440.12 [M+Na]+.
= +39.0 (c = 0.6 in chloroform); 1H-NMR (CDCl3): δ = 2.0, 2.05, 2.11, 2.16 (4s, 12H, 4CH3); 3.47 (m, 2H, CH2N3); 3.67 (m, 1H, CH2CH2N3); 3.87 (m, 1H, CH2CH2N3); 4.05 (ddd, 1H, J = 2.4 Hz, J = 5.2 Hz, J = 9.7 Hz, H5); 4.13 (dd, 1H, J = 2.6 Hz, J = 12.2 Hz, H6a); 4.29 (dd, 1H, J = 5.2 Hz, J = 12.4 Hz, H6b); 4.87 (d, 1H, J = 1.6 Hz, H1); 5.30 (t, 1H, J = 10.0 Hz, H4); 5.28 (dd, 1H, J = 2.0 Hz, J = 3.2 Hz, H2); 5.36 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3) : δ = 20.63, 20.68, 20.71, 20.84 (4CH3); 50.32 (CH2N3); 62.42 (C6); 65.96 (C4); 67.02 (CH2CH2N3); 68.82 (C5 and C3); 69.36 (C2); 97.71 (C1); 169.73, 169.78, 169.98, 170.59 ppm (4CO); MS (ESI) m/z: 440.12 [M+Na]+.2'-Azidoethyl-α-d-mannopyranoside (3): Compound 2 (16.0 g, 38.36 mmol, 1 eq.) and NaOMe (2.07 g, 38.36 mmol, 1 eq.) were added to methanol (100 mL). After 30 min stirring at RT, the mixture was neutralized with Amberlite IRC-50 H+ resins, filtered and concentrated in vacuo. Purification by chromatography on silica gel (CH2Cl2/MeOH 9:1) gave a white powder (65%). Rf = 0.40 (CH2Cl2/MeOH 4:1);  = + 54.9 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 3.41 (t, 2H, J = 5.0 Hz, CH2N3); 3.60 (m, 3H, H3, H5 and CH2CH2N3); 3.71 (m, 2H, H4 and H6a); 3.85 (m, 2H, H2 and H6b); 3.92 (m, 1H, CH2CH2N3); 4.81 ppm (d, 1H, J = 1.2 Hz, H1); MS (ESI) m/z: 272.11 [M+Na]+, 288.02 [M+K]+, 521.19 [2M+Na]+.
= + 54.9 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 3.41 (t, 2H, J = 5.0 Hz, CH2N3); 3.60 (m, 3H, H3, H5 and CH2CH2N3); 3.71 (m, 2H, H4 and H6a); 3.85 (m, 2H, H2 and H6b); 3.92 (m, 1H, CH2CH2N3); 4.81 ppm (d, 1H, J = 1.2 Hz, H1); MS (ESI) m/z: 272.11 [M+Na]+, 288.02 [M+K]+, 521.19 [2M+Na]+.
 = + 54.9 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 3.41 (t, 2H, J = 5.0 Hz, CH2N3); 3.60 (m, 3H, H3, H5 and CH2CH2N3); 3.71 (m, 2H, H4 and H6a); 3.85 (m, 2H, H2 and H6b); 3.92 (m, 1H, CH2CH2N3); 4.81 ppm (d, 1H, J = 1.2 Hz, H1); MS (ESI) m/z: 272.11 [M+Na]+, 288.02 [M+K]+, 521.19 [2M+Na]+.
= + 54.9 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 3.41 (t, 2H, J = 5.0 Hz, CH2N3); 3.60 (m, 3H, H3, H5 and CH2CH2N3); 3.71 (m, 2H, H4 and H6a); 3.85 (m, 2H, H2 and H6b); 3.92 (m, 1H, CH2CH2N3); 4.81 ppm (d, 1H, J = 1.2 Hz, H1); MS (ESI) m/z: 272.11 [M+Na]+, 288.02 [M+K]+, 521.19 [2M+Na]+.2'-Azidoethyl-2,3-O-isopropylidene-α-d-mannopyranoside (4): A solution of compound 3 (9.5 g, 38.15 mmol, 1 eq.), 2,2-dimethoxypropane (23.4 mL, 190.76 mmol, 5 eq.) and para-toluenesulfonic acid (362 mg, 1.90 mmol, 0.05 eq.) in acetone (40 mL) was stirred for 4 h at RT. The para-toluenesulfonic acid was neutralized with 5% aq NaHCO3. Acetone was removed in vacuo and the aqueous phase was washed with petroleum ether to remove the diisopropylidene species. This organic layer was dried (Na2SO4) and concentrated in vacuo. Then the aqueous layer containing the monoisopropylidene was lyophilized. The diisopropylidene compound (7.2 g, 21.88 mmol, 1 eq.) was stirred in a solution of acetic acid/water 8:2 (60 mL) at 35 °C. After 2 h, solvents were evaporated, and then coevaporated with toluene several times. The crude product obtained was purified by chromatography on silica gel (petroleum ether/EtOAc 2:3) to give a yellow oil (85% monoisopropylidene compound, over two steps).
Diisopropylidene derivative: Rf = 0.63 (EtOAc/petroleum ether 1:1); 1H-NMR (acetone-d6): δ = 1.31, 1.32 (2s, 6H, 2CH3); 1.47, 1.48 (2s, 6H, 2CH3); 3.50 (t, 2H, J = 4.8 Hz, CH2N3); 3.53 (m, 1H, H5); 3.72 (m, 3H, H6a, H4 and CH2CH2N3); 3.82 (dd, 1H, J = 5.8 Hz, J = 10.6 Hz, H6b); 3.93 (qt, 1H, J = 5.2 Hz, CH2CH2N3); 4.03 (dd, 1H, J = 5.6 Hz, J = 8.0 Hz, H3); 4.18 (d, 1H, J = 5.6 Hz, H2); 5.09 (s, 1H, H1); 13C-NMR (acetone-d6): δ = 20.11, 29.38, 27.45, 30.50 (4CH3); 52.18 (CH2N3); 63.48, 63.53 (C5 and C6); 68.17 (CH2CH2N3); 74.47 (C4); 76.78 (C3); 77.83 (C2); 99.68 (C1); 100.10,109.76 ppm (2C(CH3)2); MS (ESI) m/z: 352.20 [M+Na]+, 368.02 [M+K]+.
Monoisopropylidene derivative: Rf = 0.26 (EtOAc/petroleum ether 3/2); 1H-NMR (acetone-d6 + D2O): δ = 1.27, 1.41 (2s, 6H, 2CH3); 3.45 (t, 2H, J = 5.0 Hz, CH2N3); 3.52 (m, 2H, H4 and H5); 3.62 (dd, 1H, J = 5.2 Hz, J = 11.6 Hz, H6a); 3.67 (m, 1H, CH2CH2N3); 3.80 (m, 1H, H6b); 3.93 (m, 1H, CH2CH2N3); 4.02 (m, 1H, H3); 4.09 (d, 1H, J = 5.6 Hz, H2); 5.03 (s, 1H, H1); 13C-NMR (acetone-d6 + D2O): δ = 20.34, 29.11 (2CH3); 51.95 (CH2N3); 62.97 (C6); 67.74 (CH2CH2N3); 70.41, 72.62 (C4 and C5); 77.30 (C2); 80.42 (C3); 98.60 (C1); 110.60 (C(CH3)2); MS (ESI) m/z: 312.12 [M+Na]+, 328.15 [M+K]+, 324.12 [M+Cl]−.
2'-Azidoethyl-2,3-O-isopropylidene-α-d-mannopyranoside-4,6-cyclic sulfate (5): Compound 4 (100 mg, 0.35 mmol, 1 eq.) and Et3N (144 µL, 1.04 mmol, 3 eq.) in CH2Cl2 (2 mL) were stirred for 5 min at 0 °C. Then SOCl2 (27 µL, 0.38 mmol, 1.1 eq.) was added dropwise to the mixture. After 10 min, the solution was filtered. Impurities were removed with water and the organic layer was washed with 1N HCl, dried (Na2SO4) and concentrated in vacuo to give a brown solid. The crude sulfite obtained was then reacted with NaIO4 (81 mg, 0.38 mmol, 1.1 eq.), water (0.5 mL) and RuCl3 (1.38.10−3 mmol, 0.004 eq.) in CH2Cl2/CH3CN 1:1 (2 mL). After 1h at RT, the solution was filtered before adding water. After extraction, the organic layer was dried (Na2SO4) and concentrated in vacuo. Filtration on silica gel and washes with CH2Cl2 gave a white solid (66%). Rf = 0.58 (EtOAc/petroleum ether 1:1); mp: 80–82 °C; 1H-NMR (acetone-d6): δ = 1.37, 1.52 (2s, 6H, 2CH3); 3.55 (m, 2H, CH2N3); 3.80 (m, 1H, CH2CH2N3); 4.29 (m, 1H, CH2CH2N3); 4.26 (td, 1H, J = 10.7 Hz, J = 5.5 Hz, H5); 4.36 (d, 1H, J = 6.0 Hz, H2); 4.43 (dd, 1H, J = 5.6 Hz, J = 8.0 Hz, H3); 4.6 (dd, 1H, J = 7.6 Hz, J = 10.8 Hz, H4); 4.63 (t, 1H, J = 10.8 Hz, H6a); 4.84 (dd, 1H, J = 5.6 Hz, J = 10.4 Hz, H6b); 5.28 ppm (s, 1H, H1); 13C-NMR (acetone-d6): δ = 27.16, 29.13 (2CH3); 52.06 (CH2N3); 60.35 (C5); 68.75 (CH2CH2N3); 74.34 (C6); 74.95 (C3); 77.88 (C2); 86.65 (C4); 99.70 (C1); 112.07 ppm (C(CH3)2); MS (ESI) m/z: 374.13 [M+Na]+, 386.08 [M+Cl]−.
3,6,9,12,15,18-Hexaoxatricos-22-en-1-ol (6): A mixture of 50% aqueous sodium hydroxide (1.93 mL, 24.18 mmol, 1.1 eq.) and hexa(ethylene glycol) (25 g, 88.55 mmol, 4.12 eq.) was stirred for 30 min at 100 °C, before adding 5-bromopent-1-ene (2.55 mL, 21.50 mmol, 1 eq.). After 15 min, the reaction mixture was cooled, diluted in CH2Cl2 and washed with water. The organic phase was dried (Na2SO4), filtered and concentrated in vacuo. Purification by chromatography on silica gel (EtOAc/petroleum ether 9:1 to EtOAc/MeOH 9:1) gave a yellow oil (99%). Rf = 0.14 (AcOEt/petroleum ether 5:5);  = + 54.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.68 (m, 2H, CH2CH2CH=CH2); 2.09 (m, 2H, CH2CH=CH2); 3.46 (t, 2H, J = 6.6 Hz, CH2CH2CH2CH=CH2); 3.56–3.73 (m, 24 h, 12CH2O); 4.99 (m, 2H, CH=CH2); 5.81 ppm (m, 2H, CH=CH2); 13C-NMR (CDCl3): δ = 28.66 (CH2CH2CH=CH2); 30.12 (CH2CH=CH2); 61.51–72.58 (13CH2O); 114.59 (CH=CH2); 138.18 ppm (CH=CH2); MS (ESI) m/z: 373.27 [M+Na]+, 389.20 [M+K]+.
= + 54.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.68 (m, 2H, CH2CH2CH=CH2); 2.09 (m, 2H, CH2CH=CH2); 3.46 (t, 2H, J = 6.6 Hz, CH2CH2CH2CH=CH2); 3.56–3.73 (m, 24 h, 12CH2O); 4.99 (m, 2H, CH=CH2); 5.81 ppm (m, 2H, CH=CH2); 13C-NMR (CDCl3): δ = 28.66 (CH2CH2CH=CH2); 30.12 (CH2CH=CH2); 61.51–72.58 (13CH2O); 114.59 (CH=CH2); 138.18 ppm (CH=CH2); MS (ESI) m/z: 373.27 [M+Na]+, 389.20 [M+K]+.
 = + 54.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.68 (m, 2H, CH2CH2CH=CH2); 2.09 (m, 2H, CH2CH=CH2); 3.46 (t, 2H, J = 6.6 Hz, CH2CH2CH2CH=CH2); 3.56–3.73 (m, 24 h, 12CH2O); 4.99 (m, 2H, CH=CH2); 5.81 ppm (m, 2H, CH=CH2); 13C-NMR (CDCl3): δ = 28.66 (CH2CH2CH=CH2); 30.12 (CH2CH=CH2); 61.51–72.58 (13CH2O); 114.59 (CH=CH2); 138.18 ppm (CH=CH2); MS (ESI) m/z: 373.27 [M+Na]+, 389.20 [M+K]+.
= + 54.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.68 (m, 2H, CH2CH2CH=CH2); 2.09 (m, 2H, CH2CH=CH2); 3.46 (t, 2H, J = 6.6 Hz, CH2CH2CH2CH=CH2); 3.56–3.73 (m, 24 h, 12CH2O); 4.99 (m, 2H, CH=CH2); 5.81 ppm (m, 2H, CH=CH2); 13C-NMR (CDCl3): δ = 28.66 (CH2CH2CH=CH2); 30.12 (CH2CH=CH2); 61.51–72.58 (13CH2O); 114.59 (CH=CH2); 138.18 ppm (CH=CH2); MS (ESI) m/z: 373.27 [M+Na]+, 389.20 [M+K]+.S-(23-Hydroxy-6,9,12,15,18,21-hexaoxatricos-1-yl)ethane-thioate (7): A solution containing compound 6 (3.1 g, 8.85 mmol, 1 eq.), thiolacetic acid (3.17 mL, 44.28 mmol, 5 eq.) and AIBN (100 mg) in anhydrous THF (12 mL) was refluxed for 1 h under nitrogen. The mixture was diluted with EtOAc, washed with a saturated solution of NaHCO3. The organic layer was dried (Na2SO4), filtered and reduced in vacuo. Purification by chromatography on silica gel (EtOAc/petroleum ether 9:1 to EtOAc/MeOH 9:1) gave a yellow oil (71%). Rf = 0.27 (EtOAc/MeOH 9:1); 1H-NMR (CDCl3): δ = 1.40 (m, 2H, CH2(CH2)2S); 1.58 (m, 4 h, CH2(CH2)3S and CH2CH2S); 1.83 (s, 1H, OH); 2.32 (s, 3H, CH3); 2.86 (t, 2H, J = 7.2 Hz, CH2S); 3.44 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.56–3.73 ppm (m, 24 h, 12CH2O); 13C-NMR (CDCl3): δ = 25.25 (CH2(CH2)2S); 28.90 (CH2S); 28.99, 29.24 (CH2(CH2)3S and CH2CH2S); 30.52 (CH3); 61.55–72.43 (13CH2O); 195.84 ppm (CO); MS (ESI) m/z: 449.26 [M+Na]+, 461.17 [M+Cl]−.
1-(Methoxytritylthio)-8,11,14,17,20,23-hexaoxa-2-thiapentacosan-25-ol (8): Compound 7 (2.6 g, 6.1 mmol, 1 eq.) and a concentrated solution of HCl (3 mL) were stirred in EtOH (65 mL). After 20 h reaction at 60 °C, the mixture was neutralized with ammonia then reduced under pressure. The obtained solution was diluted with EtOAc, and the organic layer was quickly washed with water, dried (Na2SO4), and concentrated in vacuo. The crude product was directly put in reaction with MeOTrCl (2.83 g, 9.15 mmol, 1.5 eq.) in anhydrous THF (60 mL). After 24 h stirring at RT, the solution was concentrated in vacuo and purified by chromatography on silica gel (EtOAc/MeOH 9:1) to give a yellow oil (91%): Rf = 0.40 (EtOAc/MeOH 7:3); 1H-NMR (acetone-d6): δ = 1.31 (m, 2H, CH2(CH2)2S); 1.40 (m, 4 h, CH2(CH2)3S and CH2CH2S); 2.17 (t, 2H, J = 7.4 Hz, CH2S); 2.87 (s, 1H, OH); 3.35 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.47–3.63 (m, 24 h, 12CH2O); 3.79 (s, 3H, CH3); 6.86–7.42 ppm (m, 14 h, CHAr); 13C-NMR (acetone-d6): δ = 27.37, 30.16 (CH2CH2CH2CH2S); 33.54 (CH2S); 56.50 (CH3); 62.94–72.33 (13CH2O); 74.48 (SC); 114.86–132.54 (14CHAr); 138.81, 147.37 (3SCCAr); 160.12 ppm (COCH3); MS (ESI) m/z: 679.34 [M+Na]+.
1-(Methoxytritylthio)-8,11,14,17,20,23-hexaoxa-2-thiahexacos-25-yne (9): NaH (7.3 mg, 0.30 mmol, 2 eq.) and 2-bromopropyne (19 µL, 0.21 mmol, 1.4 eq.) were added to a solution containing compound 8 (100 mg, 0.15 mmol, 1 eq.) in anhydrous THF (3 mL) at 0 °C. After 18 h stirring at RT, the mixture was concentrated then purified by chromatography on silica gel (EtOAc/petroleum ether 8:2) to give a white oil (97%): Rf = 0.34 (EtOAc); 1H-NMR (CDCl3): δ = 1.28 (m, 2H, CH2(CH2)2S); 1.42 (m, 4 h, CH2(CH2)3S and CH2CH2S); 2.14 (t, 2H, J = 7.4 Hz, CH2S); 2.43 (t, 1H, J = 2.4 Hz, CH); 3.36 (t, 2H, J = 6.8 Hz, CH2(CH2)4S); 3.52–3.71 (m, 24 h, 12CH2O); 3.79 (s, 3H, CH3); 4.20 (d, 2H, J = 2.4 Hz, CH2CCH); 6.79–7.40 ppm (m, 14 h, CHAr); 13C-NMR (CDCl3): δ = 25.59 (CH2(CH2)2S); 28.46, 29.19 (CH2(CH2)3S and CH2CH2S); 31.96 (CH2S); 55.20 (CH3); 58.39 (CH2CCH); 65.85 (CH); 69.10–71.17 (13CH2O); 74.51 (SC); 113.03–130.73 (14CHAr); 137.12, 145.32 (3SCCAr); 157.94 ppm (COCH3); MS (ESI) m/z: 717.39 [M+Na]+.
2-{4-[27-(4-Methoxyphenyl)-27,27-diphenyl-2,5,8,11,14,17,20-heptaoxa-26-thiaheptacos-1-yl]-2,3-dihydro-1H-1,2,3-triazol-1-yl}ethyl-6-deoxy-2,3-O-(1-methylethylidene)-4,6-cyclic sulfate-α-d-manno-pyranoside (10): Compounds 5 (40 mg, 0.11 mmol, 1 eq.) and 9 (87 mg, 0.13 mmol, 1.1 eq.) were suspended in a mixture of t-BuOH/H2O 1:1 (4 mL). Cu(0) nanosize activated powder (4 mg, 0.06 mmol, 0.5 eq.) and NHEt3Cl (32 mg, 0.23 mmol, 2 eq.) were added, and the heterogeneous mixture was stirred vigorously for 20 h at RT. The reaction mixture was diluted in EtOAc then washed with water. The organic layer was dried, filtered and concentrated in vacuo. Purification by chromatography on silica gel (CH2Cl2/MeOH 99:1 to 9:1) gave a colourless oil (60%): Rf = 0.40 (CH2Cl2/MeOH 9:1);  = + 36.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.36 (m, 6H, CH2CH2CH2CH2S); 1.34, 1.49 (2s, 6H, 2CCH3); 2.14 (t, 2H, J = 7.2 Hz, CH2S); 3.38 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.44–3.66 (m, 25 h, H5 and 12CH2O); 3.78 (s, 3H, OCH3); 3.97 (m, 1H, CH2CH2N); 4.15 (m, 1H, CH2CH2N); 4.27 (m, 3H, H2, H3 and H6a); 4.50 (m, 2H, H6b and H4); 4.64 (m, 4 h, CH2N and CH2C=CH); 5.12 (s, 1H, H1); 6.81–7.39 (m, 14 h, CHAr); 8.07 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.34, 28.20 (2CCH3); 26.71 (CH2(CH2)2S); 29.62 (CH2CH2S); 30.23 (CH2(CH2)3S); 33.03 (SCH2); 51.19 (CH2N); 55.79 (OCH3); 59.79 (C5); 65.14 (CH2C=CH); 67.42 (CH2CH2N); 70.95, 71.19, 71.49, 71.58, 72.03 (13CH2O); 73.53 (C6); 74.46, 77.23 (C2 and C3); 85.66 (C4); 98.96 (C1); 108.26, 111.66 (SC and C(CH3)2); 114.11, 127.66, 128.86, 130.73, 132.02 (14CHAr); 126.04 (NCH); 138.40, 146.86 (3SCCAr and C=CH); 159.71 ppm (COCH3); MS (ESI) m/z: 1068.62 [M+Na]+, 1080.77 [M+Cl]−.
= + 36.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.36 (m, 6H, CH2CH2CH2CH2S); 1.34, 1.49 (2s, 6H, 2CCH3); 2.14 (t, 2H, J = 7.2 Hz, CH2S); 3.38 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.44–3.66 (m, 25 h, H5 and 12CH2O); 3.78 (s, 3H, OCH3); 3.97 (m, 1H, CH2CH2N); 4.15 (m, 1H, CH2CH2N); 4.27 (m, 3H, H2, H3 and H6a); 4.50 (m, 2H, H6b and H4); 4.64 (m, 4 h, CH2N and CH2C=CH); 5.12 (s, 1H, H1); 6.81–7.39 (m, 14 h, CHAr); 8.07 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.34, 28.20 (2CCH3); 26.71 (CH2(CH2)2S); 29.62 (CH2CH2S); 30.23 (CH2(CH2)3S); 33.03 (SCH2); 51.19 (CH2N); 55.79 (OCH3); 59.79 (C5); 65.14 (CH2C=CH); 67.42 (CH2CH2N); 70.95, 71.19, 71.49, 71.58, 72.03 (13CH2O); 73.53 (C6); 74.46, 77.23 (C2 and C3); 85.66 (C4); 98.96 (C1); 108.26, 111.66 (SC and C(CH3)2); 114.11, 127.66, 128.86, 130.73, 132.02 (14CHAr); 126.04 (NCH); 138.40, 146.86 (3SCCAr and C=CH); 159.71 ppm (COCH3); MS (ESI) m/z: 1068.62 [M+Na]+, 1080.77 [M+Cl]−.
 = + 36.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.36 (m, 6H, CH2CH2CH2CH2S); 1.34, 1.49 (2s, 6H, 2CCH3); 2.14 (t, 2H, J = 7.2 Hz, CH2S); 3.38 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.44–3.66 (m, 25 h, H5 and 12CH2O); 3.78 (s, 3H, OCH3); 3.97 (m, 1H, CH2CH2N); 4.15 (m, 1H, CH2CH2N); 4.27 (m, 3H, H2, H3 and H6a); 4.50 (m, 2H, H6b and H4); 4.64 (m, 4 h, CH2N and CH2C=CH); 5.12 (s, 1H, H1); 6.81–7.39 (m, 14 h, CHAr); 8.07 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.34, 28.20 (2CCH3); 26.71 (CH2(CH2)2S); 29.62 (CH2CH2S); 30.23 (CH2(CH2)3S); 33.03 (SCH2); 51.19 (CH2N); 55.79 (OCH3); 59.79 (C5); 65.14 (CH2C=CH); 67.42 (CH2CH2N); 70.95, 71.19, 71.49, 71.58, 72.03 (13CH2O); 73.53 (C6); 74.46, 77.23 (C2 and C3); 85.66 (C4); 98.96 (C1); 108.26, 111.66 (SC and C(CH3)2); 114.11, 127.66, 128.86, 130.73, 132.02 (14CHAr); 126.04 (NCH); 138.40, 146.86 (3SCCAr and C=CH); 159.71 ppm (COCH3); MS (ESI) m/z: 1068.62 [M+Na]+, 1080.77 [M+Cl]−.
= + 36.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.36 (m, 6H, CH2CH2CH2CH2S); 1.34, 1.49 (2s, 6H, 2CCH3); 2.14 (t, 2H, J = 7.2 Hz, CH2S); 3.38 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.44–3.66 (m, 25 h, H5 and 12CH2O); 3.78 (s, 3H, OCH3); 3.97 (m, 1H, CH2CH2N); 4.15 (m, 1H, CH2CH2N); 4.27 (m, 3H, H2, H3 and H6a); 4.50 (m, 2H, H6b and H4); 4.64 (m, 4 h, CH2N and CH2C=CH); 5.12 (s, 1H, H1); 6.81–7.39 (m, 14 h, CHAr); 8.07 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.34, 28.20 (2CCH3); 26.71 (CH2(CH2)2S); 29.62 (CH2CH2S); 30.23 (CH2(CH2)3S); 33.03 (SCH2); 51.19 (CH2N); 55.79 (OCH3); 59.79 (C5); 65.14 (CH2C=CH); 67.42 (CH2CH2N); 70.95, 71.19, 71.49, 71.58, 72.03 (13CH2O); 73.53 (C6); 74.46, 77.23 (C2 and C3); 85.66 (C4); 98.96 (C1); 108.26, 111.66 (SC and C(CH3)2); 114.11, 127.66, 128.86, 130.73, 132.02 (14CHAr); 126.04 (NCH); 138.40, 146.86 (3SCCAr and C=CH); 159.71 ppm (COCH3); MS (ESI) m/z: 1068.62 [M+Na]+, 1080.77 [M+Cl]−.2-{4-[27-(4-Methoxyphenyl)-27,27-diphenyl-2,5,8,11,14,17,20-heptaoxa-26-thiaheptacos-1-yl]-2,3-dihydro-1H-1,2,3,-triazol-1-yl}ethyl-6-deoxy-2,3-O-(1-methylethylidene)-4-sodiumsulfate-6-azido-α-d-mannopyranoside (11): The procedure described for compound 2 was applied to 10 to give compound 11 as a yellow oil (62%). Rf = 0.15 (CH2Cl2/MeOH 8.5:1.5);  = +17.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.37 (m, 6H, CH2CH2CH2CH2S); 1.30, 1.45 (2s, 6H, 2CCH3); 2.17 (t, 2H, J = 7.2 Hz, CH2S); 3.37 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.42–3.67 (m, 27H, H2 and 13CH2O); 3.79 (s, 3H, OCH3); 3.96 (m, 1H, CH2CH2N); 4.12 (m, 1H, CH2CH2N); 4.07 (d, 1H, J = 6.0 Hz, H5); 4.25 (m, 1H, H3); 4.38 (t, 1H, J = 5.8 Hz, H4); 4.70 (m, 6H, H6, CH2N and CH2C=CH); 4.93 (s, 1H, H1); 6.87–7.41 (m, 14 h, CHAr); 9.19 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 27.20, 28.67 (2CCH3); 27.33, 30.16, 30.87 (CH2CH2CH2CH2S); 33.55 (CH2S); 51.66 (C6 and CH2(CH2)4S); 54.55 (CH2N); 56.53 (OCH3); 65.58 (CH2C=CH); 67.89 (CH2CH2N); 70.39–72.33 (12CH2O); 72.13 (C2); 73.88 (C3); 76.31 (C5); 77.37 (C4); 99.59 (C1); 110.79 (SC); 114.87–132.54 (14CHAr); 126.23 (NCH); 138.80–160.13 (3SCCAr and C=CH); 158.40 ppm (COCH3); MS(ESI) m/z:1134.58 [M+Na]+.
= +17.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.37 (m, 6H, CH2CH2CH2CH2S); 1.30, 1.45 (2s, 6H, 2CCH3); 2.17 (t, 2H, J = 7.2 Hz, CH2S); 3.37 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.42–3.67 (m, 27H, H2 and 13CH2O); 3.79 (s, 3H, OCH3); 3.96 (m, 1H, CH2CH2N); 4.12 (m, 1H, CH2CH2N); 4.07 (d, 1H, J = 6.0 Hz, H5); 4.25 (m, 1H, H3); 4.38 (t, 1H, J = 5.8 Hz, H4); 4.70 (m, 6H, H6, CH2N and CH2C=CH); 4.93 (s, 1H, H1); 6.87–7.41 (m, 14 h, CHAr); 9.19 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 27.20, 28.67 (2CCH3); 27.33, 30.16, 30.87 (CH2CH2CH2CH2S); 33.55 (CH2S); 51.66 (C6 and CH2(CH2)4S); 54.55 (CH2N); 56.53 (OCH3); 65.58 (CH2C=CH); 67.89 (CH2CH2N); 70.39–72.33 (12CH2O); 72.13 (C2); 73.88 (C3); 76.31 (C5); 77.37 (C4); 99.59 (C1); 110.79 (SC); 114.87–132.54 (14CHAr); 126.23 (NCH); 138.80–160.13 (3SCCAr and C=CH); 158.40 ppm (COCH3); MS(ESI) m/z:1134.58 [M+Na]+.
 = +17.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.37 (m, 6H, CH2CH2CH2CH2S); 1.30, 1.45 (2s, 6H, 2CCH3); 2.17 (t, 2H, J = 7.2 Hz, CH2S); 3.37 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.42–3.67 (m, 27H, H2 and 13CH2O); 3.79 (s, 3H, OCH3); 3.96 (m, 1H, CH2CH2N); 4.12 (m, 1H, CH2CH2N); 4.07 (d, 1H, J = 6.0 Hz, H5); 4.25 (m, 1H, H3); 4.38 (t, 1H, J = 5.8 Hz, H4); 4.70 (m, 6H, H6, CH2N and CH2C=CH); 4.93 (s, 1H, H1); 6.87–7.41 (m, 14 h, CHAr); 9.19 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 27.20, 28.67 (2CCH3); 27.33, 30.16, 30.87 (CH2CH2CH2CH2S); 33.55 (CH2S); 51.66 (C6 and CH2(CH2)4S); 54.55 (CH2N); 56.53 (OCH3); 65.58 (CH2C=CH); 67.89 (CH2CH2N); 70.39–72.33 (12CH2O); 72.13 (C2); 73.88 (C3); 76.31 (C5); 77.37 (C4); 99.59 (C1); 110.79 (SC); 114.87–132.54 (14CHAr); 126.23 (NCH); 138.80–160.13 (3SCCAr and C=CH); 158.40 ppm (COCH3); MS(ESI) m/z:1134.58 [M+Na]+.
= +17.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.37 (m, 6H, CH2CH2CH2CH2S); 1.30, 1.45 (2s, 6H, 2CCH3); 2.17 (t, 2H, J = 7.2 Hz, CH2S); 3.37 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.42–3.67 (m, 27H, H2 and 13CH2O); 3.79 (s, 3H, OCH3); 3.96 (m, 1H, CH2CH2N); 4.12 (m, 1H, CH2CH2N); 4.07 (d, 1H, J = 6.0 Hz, H5); 4.25 (m, 1H, H3); 4.38 (t, 1H, J = 5.8 Hz, H4); 4.70 (m, 6H, H6, CH2N and CH2C=CH); 4.93 (s, 1H, H1); 6.87–7.41 (m, 14 h, CHAr); 9.19 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 27.20, 28.67 (2CCH3); 27.33, 30.16, 30.87 (CH2CH2CH2CH2S); 33.55 (CH2S); 51.66 (C6 and CH2(CH2)4S); 54.55 (CH2N); 56.53 (OCH3); 65.58 (CH2C=CH); 67.89 (CH2CH2N); 70.39–72.33 (12CH2O); 72.13 (C2); 73.88 (C3); 76.31 (C5); 77.37 (C4); 99.59 (C1); 110.79 (SC); 114.87–132.54 (14CHAr); 126.23 (NCH); 138.80–160.13 (3SCCAr and C=CH); 158.40 ppm (COCH3); MS(ESI) m/z:1134.58 [M+Na]+.2-{4-[27-(4-Methoxyphenyl)-27,27-diphenyl-2,5,8,11,14,17,20-heptaoxa-26-thiaheptacos-1-yl]-2,3-dihydro-1H-1,2,3,-triazol-1-yl}ethyl-6-deoxy-6-azido-α-d-mannopyranoside (12): Firstly, compound 11 (200 mg, 0.18 mmol, 1 eq.) and CAN (50 mg, 0.09 mmol, 0.5 eq.) were added to a mixture of CH3CN/H2O 95:5 (4 mL). After 4 h stirring at 60 °C, the solution was diluted in CH2Cl2, washed several times with water and the aqueous layer was lyophilized. Purification by chromatography on silica gel (CH2Cl2/MeOH 9:1 to CH2Cl2/MeOH 8:2) gave the product as a colourless oil (72%). Secondly, this intermediate was dissolved in a mixture of MeOH/THF 1:1 (6 mL) before adding Amberlyst H+ resins. After 24 h at RT, the resins were filtered, and the solution was neutralized with a saturated solution of NaHCO3. Organic solvents were evaporated and water lyophilized. The crude product was dissolved in methanol and the insoluble NaHCO3 was filtered. Purification by chromatography on silica gel (CH2Cl2/MeOH 9:1) gave the product as a colourless oil (53%): Rf = 0.25 (CH2Cl2/MeOH 9:1);  = −2.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.47 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.71 (m, 2H, CH2CH2S); 2.70 (t, 2H, J = 7.2 Hz, CH2S); 3.48 (t, 2H, J = 6.2 Hz, CH2(CH2)4S); 3.19–3.78 (m, 30H, H2-6 and 12CH2O); 3.88 (m, 1H, CH2CH2N); 4.13 (m, 1H, CH2CH2N); 4.63 (m, 4 h, CH2N and CH2C=CH); 4.72 (s, 1H, H1); 8.03 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.13 (CH2(CH2)3S); 30.07 (CH2CH2S); 30.36 (CH2(CH2)3S); 39.66 (CH2S); 51.34 (CH2N); 62.85 (C6); 65.05 (CH2C=CH); 66.79 (CH2CH2N); 68.38, 70.81, 71.24, 71.59, 71.93, 72.15, 72.51, 75.01 (C2-5 and 13CH2O); 101.70 (C1); 132.57 (NCH); 161.04 ppm (CH=C); MS (ESI) m/z: 798.62 [M+Na]+.
= −2.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.47 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.71 (m, 2H, CH2CH2S); 2.70 (t, 2H, J = 7.2 Hz, CH2S); 3.48 (t, 2H, J = 6.2 Hz, CH2(CH2)4S); 3.19–3.78 (m, 30H, H2-6 and 12CH2O); 3.88 (m, 1H, CH2CH2N); 4.13 (m, 1H, CH2CH2N); 4.63 (m, 4 h, CH2N and CH2C=CH); 4.72 (s, 1H, H1); 8.03 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.13 (CH2(CH2)3S); 30.07 (CH2CH2S); 30.36 (CH2(CH2)3S); 39.66 (CH2S); 51.34 (CH2N); 62.85 (C6); 65.05 (CH2C=CH); 66.79 (CH2CH2N); 68.38, 70.81, 71.24, 71.59, 71.93, 72.15, 72.51, 75.01 (C2-5 and 13CH2O); 101.70 (C1); 132.57 (NCH); 161.04 ppm (CH=C); MS (ESI) m/z: 798.62 [M+Na]+.
 = −2.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.47 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.71 (m, 2H, CH2CH2S); 2.70 (t, 2H, J = 7.2 Hz, CH2S); 3.48 (t, 2H, J = 6.2 Hz, CH2(CH2)4S); 3.19–3.78 (m, 30H, H2-6 and 12CH2O); 3.88 (m, 1H, CH2CH2N); 4.13 (m, 1H, CH2CH2N); 4.63 (m, 4 h, CH2N and CH2C=CH); 4.72 (s, 1H, H1); 8.03 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.13 (CH2(CH2)3S); 30.07 (CH2CH2S); 30.36 (CH2(CH2)3S); 39.66 (CH2S); 51.34 (CH2N); 62.85 (C6); 65.05 (CH2C=CH); 66.79 (CH2CH2N); 68.38, 70.81, 71.24, 71.59, 71.93, 72.15, 72.51, 75.01 (C2-5 and 13CH2O); 101.70 (C1); 132.57 (NCH); 161.04 ppm (CH=C); MS (ESI) m/z: 798.62 [M+Na]+.
= −2.1 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.47 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.71 (m, 2H, CH2CH2S); 2.70 (t, 2H, J = 7.2 Hz, CH2S); 3.48 (t, 2H, J = 6.2 Hz, CH2(CH2)4S); 3.19–3.78 (m, 30H, H2-6 and 12CH2O); 3.88 (m, 1H, CH2CH2N); 4.13 (m, 1H, CH2CH2N); 4.63 (m, 4 h, CH2N and CH2C=CH); 4.72 (s, 1H, H1); 8.03 ppm (s, 1H, NCH); 13C-NMR (CD3OD): δ = 26.13 (CH2(CH2)3S); 30.07 (CH2CH2S); 30.36 (CH2(CH2)3S); 39.66 (CH2S); 51.34 (CH2N); 62.85 (C6); 65.05 (CH2C=CH); 66.79 (CH2CH2N); 68.38, 70.81, 71.24, 71.59, 71.93, 72.15, 72.51, 75.01 (C2-5 and 13CH2O); 101.70 (C1); 132.57 (NCH); 161.04 ppm (CH=C); MS (ESI) m/z: 798.62 [M+Na]+.2-{4-[27-(4-Methoxyphenyl)-27,27-diphenyl-2,5,8,11,14,17,20-heptaoxa-26-thiaheptacos-1-yl]-2,3-dihydro-1H-1,2,3,-triazol-1-yl}ethyl-6-deoxy-2,3-O-(1-methylethylidene)-4-sodium sulfate-6-cyano-α-d-mannopyranoside (13): Sodium cyanide (15 mg, 0.31 mmol, 2 eq.) was added to a suspension of compound 10 (160 mg, 0.15 mmol, 1 eq.) in DMF (1.5 mL). After 4 h stirring at RT, the mixture was poured into brine and extracted with CH2Cl2. The organic layers were dried (Na2SO4) and concentrated in vacuo. The residue was purified by chromatography on silica gel (CH2Cl2/MeOH 9:1) to give the appropriated intermediate as a colourless oil (65%): Rf = 0.24 (CH2Cl2/MeOH 9:1); 1H-NMR (CD3OD): δ = 1.31, 1.50 (2s, 6H, 2CCH3); 1.38 (m, 6H, CH2CH2CH2CH2S); 2.15 (t, 2H, J = 7.2 Hz, CH2S); 2.70 (dd, 1H, J = 8.8 Hz, J = 17.2 Hz, H6a); 3.02 (dd, 1H, J = 3.0 Hz, J = 17.0 Hz, H6b); 3.38 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.48–3.66 (m, 26H, H4,5 and 12CH2O); 3.78 (s, 3H, OCH3); 3.93 (m, 1H, CH2CH2N); 4.10 (d, 1H, J = 4.8 Hz, H2); 4.19 (m, 2H, CH2CH2N and H3); 4.65 (m, 4 h, CH2N and CH2C=CH); 5.00 (s, 1H, H1); 6.82–7.39 (m, 14 h, CHAr); 8.04 ppm (s, 1H, NCH); 13C-NMR (100.62 MHz, CD3OD): δ = 21.83 (C6); 26.60, 28.05 (2CCH3); 26.69 (CH2(CH2)2S); 29.62 (CH2CH2S); 30.17 (CH2(CH2)3S); 33.04 (CH2S); 51.17 (CH2N); 55.81 (OCH3); 64.87 (CH2C=CH); 66.66, 76.70 (C4 and C5); 67.02 (CH2CH2N); 70.47–72.02 (13CH2O); 77.54 (C2); 77.92 (C3); 98.52 (C1); 110.98 (SC and C(CH3)2); 118.96 (CH2CN); 114.12–132.01 (14CHAr); 125.87 (NCH); 138.38, 146.00, 146.84 (3SCCAr and C=CH); 159.71 ppm (COCH3); MS (ESI) m/z: 1117.77 [M+Na]+,1071.63 [M-Na]−.
2-{4-[27-(4-Methoxyphenyl)-27,27-diphenyl-2,5,8,11,14,17,20-heptaoxa-26-thiaheptacos-1-yl]-2,3-dihydro-1H-1,2,3,-triazol-1-yl}ethyl-6-deoxy-α-d-heptomannopyranouronic acid (14): Firstly, NaOH (60 mg, 1.46 mmol, 8 eq.) was added to a solution of compound 13 (200 mg, 0.18 mmol, 1 eq.) and H2O2 at 30% (1.5 mL). 1.5 mL of H2O2 at 30% and 60 mg of NaOH were added to the mixture after 12 h stirring at RT and again after 24 h stirring at RT. After 48 h, the solution was neutralized with Amberlite IRC-50 (H+) resin, filtered, and concentrated in vacuo. The crude product was purified by chromatography on silica gel (CH2Cl2/MeOH 9:1 to NH4OH/iPrOH 1:1) to give a yellow oil (52%): Rf = 0.15 (AcOEt/MeOH 5:5);  = −9.0 (c = 1.00 in chloroform); 1H-NMR (D2O): δ = 1.35, 1.52 (2s, 6H, 2CCH3); 1.44 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.73 (m, 2H, CH2CH2S); 2.29 (dd, 1H, J = 10.6 Hz, J = 15.0Hz, H6a); 2.80 (dd, 1H, J = 2.0 Hz, J = 15.2 Hz, H6b); 2.89 (t, 2H, J = 8.0 Hz, CH2S); 3.53 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.50–3.69 (m, 25 h, H5 and 12CH2O); 3.75 (s, 3H, OCH3); 3.88 (m, 1H, CH2CH2N); 4.17 (m, 2H, CH2CH2N and H4); 4.19 (d, 1H, J = 5.6 Hz, H2); 4.30 (m, 1H, H3); 4.68 (m, 4 h, CH2N and CH2C=CH); 4.95 (s, 1H, H1); 6.80–7.37 (m, 14 h, CHAr); 8.12 ppm (s, 1H, NCH); 13C-NMR (D2O): δ = 23.80 (CH2CH2S); 24.24 (CH2(CH2)2S); 25.45, 26.80 (2CCH3); 28.06 (CH2(CH2)3S); 38.92 (C6); 49.99 (CH2N); 50.89 (CH2S); 55.86 (OCH3); 62.95 (CH2C=CH); 65.47 (CH2CH2N); 66.41 (C5); 66.54, 68.69, 69.07, 69.40, 69.53, 70.70 (13CH2O); 75.21 (C2); 76.00 (C3); 78.30 (C4); 95.99 (C1); 110.39 (SC and C(CH3)2); 114.14–132.02 (14CHAr); 125.55 (NCH); 143.85 (C=CH); 138.38, 146.00, 146.84 (3SCCAr); 159.71 (COCH3);177.75 (CO2H); MS (ESI) m/z: 1037.34 [M+Na]+.
= −9.0 (c = 1.00 in chloroform); 1H-NMR (D2O): δ = 1.35, 1.52 (2s, 6H, 2CCH3); 1.44 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.73 (m, 2H, CH2CH2S); 2.29 (dd, 1H, J = 10.6 Hz, J = 15.0Hz, H6a); 2.80 (dd, 1H, J = 2.0 Hz, J = 15.2 Hz, H6b); 2.89 (t, 2H, J = 8.0 Hz, CH2S); 3.53 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.50–3.69 (m, 25 h, H5 and 12CH2O); 3.75 (s, 3H, OCH3); 3.88 (m, 1H, CH2CH2N); 4.17 (m, 2H, CH2CH2N and H4); 4.19 (d, 1H, J = 5.6 Hz, H2); 4.30 (m, 1H, H3); 4.68 (m, 4 h, CH2N and CH2C=CH); 4.95 (s, 1H, H1); 6.80–7.37 (m, 14 h, CHAr); 8.12 ppm (s, 1H, NCH); 13C-NMR (D2O): δ = 23.80 (CH2CH2S); 24.24 (CH2(CH2)2S); 25.45, 26.80 (2CCH3); 28.06 (CH2(CH2)3S); 38.92 (C6); 49.99 (CH2N); 50.89 (CH2S); 55.86 (OCH3); 62.95 (CH2C=CH); 65.47 (CH2CH2N); 66.41 (C5); 66.54, 68.69, 69.07, 69.40, 69.53, 70.70 (13CH2O); 75.21 (C2); 76.00 (C3); 78.30 (C4); 95.99 (C1); 110.39 (SC and C(CH3)2); 114.14–132.02 (14CHAr); 125.55 (NCH); 143.85 (C=CH); 138.38, 146.00, 146.84 (3SCCAr); 159.71 (COCH3);177.75 (CO2H); MS (ESI) m/z: 1037.34 [M+Na]+.
 = −9.0 (c = 1.00 in chloroform); 1H-NMR (D2O): δ = 1.35, 1.52 (2s, 6H, 2CCH3); 1.44 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.73 (m, 2H, CH2CH2S); 2.29 (dd, 1H, J = 10.6 Hz, J = 15.0Hz, H6a); 2.80 (dd, 1H, J = 2.0 Hz, J = 15.2 Hz, H6b); 2.89 (t, 2H, J = 8.0 Hz, CH2S); 3.53 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.50–3.69 (m, 25 h, H5 and 12CH2O); 3.75 (s, 3H, OCH3); 3.88 (m, 1H, CH2CH2N); 4.17 (m, 2H, CH2CH2N and H4); 4.19 (d, 1H, J = 5.6 Hz, H2); 4.30 (m, 1H, H3); 4.68 (m, 4 h, CH2N and CH2C=CH); 4.95 (s, 1H, H1); 6.80–7.37 (m, 14 h, CHAr); 8.12 ppm (s, 1H, NCH); 13C-NMR (D2O): δ = 23.80 (CH2CH2S); 24.24 (CH2(CH2)2S); 25.45, 26.80 (2CCH3); 28.06 (CH2(CH2)3S); 38.92 (C6); 49.99 (CH2N); 50.89 (CH2S); 55.86 (OCH3); 62.95 (CH2C=CH); 65.47 (CH2CH2N); 66.41 (C5); 66.54, 68.69, 69.07, 69.40, 69.53, 70.70 (13CH2O); 75.21 (C2); 76.00 (C3); 78.30 (C4); 95.99 (C1); 110.39 (SC and C(CH3)2); 114.14–132.02 (14CHAr); 125.55 (NCH); 143.85 (C=CH); 138.38, 146.00, 146.84 (3SCCAr); 159.71 (COCH3);177.75 (CO2H); MS (ESI) m/z: 1037.34 [M+Na]+.
= −9.0 (c = 1.00 in chloroform); 1H-NMR (D2O): δ = 1.35, 1.52 (2s, 6H, 2CCH3); 1.44 (m, 2H, CH2(CH2)2S); 1.60 (m, 2H, CH2(CH2)3S); 1.73 (m, 2H, CH2CH2S); 2.29 (dd, 1H, J = 10.6 Hz, J = 15.0Hz, H6a); 2.80 (dd, 1H, J = 2.0 Hz, J = 15.2 Hz, H6b); 2.89 (t, 2H, J = 8.0 Hz, CH2S); 3.53 (t, 2H, J = 6.6 Hz, CH2(CH2)4S); 3.50–3.69 (m, 25 h, H5 and 12CH2O); 3.75 (s, 3H, OCH3); 3.88 (m, 1H, CH2CH2N); 4.17 (m, 2H, CH2CH2N and H4); 4.19 (d, 1H, J = 5.6 Hz, H2); 4.30 (m, 1H, H3); 4.68 (m, 4 h, CH2N and CH2C=CH); 4.95 (s, 1H, H1); 6.80–7.37 (m, 14 h, CHAr); 8.12 ppm (s, 1H, NCH); 13C-NMR (D2O): δ = 23.80 (CH2CH2S); 24.24 (CH2(CH2)2S); 25.45, 26.80 (2CCH3); 28.06 (CH2(CH2)3S); 38.92 (C6); 49.99 (CH2N); 50.89 (CH2S); 55.86 (OCH3); 62.95 (CH2C=CH); 65.47 (CH2CH2N); 66.41 (C5); 66.54, 68.69, 69.07, 69.40, 69.53, 70.70 (13CH2O); 75.21 (C2); 76.00 (C3); 78.30 (C4); 95.99 (C1); 110.39 (SC and C(CH3)2); 114.14–132.02 (14CHAr); 125.55 (NCH); 143.85 (C=CH); 138.38, 146.00, 146.84 (3SCCAr); 159.71 (COCH3);177.75 (CO2H); MS (ESI) m/z: 1037.34 [M+Na]+. Next, the procedure described for compound 12 was applied to the preceeding intermediate to give compound 14 as a colourless oil (80%). Rf = 0.18 (EtOAc/MeOH 5:5);  = +10.2 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.49 (m, 2H, CH2(CH2)2S); 1.61 (m, 2H, CH2(CH2)3S); 1.80 (m, 2H, CH2CH2S); 2.41 (dd, 1H, J = 10.2 Hz, J = 16.2 Hz, H6a); 2.84 (m, 3H, CH2S and H6b); 3.49 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.40–3.79 (m, 28 h, H2-5 and 12 CH2O); 3.92 (m, 1H, CH2CH2N); 4.22 (m, 1H, CH2CH2N); 4.71 (d, 1H, J = 1.2 Hz, H1); 4.87 (m, 2H, CH2N); 4.92 (m, 2H, CH2C=CH); 8.65 (s, 1H, NCH); 13C-NMR (D2O): δ = 23.79 (CH2CH2S); 24.23 (CH2(CH2)2S); 28.06 (CH2(CH2)3S); 36.51 (C6); 50.67 (CH2N); 50.90 (CH2S); 62.55 (CH2C=CH); 65.29 (CH2N); 67.88, 69.08, 69.54, 70.70 (13CH2O); 52.32, 69.36, 69.82, 70.16 (C2-5); 99.48 (C1); 109.39 (C=CH); 146.74 (NCH); 175.27 ppm (CO2H); MS (ESI) m/z: 765.86 [M-3H+3Na]+.
= +10.2 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.49 (m, 2H, CH2(CH2)2S); 1.61 (m, 2H, CH2(CH2)3S); 1.80 (m, 2H, CH2CH2S); 2.41 (dd, 1H, J = 10.2 Hz, J = 16.2 Hz, H6a); 2.84 (m, 3H, CH2S and H6b); 3.49 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.40–3.79 (m, 28 h, H2-5 and 12 CH2O); 3.92 (m, 1H, CH2CH2N); 4.22 (m, 1H, CH2CH2N); 4.71 (d, 1H, J = 1.2 Hz, H1); 4.87 (m, 2H, CH2N); 4.92 (m, 2H, CH2C=CH); 8.65 (s, 1H, NCH); 13C-NMR (D2O): δ = 23.79 (CH2CH2S); 24.23 (CH2(CH2)2S); 28.06 (CH2(CH2)3S); 36.51 (C6); 50.67 (CH2N); 50.90 (CH2S); 62.55 (CH2C=CH); 65.29 (CH2N); 67.88, 69.08, 69.54, 70.70 (13CH2O); 52.32, 69.36, 69.82, 70.16 (C2-5); 99.48 (C1); 109.39 (C=CH); 146.74 (NCH); 175.27 ppm (CO2H); MS (ESI) m/z: 765.86 [M-3H+3Na]+.
 = +10.2 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.49 (m, 2H, CH2(CH2)2S); 1.61 (m, 2H, CH2(CH2)3S); 1.80 (m, 2H, CH2CH2S); 2.41 (dd, 1H, J = 10.2 Hz, J = 16.2 Hz, H6a); 2.84 (m, 3H, CH2S and H6b); 3.49 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.40–3.79 (m, 28 h, H2-5 and 12 CH2O); 3.92 (m, 1H, CH2CH2N); 4.22 (m, 1H, CH2CH2N); 4.71 (d, 1H, J = 1.2 Hz, H1); 4.87 (m, 2H, CH2N); 4.92 (m, 2H, CH2C=CH); 8.65 (s, 1H, NCH); 13C-NMR (D2O): δ = 23.79 (CH2CH2S); 24.23 (CH2(CH2)2S); 28.06 (CH2(CH2)3S); 36.51 (C6); 50.67 (CH2N); 50.90 (CH2S); 62.55 (CH2C=CH); 65.29 (CH2N); 67.88, 69.08, 69.54, 70.70 (13CH2O); 52.32, 69.36, 69.82, 70.16 (C2-5); 99.48 (C1); 109.39 (C=CH); 146.74 (NCH); 175.27 ppm (CO2H); MS (ESI) m/z: 765.86 [M-3H+3Na]+.
= +10.2 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.49 (m, 2H, CH2(CH2)2S); 1.61 (m, 2H, CH2(CH2)3S); 1.80 (m, 2H, CH2CH2S); 2.41 (dd, 1H, J = 10.2 Hz, J = 16.2 Hz, H6a); 2.84 (m, 3H, CH2S and H6b); 3.49 (t, 2H, J = 6.4 Hz, CH2(CH2)4S); 3.40–3.79 (m, 28 h, H2-5 and 12 CH2O); 3.92 (m, 1H, CH2CH2N); 4.22 (m, 1H, CH2CH2N); 4.71 (d, 1H, J = 1.2 Hz, H1); 4.87 (m, 2H, CH2N); 4.92 (m, 2H, CH2C=CH); 8.65 (s, 1H, NCH); 13C-NMR (D2O): δ = 23.79 (CH2CH2S); 24.23 (CH2(CH2)2S); 28.06 (CH2(CH2)3S); 36.51 (C6); 50.67 (CH2N); 50.90 (CH2S); 62.55 (CH2C=CH); 65.29 (CH2N); 67.88, 69.08, 69.54, 70.70 (13CH2O); 52.32, 69.36, 69.82, 70.16 (C2-5); 99.48 (C1); 109.39 (C=CH); 146.74 (NCH); 175.27 ppm (CO2H); MS (ESI) m/z: 765.86 [M-3H+3Na]+.Methyl-2,3-O-isopropylidene-4,6-cyclic sulfate-α-d-mannopyranoside (15): Firstly, the procedure described for compound 4 was applied to methyl α-d-mannopyranoside to give the appropriate intermediate as a white solid (63%): Rf = 0.53 (EtOAc); mp: 103–105 °C; 1H-NMR (acetone-d6): δ = 1.28, 1.42 (2s, 6H,2CCH3); 3.35 (s, 3H, OCH3); 3.46 (ddd, 1H, J = 2.6 Hz, J = 5.6 Hz, J = 10.2 Hz, H5); 3.52 (dd, 1H,J = 6.9 Hz, J = 10.2 Hz, H4); 3.65 (dd, 1H, J = 5.8 Hz, J = 12.0 Hz, H6a); 3.81 (dd, 1H, J = 2.6 Hz, J = 11.9Hz, H6b); 4.01 (dd, 1H, J = 5.7 Hz, J = 6.9 Hz, H3); 4.06 (dd, 1H, J = 0.8 Hz, J = 5.7 Hz, H2); 5.01 ppm (s, 1H,H1); 13C-NMR (acetone-d6+D2O): δ = 26.50, 28.31 (2CCH3), 55.57 (OCH3), 62.79 (C6), 69.74, 70.02 (C4,C5), 75.97 (C2), 78.83 (C3), 98.80 (C1), 110.07 ppm (C(CH3)2); MS (ESI): m/z [M+Na]+ calcd. for C10H18O6 Na: 257.10, found: 257.21.
Next, the procedure described for compound 5 was applied to the preceeding intermediate to give compound 15 as a white solid (85%). mp: 80–82 °C; Rf = 0.48 (EtOAc/petroleum ether 3:7); 1H-NMR (acetone-d6): δ = 1.38, 1.53 (2s, 6H, 2CCH3); 3.46 (s, 3H, OCH3); 4.17 (td, 1H, J = 5.5 Hz, J = 10.6 Hz, H5); 4.32 (dd, 1H, J = 0.4 Hz, J = 5.6 Hz, H2); 4.42 (dd, 1H, J = 5.6 Hz, J = 7.7 Hz, H3); 4.59 (dd, 1H, J = 7.8 Hz, J = 10.4 Hz, H4); 4.64 (t, 1H, J = 10.7 Hz, H6a); 4.87 (dd, 1H, J = 5.5 Hz, J = 10.5 Hz, H6b); 5.01 (d, 1H, J = 0.5 Hz, H1); 13C-NMR (CDCl3): δ = 26.44, 28.45 (2CCH3); 56.14 (OCH3); 58.94 (C5); 72.32 (C6); 76.37 (C2); 73.61 (C3); 84.67 (C4); 99.42 (C1); 111.08 ppm (C(CH3)2); MS (ESI) m/z: 297.37 [M+H]+, 319.32 [M+Na]+.
Methyl-6-deoxy-6-azido-2,3,4-tri-O-acetyl-α-d-mannopyranoside (16): First, the procedures described for compounds 2 and 4 were applied to 15 to give 16 as a white solid: Rf = 0.50 (CH2Cl2/MeOH 9:1);  = +54.8 (c = 1.00 in methanol); 1H-NMR (D2O): δ = 3.40 (s, 3H, OCH3); 3.54 (dd, 1H, J = 6.2 Hz, J = 13.3 Hz, H6a); 3.60–3.73 (m, 4 h, H6b, H5, H4 and H3); 3.91 (dd, 1H, J = 3.3 Hz, J = 1.7 Hz, H2); 4.73 (d, 1H, J = 1.6 Hz, H1); 13C-NMR (D2O): δ = 51.4 (C6); 55.2 (OCH3); 67.8 (C5); 70.2 (C2); 70.7 (C3); 71.6 (C4); 101.4 ppm (C1); MS(ESI) m/z:242.31 [M+Na]+, 218.14 [M-H]−.
= +54.8 (c = 1.00 in methanol); 1H-NMR (D2O): δ = 3.40 (s, 3H, OCH3); 3.54 (dd, 1H, J = 6.2 Hz, J = 13.3 Hz, H6a); 3.60–3.73 (m, 4 h, H6b, H5, H4 and H3); 3.91 (dd, 1H, J = 3.3 Hz, J = 1.7 Hz, H2); 4.73 (d, 1H, J = 1.6 Hz, H1); 13C-NMR (D2O): δ = 51.4 (C6); 55.2 (OCH3); 67.8 (C5); 70.2 (C2); 70.7 (C3); 71.6 (C4); 101.4 ppm (C1); MS(ESI) m/z:242.31 [M+Na]+, 218.14 [M-H]−.
 = +54.8 (c = 1.00 in methanol); 1H-NMR (D2O): δ = 3.40 (s, 3H, OCH3); 3.54 (dd, 1H, J = 6.2 Hz, J = 13.3 Hz, H6a); 3.60–3.73 (m, 4 h, H6b, H5, H4 and H3); 3.91 (dd, 1H, J = 3.3 Hz, J = 1.7 Hz, H2); 4.73 (d, 1H, J = 1.6 Hz, H1); 13C-NMR (D2O): δ = 51.4 (C6); 55.2 (OCH3); 67.8 (C5); 70.2 (C2); 70.7 (C3); 71.6 (C4); 101.4 ppm (C1); MS(ESI) m/z:242.31 [M+Na]+, 218.14 [M-H]−.
= +54.8 (c = 1.00 in methanol); 1H-NMR (D2O): δ = 3.40 (s, 3H, OCH3); 3.54 (dd, 1H, J = 6.2 Hz, J = 13.3 Hz, H6a); 3.60–3.73 (m, 4 h, H6b, H5, H4 and H3); 3.91 (dd, 1H, J = 3.3 Hz, J = 1.7 Hz, H2); 4.73 (d, 1H, J = 1.6 Hz, H1); 13C-NMR (D2O): δ = 51.4 (C6); 55.2 (OCH3); 67.8 (C5); 70.2 (C2); 70.7 (C3); 71.6 (C4); 101.4 ppm (C1); MS(ESI) m/z:242.31 [M+Na]+, 218.14 [M-H]−.Secondly, Ac2O (1.72 mL, 18.26 mmol, 5 eq.) and DMAP (134 mg, 1.10 mmol, 0.3 eq.) were added to a solution of pyridine (15 mL) and methyl 6-azido-6-deoxy-α-d-mannopyranoside (800 mg, 3.65 mmol, 1 eq.). After 4 h stirring, the mixture was diluted in ethyl acetate and washed with a solution of HCl 2N (until pH = 1), a solution of NaHCO3 5%, water (until pH = 7) and with a saturated solution of NaCl. The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. The residue was purified by chromatography on silica gel (EtOAc/petroleum ether 2:3) to give a yellow powder (97%). Rf = 0.62 (EtOAc/petroleum ether 1:1); mp: 98–100 °C (lit. 99–100 °C);  = +65.7 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.97, 2.05, 2.13 (3s, 9H, COCH3); 3.16 (dd, 1H, J = 8.8 Hz, J = 10.8 Hz, H6a); 3.29 (dd, 1H, J = 2.6 Hz, J = 11.0 Hz, H6b); 3.46 (s, 3H, OCH3); 3.78 (td, 1H, J = 2.4 Hz, J = 9.2 Hz, H5); 4.71 (s, 1H, J = 1.2 Hz, H1); 5.09 (t, 1H, J = 9.8 Hz, H4); 5.20 (m, 1H, H2); 5.29 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 3.85 (C6); 20.60, 20.73, 20.80 (3COCH3); 55.49 (OCH3); 68.60 (C3); 69.52 (C2); 69.90 (C4); 70.07 (C5); 98.44 (C1); 169.77, 169.80, 169.95 ppm (3C=O); MS (ESI) m/z: 368.24 [M+Na]+.
= +65.7 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.97, 2.05, 2.13 (3s, 9H, COCH3); 3.16 (dd, 1H, J = 8.8 Hz, J = 10.8 Hz, H6a); 3.29 (dd, 1H, J = 2.6 Hz, J = 11.0 Hz, H6b); 3.46 (s, 3H, OCH3); 3.78 (td, 1H, J = 2.4 Hz, J = 9.2 Hz, H5); 4.71 (s, 1H, J = 1.2 Hz, H1); 5.09 (t, 1H, J = 9.8 Hz, H4); 5.20 (m, 1H, H2); 5.29 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 3.85 (C6); 20.60, 20.73, 20.80 (3COCH3); 55.49 (OCH3); 68.60 (C3); 69.52 (C2); 69.90 (C4); 70.07 (C5); 98.44 (C1); 169.77, 169.80, 169.95 ppm (3C=O); MS (ESI) m/z: 368.24 [M+Na]+.
 = +65.7 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.97, 2.05, 2.13 (3s, 9H, COCH3); 3.16 (dd, 1H, J = 8.8 Hz, J = 10.8 Hz, H6a); 3.29 (dd, 1H, J = 2.6 Hz, J = 11.0 Hz, H6b); 3.46 (s, 3H, OCH3); 3.78 (td, 1H, J = 2.4 Hz, J = 9.2 Hz, H5); 4.71 (s, 1H, J = 1.2 Hz, H1); 5.09 (t, 1H, J = 9.8 Hz, H4); 5.20 (m, 1H, H2); 5.29 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 3.85 (C6); 20.60, 20.73, 20.80 (3COCH3); 55.49 (OCH3); 68.60 (C3); 69.52 (C2); 69.90 (C4); 70.07 (C5); 98.44 (C1); 169.77, 169.80, 169.95 ppm (3C=O); MS (ESI) m/z: 368.24 [M+Na]+.
= +65.7 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.97, 2.05, 2.13 (3s, 9H, COCH3); 3.16 (dd, 1H, J = 8.8 Hz, J = 10.8 Hz, H6a); 3.29 (dd, 1H, J = 2.6 Hz, J = 11.0 Hz, H6b); 3.46 (s, 3H, OCH3); 3.78 (td, 1H, J = 2.4 Hz, J = 9.2 Hz, H5); 4.71 (s, 1H, J = 1.2 Hz, H1); 5.09 (t, 1H, J = 9.8 Hz, H4); 5.20 (m, 1H, H2); 5.29 ppm (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 3.85 (C6); 20.60, 20.73, 20.80 (3COCH3); 55.49 (OCH3); 68.60 (C3); 69.52 (C2); 69.90 (C4); 70.07 (C5); 98.44 (C1); 169.77, 169.80, 169.95 ppm (3C=O); MS (ESI) m/z: 368.24 [M+Na]+.(6-Deoxy-6-azido-1,2,3,4-tetra-O-acetyl-α-d-mannopyranose 17): Compound 16 (500 mg, 1.45 mmol, 1 eq.) dissolved in acetic anhydride (10 mL) was added dropwise to a solution of Ac2O/AcOH/H2SO4 5:4:1 (12.5 mL) at 0 °C. After 4 h at RT, the mixture was diluted with EtOAc then ice was added slowly. The obtained organic layer was washed with a solution of NaHCO3 5% then water, dried (Na2SO4), filtered and concentrated in vacuo. The beige oil was used without purification (83%): Rf = 0.83 (CH2Cl2/MeOH 9:1);  = −42.7 (c = 1.01 in chloroform); 1H-NMR (CDCl3): δ = 1.98, 2.03, 2.14, 2.15 (4s, 12H, 4CH3); 3.28 (dd, 1H, J = 5.6 Hz, J = 13.6 Hz, H6a); 3.37 (dd, 1H, J = 2.4 Hz, J = 13.2 Hz, H6b); 3.97 (m, 1H, H5); 5.22 (s, 1H, H2); 5.31 (m, 2H, H3 and H4); 6.06 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 20.53, 20.56, 20.63, 20.72 (4CH3); 50.55 (C6); 66.30, 68.43 (C3 and C4); 68.14 (C2); 71.70 (C5); 90.19 (C1); 167.96, 169.49, 169.68, 169.92 ppm (4C=O); MS (ESI) m/z: 396.22 [M+Na]+, 408.35 [M-Cl]−.
= −42.7 (c = 1.01 in chloroform); 1H-NMR (CDCl3): δ = 1.98, 2.03, 2.14, 2.15 (4s, 12H, 4CH3); 3.28 (dd, 1H, J = 5.6 Hz, J = 13.6 Hz, H6a); 3.37 (dd, 1H, J = 2.4 Hz, J = 13.2 Hz, H6b); 3.97 (m, 1H, H5); 5.22 (s, 1H, H2); 5.31 (m, 2H, H3 and H4); 6.06 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 20.53, 20.56, 20.63, 20.72 (4CH3); 50.55 (C6); 66.30, 68.43 (C3 and C4); 68.14 (C2); 71.70 (C5); 90.19 (C1); 167.96, 169.49, 169.68, 169.92 ppm (4C=O); MS (ESI) m/z: 396.22 [M+Na]+, 408.35 [M-Cl]−.
 = −42.7 (c = 1.01 in chloroform); 1H-NMR (CDCl3): δ = 1.98, 2.03, 2.14, 2.15 (4s, 12H, 4CH3); 3.28 (dd, 1H, J = 5.6 Hz, J = 13.6 Hz, H6a); 3.37 (dd, 1H, J = 2.4 Hz, J = 13.2 Hz, H6b); 3.97 (m, 1H, H5); 5.22 (s, 1H, H2); 5.31 (m, 2H, H3 and H4); 6.06 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 20.53, 20.56, 20.63, 20.72 (4CH3); 50.55 (C6); 66.30, 68.43 (C3 and C4); 68.14 (C2); 71.70 (C5); 90.19 (C1); 167.96, 169.49, 169.68, 169.92 ppm (4C=O); MS (ESI) m/z: 396.22 [M+Na]+, 408.35 [M-Cl]−.
= −42.7 (c = 1.01 in chloroform); 1H-NMR (CDCl3): δ = 1.98, 2.03, 2.14, 2.15 (4s, 12H, 4CH3); 3.28 (dd, 1H, J = 5.6 Hz, J = 13.6 Hz, H6a); 3.37 (dd, 1H, J = 2.4 Hz, J = 13.2 Hz, H6b); 3.97 (m, 1H, H5); 5.22 (s, 1H, H2); 5.31 (m, 2H, H3 and H4); 6.06 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 20.53, 20.56, 20.63, 20.72 (4CH3); 50.55 (C6); 66.30, 68.43 (C3 and C4); 68.14 (C2); 71.70 (C5); 90.19 (C1); 167.96, 169.49, 169.68, 169.92 ppm (4C=O); MS (ESI) m/z: 396.22 [M+Na]+, 408.35 [M-Cl]−.4-Bromobut-2-en-1-yl-6-deoxy-6-azido-2,3,4-tri-O-acetyl-α-d-mannopyranoside (18): The procedure described for compound 1 was applied to 17 and 4-bromo-but-2-en-1-ol to give compound 18 as a beige oil (78%): Rf = 0.59 (EtOAc/petroleum ether 1:1); 1H-NMR (CDCl3): δ = 1.97, 2.03, 2.13 (3s, 9H, 3CH3); 3.25 (dd, 1H, J = 5.6 Hz, J = 13.5 Hz, H6a); 3.32 (dd, 1H, J = 2.4 Hz, J = 13.5 Hz, H6b); 3.99 (m, 1H, H5); 4.01 (d, 2H, J = 8.4 Hz, CH2Br); 4.68 (m, 2H, CH2O); 4.96 (d, 1H, J = 1.6 Hz, H1); 5.45 (m, 2H, H3 and H4); 5.62 (s, 1H, H2); 5.71 (m, 1H, CHCH2O); 5.93 ppm (m, 1H, CHCH2Br); 13C-NMR (CDCl3): δ = 20.51, 20.54, 20.62 (3CH3); 25.66 (CH2Br); 50.54 (C6); 59.12 (CH2O); 66.33, 68.46 (C3 and C4); 68.34 (C2); 71.71 (C5); 90.23 (C1); 128.10 (CHCH2O); 129.75 (CHCH2Br); 167.97, 169.52, 169.70 ppm (3C=O); MS (ESI) m/z: 487.45 [M+Na]+.
Methyl 6-deoxy-2,3-O-(1-methylethylidene)-4-O-sodiumsulfate-α-d-heptomanno-pyranosiduronic acid (19): Firstly, the procedure described for compound 13 was applied to 15 to give the appropriate intermediate as a yellow solid (quantitative): Rf = 0.49 (CH2Cl2/MeOH 8.5:1.5);  = + 37.7 (c = 1.00 in chloroform); 1H-NMR (acetone-d6): δ = 1.24, 1.41 (2s, 6H, 2CCH3); 2.76 (dd, 1H, J = 9.3 Hz, J = 17.3 Hz, H6a); 3.18 (dd, 1H, J = 2.8 Hz, J = 17.3 Hz, H6b); 3.46 (s, 3H, OCH3); 3.86 (td, 1H, J = 9.6 Hz, J = 2.8 Hz, H5); 4.15 (d, 1H, J = 7.4 Hz, H2); 4.21 (dd, 1H, J = 9.9 Hz, J = 7.0 Hz, H4); 4.44 (m, 1H, H3); 4.93 ppm (s, 1H, H1); 13C-NMR (acetone-d6): δ = 20.60 (C6); 25.5, 27.10 (2CCH3); 54.5 (OCH3); 64.90 (C5); 75.62 (C2); 76.34 (C4); 76.90 (C3); 98.17 (C1); 109.88 (C(CH3)2); 118.13 ppm (CN); MS (ESI) m/z: 384.23 [M+Na]+, 322.42 [M-Na]−.
= + 37.7 (c = 1.00 in chloroform); 1H-NMR (acetone-d6): δ = 1.24, 1.41 (2s, 6H, 2CCH3); 2.76 (dd, 1H, J = 9.3 Hz, J = 17.3 Hz, H6a); 3.18 (dd, 1H, J = 2.8 Hz, J = 17.3 Hz, H6b); 3.46 (s, 3H, OCH3); 3.86 (td, 1H, J = 9.6 Hz, J = 2.8 Hz, H5); 4.15 (d, 1H, J = 7.4 Hz, H2); 4.21 (dd, 1H, J = 9.9 Hz, J = 7.0 Hz, H4); 4.44 (m, 1H, H3); 4.93 ppm (s, 1H, H1); 13C-NMR (acetone-d6): δ = 20.60 (C6); 25.5, 27.10 (2CCH3); 54.5 (OCH3); 64.90 (C5); 75.62 (C2); 76.34 (C4); 76.90 (C3); 98.17 (C1); 109.88 (C(CH3)2); 118.13 ppm (CN); MS (ESI) m/z: 384.23 [M+Na]+, 322.42 [M-Na]−.
 = + 37.7 (c = 1.00 in chloroform); 1H-NMR (acetone-d6): δ = 1.24, 1.41 (2s, 6H, 2CCH3); 2.76 (dd, 1H, J = 9.3 Hz, J = 17.3 Hz, H6a); 3.18 (dd, 1H, J = 2.8 Hz, J = 17.3 Hz, H6b); 3.46 (s, 3H, OCH3); 3.86 (td, 1H, J = 9.6 Hz, J = 2.8 Hz, H5); 4.15 (d, 1H, J = 7.4 Hz, H2); 4.21 (dd, 1H, J = 9.9 Hz, J = 7.0 Hz, H4); 4.44 (m, 1H, H3); 4.93 ppm (s, 1H, H1); 13C-NMR (acetone-d6): δ = 20.60 (C6); 25.5, 27.10 (2CCH3); 54.5 (OCH3); 64.90 (C5); 75.62 (C2); 76.34 (C4); 76.90 (C3); 98.17 (C1); 109.88 (C(CH3)2); 118.13 ppm (CN); MS (ESI) m/z: 384.23 [M+Na]+, 322.42 [M-Na]−.
= + 37.7 (c = 1.00 in chloroform); 1H-NMR (acetone-d6): δ = 1.24, 1.41 (2s, 6H, 2CCH3); 2.76 (dd, 1H, J = 9.3 Hz, J = 17.3 Hz, H6a); 3.18 (dd, 1H, J = 2.8 Hz, J = 17.3 Hz, H6b); 3.46 (s, 3H, OCH3); 3.86 (td, 1H, J = 9.6 Hz, J = 2.8 Hz, H5); 4.15 (d, 1H, J = 7.4 Hz, H2); 4.21 (dd, 1H, J = 9.9 Hz, J = 7.0 Hz, H4); 4.44 (m, 1H, H3); 4.93 ppm (s, 1H, H1); 13C-NMR (acetone-d6): δ = 20.60 (C6); 25.5, 27.10 (2CCH3); 54.5 (OCH3); 64.90 (C5); 75.62 (C2); 76.34 (C4); 76.90 (C3); 98.17 (C1); 109.88 (C(CH3)2); 118.13 ppm (CN); MS (ESI) m/z: 384.23 [M+Na]+, 322.42 [M-Na]−.Secondly, the procedure described for compound 14 was applied to the precedent intermediate to give compound 19 as a colourless oil (quantitative): Rf = 0.61 (EtOAc/MeOH 1:1);  = +17.23 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.33, 1.53 (2s, 6H, 2CCH3); 2.40 (dd, 1H, J = 9.8 Hz, J = 15.8 Hz, H6a); 3.09 (dd, 1H, J = 2.2 Hz, J = 16.2 Hz, H6b); 3.41 (s, 3H, OCH3); 4.09 (m, 2H, H2 and H5); 4.21 (m, 2H, H3 and H4); 4.81 ppm (s, 1H, H1); 13C-NMR (CD3OD): δ = 26.57, 28.12 (2CCH3); 38.32 (C6); 55.76 (OCH3); 66.91 (C2); 77.19, 78.07, 79.05 (C3, C4 and C5); 99.42 (C1); 110.75 (C(CH3)2); 175.20 ppm (CO2H); MS (ESI) m/z: 387.99 [M+Na]+, 363.12 [M-H]−.
= +17.23 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.33, 1.53 (2s, 6H, 2CCH3); 2.40 (dd, 1H, J = 9.8 Hz, J = 15.8 Hz, H6a); 3.09 (dd, 1H, J = 2.2 Hz, J = 16.2 Hz, H6b); 3.41 (s, 3H, OCH3); 4.09 (m, 2H, H2 and H5); 4.21 (m, 2H, H3 and H4); 4.81 ppm (s, 1H, H1); 13C-NMR (CD3OD): δ = 26.57, 28.12 (2CCH3); 38.32 (C6); 55.76 (OCH3); 66.91 (C2); 77.19, 78.07, 79.05 (C3, C4 and C5); 99.42 (C1); 110.75 (C(CH3)2); 175.20 ppm (CO2H); MS (ESI) m/z: 387.99 [M+Na]+, 363.12 [M-H]−.
 = +17.23 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.33, 1.53 (2s, 6H, 2CCH3); 2.40 (dd, 1H, J = 9.8 Hz, J = 15.8 Hz, H6a); 3.09 (dd, 1H, J = 2.2 Hz, J = 16.2 Hz, H6b); 3.41 (s, 3H, OCH3); 4.09 (m, 2H, H2 and H5); 4.21 (m, 2H, H3 and H4); 4.81 ppm (s, 1H, H1); 13C-NMR (CD3OD): δ = 26.57, 28.12 (2CCH3); 38.32 (C6); 55.76 (OCH3); 66.91 (C2); 77.19, 78.07, 79.05 (C3, C4 and C5); 99.42 (C1); 110.75 (C(CH3)2); 175.20 ppm (CO2H); MS (ESI) m/z: 387.99 [M+Na]+, 363.12 [M-H]−.
= +17.23 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.33, 1.53 (2s, 6H, 2CCH3); 2.40 (dd, 1H, J = 9.8 Hz, J = 15.8 Hz, H6a); 3.09 (dd, 1H, J = 2.2 Hz, J = 16.2 Hz, H6b); 3.41 (s, 3H, OCH3); 4.09 (m, 2H, H2 and H5); 4.21 (m, 2H, H3 and H4); 4.81 ppm (s, 1H, H1); 13C-NMR (CD3OD): δ = 26.57, 28.12 (2CCH3); 38.32 (C6); 55.76 (OCH3); 66.91 (C2); 77.19, 78.07, 79.05 (C3, C4 and C5); 99.42 (C1); 110.75 (C(CH3)2); 175.20 ppm (CO2H); MS (ESI) m/z: 387.99 [M+Na]+, 363.12 [M-H]−.Methyl 6-deoxy-2,3-4-tri-O-acetyl-α-d-heptomannopyranosiduronic acid (20): Firstly, the procedure described for compound 4 was applied to 19 to give the appropriate intermediate as a colourless oil (78%): Rf = 0.25 (i-PrOH/NH4OH 8.5:1.5); 1H-NMR (D2O): δ = 2.86 (dd, 1H, J = 7.4 Hz, J = 17.3 Hz, H6a); 3.04 (dd, 1H, J = 3.6 Hz, J = 17.3 Hz, H6b); 3.44 (s, 3H, OCH3); 3.60 (t, 1H, J = 9.7 Hz, H4); 3.76 (dd, 1H, J = 9.6 Hz, J = 3.4 Hz, H3); 3.84 (m, 1H, H5); 3.96 (dd, 1H, J = 3.4 Hz, J = 1.7 Hz, H2); 4.78 ppm (d, 1H, J = 1.5 Hz, H1); 13C-NMR (D2O): δ = 51.44 (C6); 55.20 (OCH3); 67.89 (C5); 70.27 (C2); 70.76 (C3); 71.60 (C4); 101.42 (C1); 176.01 ppm (CO2H); MS (ESI) m/z: 245.56 [M+Na]+, 221.03 [M-H]−.
Secondly, the procedure described for compound 16 was applied to the preceeding intermediate to give compound 20 as a white powder (92%): Rf = 0.71 (EtOAc/petroleum ether 1:1); 1H-NMR (CDCl3): δ = 1.98, 2.03, 2.10 (3s, 9H, 3COCH3); 3.40 (s, 3H, OCH3); 3.96 (m, 1H, H5); 4.11 (dd, 1H, J = 2.4 Hz, J = 12.4 Hz, H6a); 4.28 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.71 (d, 1H, J = 1.6 Hz, H1); 5.23 (m, 1H, H2); 5.27 (t, 1H, J = 9.8 Hz, H4); 5.32 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.67, 20.72, 20.87 (3COCH3); 55.28 (OCH3); 62.46 (C6); 66.08 (C4); 68.32 (C5); 69.00 (C3); 69.45 (C2); 98.54 (C1); 169.88, 170.04, 170.66 (3C=O); 175.89 ppm (CO2H); MS (ESI) m/z: 371.59 [M+Na]+.
6-Deoxy-1-2,3-4-tetra-O-acetyl-α-d-heptomannopyranosiduronic acid (21): The procedure described for compound 17 was applied to 20 to give compound 21 as a beige oil (83%): Rf = 0.48 (EtOAc/petroleum ether 1:1); 1H-NMR (CDCl3): δ = 1.95, 2.06, 2.12, 2.15 (4s, 12H, 4CH3); 3.91 (m, 1H, H5); 4.17 (dd, 1H, J = 2.7 Hz, J = 12.6 Hz, H6a); 4.32 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 5.30 (t, 1H, J = 9.9 Hz, H4); 5.34 (m, 1H, H2); 5.35 (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 5.98 ppm (s, 1H, H1); 13C-NMR (CDCl3): δ = 20.67, 20.72, 20.87, 20.90 (4CH3); 62.44 (C6); 66.12 (C4); 68.36 (C5); 68.95 (C3); 69.43 (C2); 90.53 (C1); 169.72, 169.88, 170.04, 170.66 (4C=O); 176.08 ppm (CO2H); MS (ESI) m/z: 399.89 [M+Na]+.
4-Bromobut-2-en-1-yl-6-deoxy-2,3,4-tri-O-acetyl-α-d-heptomannopyranosiduronic acid (22): The procedure described for compound 18 was applied to 21 and 4-bromo-but-2-en-1-ol to give compound 22 as a beige oil (78%): Rf = 0.23 (EtOAc/petroleum ether 1:1); 1H-NMR (CDCl3): δ = 1.95, 2.06, 2.12 (3s, 9H, 3CH3); 3.91 (m, 1H, H5); 4.08 (d, 2H, J = 8.6 Hz, CH2Br); 4.17 (dd, 1H, J = 2.7 Hz, J = 12.6 Hz, H6a); 4.32 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.70 (m, 2H, CH2O); 5.30 (t, 1H, J = 9.9 Hz, H4); 5.34 (m, 1H, H2); 5.35 (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 5.77 (m, 1H, CHCH2O); 5.90 (m, 1H, CHCH2Br); 5.98 ppm (s, 1H, H1); 13C-NMR (CDCl3): δ = 20.65, 20.70, 20.80 (3CH3); 25.62 (CH2Br); 59.26 (CH2O); 62.48 (C6); 66.18 (C4); 68.39 (C5); 69.00 (C3); 69.40 (C2); 90.51 (C1); 128.15 (CHCH2O); 129.77 (CHCH2Br); 169.67, 169.83, 170.06 (3C=O); 176.09 ppm (CO2H); MS (ESI) m/z: 490.05 [M+Na]+.
(2E)-1-Bromo-3,7-dimethylocta-2,6-diene (23): PBr3 (1.1 mL, 9.51 mmol, 0.33 eq.) was added dropwise at 0 °C to geraniol (5 mL, 28.83 mmol, 1 eq.) dissolved in CH2Cl2 (50 mL). After 1 h at 0 °C, the mixture was diluted in CH2Cl2 and ice-cubes were added.The obtained organic layer was washed with a solution of NaHCO3 5% and water, dried (Na2SO4), filtered and concentrated in vacuo. Purification by chromatography on silica gel (EtOAc/petroleum ether 1:1) gave a yellow oil (93%): Rf = 0.80 (EtOAc/petroleum ether 1:1); 1H-NMR (CDCl3): δ = 1.59, 1.66, 1.67 (2s, 9H, 3CH3); 2.01,2.09 (2m, 4 h, CH2CH2); 4.13 (d, 2H, J = 7.2 Hz, CH2Br); 5.08 (m, 1H, CHC=C(CH3)2); 5.40 ppm (m, 1H, CHCH2Br); 13C-NMR (CDCl3): δ = 16.20, 17.62,25.62 (3CH3); 26.31,39.48 (CH2CH2); 59.29 (CH2Br); 123.28 (CHCH2Br); 123.83 (CHC=C(CH3)2); 131.68, 139.61 ppm (2C=CH); MS (ESI) m/z: 241.67 [M+Na]+.
{[(2E)-3,7-Dimethylocta-2,6-dien-1-yl]sulfonyl}benzene (24): Compound 23 (6 g, 27.52 mmol, 1 eq.) and NaSO2Ph (9 g, 55.05 mmol, 2eq.) were stirred in DMF (12 mL) for 2 h at RT. Then the solution was concentrated and the crude product was purified by chromatography on silica gel (EtOAc/petroleum ether 1:1) to give a yellow oil (93%): Rf = 0.45 (EtOAc /petroleum ether 3:7); 1H-NMR (CDCl3): δ = 1.24, 1.52, 1.62 (3s, 9H, 3CH3); 1.93 (s, 4 h, CH2CH2); 3.74 (d, 2H, J = 8.0 Hz, CH2SO2); 4.96 (m, 1H, CHC=C(CH3)2); 5.12 (t, 1H, J = 7.8 Hz, CHCH2SO2); 7.46 (t, 2H, J = 7.8 Hz, CHAr); 7.57 (t, 1H, J = 7.4 Hz, CHAr); 7.80 ppm (d, 2H, J = 8.0 Hz, CHAr); 13C-NMR (CDCl3): δ = 16.10, 17.65, 25.66 (3CH3); 26.13, 39.63 (CH2CH2); 56.04 (CH2SO2); 110.25 (CHCH2SO2); 123.39 (CHC=C(CH3)2); 128.52, 128.89, 133.49 (CHAr); 132.04, 138.56, 146.34 ppm (2C=CH and CSO2); MS (ESI) m/z: 301.17 [M+Na]+.
(2E,6E)-2,6-Dimethyl-8-(phenylsulfonyl)octa-2,6-dien-1-al (25): Under argon and in the dark, SeO2 (8 mg, 0.07 mmol, 0.1 eq.), t-BuOOH (233 mg, 2.59 mmol, 3.6 eq.) and salicylic acid (4-hydroxybenzoic acid) (10 mg, 0.07 mmol, 0.1 eq.) were dissolved in CH2Cl2 (1 mL). A solution of compound 24 (200 mg, 0.72 mmol, 1 eq.) in CH2Cl2 (10 mL) was added at 0 °C. The ice bath was removed after 10 min and stirring was left for 24 h. The mixture was diluted in CH2Cl2, washed with a saturated solution of NaHCO3 then with water to neutralize tBuOOH and to eliminate HSeCO3. The organic layer was dried, filtered and concentrated in vacuo. Purification by chromatography on silica gel (EtOAc/petroleum ether 3:7) gave the aldehyde and the alcohol (50:50): Rf = 0.50 (EtOAc/petroleum ether 3:7); 1H-NMR (CDCl3): δ = 1.39, 1.70 (2s, 6H, 2CH3); 2.18 (t, 2H, J = 7.4 Hz, CH2CH2); 2.38 (q, 2H, J = 7.4 Hz, J = 15.0 Hz, CH2CH2); 3.81 (d, 2H, J = 8.0 Hz, CH2SO2); 5.22 (td, 1H, J = 1.2 Hz, J = 8.0 Hz, CHCH2SO2); 6.38 (td, 1H, J = 1.2 Hz, J = 7.2 Hz, CH=CCHO); 7.52 (t, 2H, J = 7.8 Hz, CHAr); 7.63 (t, 1H, J = 7.6 Hz, CHAr); 7.84 (d, 2H, J = 7.2 Hz, CHAr); 9.36 ppm (s, 1H, CHO); 13C-NMR (CDCl3): δ = 9.16, 16.10 (2CH3); 26.78 (CH2CH2); 37.85 (CH2CH2); 55.82 (CH2SO2); 111.31 (CHCH2SO2); 128.26,129.00,133.62 (3CHAr); 152.87 (CH=CCHO); 138.64, 139.62, 144.75 (2C=CH and CSO2); 194.97 ppm (CHO); MS (ESI) m/z: 315.17 [M+Na]+.
(2E,6E)-2,6-Dimethyl-8-(phenylsulfonyl)octa-2,6-dien-1-ol (26): To a solution containing compound 25 (8 g, 27.40 mmol, 1 eq.) in EtOH (80 mL) NaBH4 (1.04 g, 27.40 mmol, 1 eq.) was added in several portions. After 10 min at 0 °C, the mixture was diluted with CH2Cl2 and then washed with water. The organic layer was dried (Na2SO4), filtered and reduced under pressure. Purification by chromatography on silica gel (EtOAc/petroleum ether 1:1) gave a colourless oil (63% in alcohol, 2 steps): Rf = 0.30 (EtOAc/petroleum ether 3:7); 1H-NMR (CDCl3): δ = 1.37, 1.66 (2s, 6H, 2CH3); 2.08 (m, 4 h, CH2CH2); 3.80 (d, 2H, J = 7.6 Hz, CH2SO2); 3.99 (s, 2H, CH2OH); 5.19 (t, 1H, J = 7.4 Hz, CHCH2SO2); 5.33 (t, 1H, J = 6.0 Hz, CH=CCH2OH); 7.54 (t, 2H, J = 7.8 Hz, CHAr); 7.64 (t, 1H, J = 7.4 Hz, CHAr); 7.87 ppm (d, 2H, J = 7.6 Hz, CHAr); 13C-NMR (CDCl3): δ = 13.68, 16.13 (2CH3); 25.39 (CH2CH2); 39.17 (CH2CH2); 56.00 (CH2SO2); 68.70 (CH2OH); 110.34 (CHCH2SO2); 124.63 (CH=CCH2OH); 128.37 (CHAr); 129.02 (CHAr); 133.58 (CHAr); 135.54, 138.81, 146.11 ppm (2C=CH and CSO2); MS (ESI) m/z: 317.19 [M+Na]+.
S-(2E,6E)-2,6-Dimethyl-8-(phenylsulfonyl)octa-2,6-dien-1-yl ethanethioate (27): Firstly, compound 26 (4 g, 13.60 mmol, 1 eq.), CBr4 (5.41 g, 16.33 mmol, 1.2 eq.) and PPh3 (5 g, 19.05 mmol, 1.4 eq.) were reacted in CH2Cl2 (7 mL) at RT. After 2h, the mixture was reduced under pressure then purified by chromatography on silica gel (EtOAc/petroleum ether 2:3) to give a yellow oil (quantitative): Rf = 0.66 (EtOAc/petroleum ether5:5); 1H-NMR (CDCl3): δ = 1.33,1.73 (2s, 6H, 2CH3); 2.05 (m, 4 h, CH2CH2); 3.81 (d, 2H, J = 8.0 Hz, CH2SO2); 3.95 (s, 2H, CH2Br); 5.19 (t, 1H, J = 8.0 Hz, CHCH2SO2); 5.50 (m, 1H, CH=CCH2Br); 7.54 (t, 2H, J = 7.6 Hz, CHAr); 7.64 (t, 1H, J = 7.4 Hz, CHAr); 7.86 ppm (d, 2H, J = 7.2 Hz, CHAr); 13C-NMR (CDCl3): δ = 14.65, 16.13 (2CH3); 26.29 (CH2CH2); 38.68 (CH2CH2); 41.42 (CH2Br); 55.95 (CH2SO2); 110.78 (CHCH2SO2); 128.35 (CHAr); 128.98 (CHAr); 129.99 (CH2Br); 133.56 (CHAr); 132.63, 138.63, 145.57 ppm (2C=CH and CSO2); MS (ESI) m/z: 379.04 and 381.09 [M+Na]+, 394.94 and 397.06 [M+K]+.
Next, to a solution of the preceeding intermediate (5 g, 14.00 mmol, 1 eq.) in DMF (50 mL) KSAc (3.2 g, 28.00 mmol, 2 eq.) was added. After 1h at RT, the solution was diluted in CH2Cl2 and washed with water. The organic layer was dried, filtered and reduced under pressure and purification by chromatography on silica gel (EtOAc/petroleum ether 1:1) gave a brown oil (quantitative): Rf = 0.66 (EtOAc/petroleum ether 1:1); 1H-NMR (CDCl3): δ = 1.30, 1.60 (2s, 6H, 2CH3); 2.01 (m, 4 h, CH2CH2); 2.32 (s, 3H, COCH3); 3.51 (s, 2H, CH2S); 3.80 (d, 2H, J = 8.0 Hz, CH2SO2); 5.17 (t, 1H, J = 8.4 Hz, CHCH2SO2); 5.31 (m, 1H, CH=CH2S); 7.53 (t, 2H, J = 7.6 Hz, CHAr); 7.63 (t, 1H, J = 7.4 Hz, CHAr); 7.86 ppm (d, 2H, J = 7.2 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.14, 16.12 (2CH3); 26.18 (CH2CH2); 30.48 (COCH3); 38.00 (CH2S); 39.09 (CH2CH2); 55.99 (CH2SO2); 110.59 (CHCH2SO2); 127.77 (CH=CCH2S); 128.47 (CHAr); 128.94 (CHAr); 133.52 (CHAr); 131.01, 138.62, 145.85 ppm (2C=CH and CSO2); 195.55 (CO); MS (ESI) m/z: 375.67 [M+Na]+.
(2Z,6E,10E)-12-(Acetylthio)-7,11-dimethyl-5-(phenylsulfonyl)dodeca-2,6,10-trien-1-yl 2,3,4-tri-O-acetyl-6-deoxy-6-azido-α-d-mannopyranoside (28): Under argon, the solution of compound 27 (200 mg, 0.57 mmol, 1.3 eq.) dissolved in anhydrous THF (4 mL) was cooled to −78 °C and LiHMDS (550 µL) was slowly added. After 10 min, a solution containing compound 18 (200 mg, 0.43 mmol, 1 eq.) was introduced dropwise. The reaction was left at −78 °C under for 4 h then 18 h at RT. The mixture was diluted in CH2Cl2 then washed with water. The organic layer was dried, filtered and reduced under pressure and purification by chromatography on silica gel (EtOAc/petroleum ether 1:1) gave a colourless oil (15%): Rf = 0.33 (EtOAc/petroleum ether 1:1);  = +10.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.59 (2s, 6H, 2CH=CCH3); 2.00 (m, 4 h, CH2CH2); 2.01, 2.09, 2.14, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 3.97 (m, 4 h, H6 and CH2CHSO2); 4.11 (m, 3H, H5 and CH2O); 4.64 (d, 1H, J = 1.2 Hz, H1); 4.74 (m, 2H, OCH2CH=CH); 5.11 (t, 1H, J = 10.2 Hz, H4); 5.20 (m, 1H, CHCHSO2); 5.30 (m, 2H, H2 and CH=CCH2S); 5.35 (dd, 1H, J = 10.4 Hz, J = 3.6 Hz, H3); 7.53 (m, 2H, CHAr); 7.64 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.2 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.11, 16.09 (2CH=CCH3); 20.58, 20.72, 20.79, 30.44 (4COCH3); 26.15, 39.05 (CH2CH2); 37.96 (CH2S); 55.53 (CHSO2); 55.96, 59.43 (C6 and CHCHSO2); 62.24 (CH2O); 63.25 (C5); 66.10 (C3); 66.82 (C4); 70.31 (C2); 98.34, 98.65 (OCH2CH=CH); 99.35 (C1); 110.57 (CHCHSO2); 127.73 (CH=CCH2S); 128.43 (CHAr); 128.91 (CHAr); 131.96 (CHAr); 133.49 (CHAr); 130.97, 138.61, 145.81 (2C=CH and CSO2); 169.63, 169.73, 170.78 ppm (4C=O); MS (ESI) m/z: 737.01 [M+H]+, 758.98 [M+Na]+.
= +10.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.59 (2s, 6H, 2CH=CCH3); 2.00 (m, 4 h, CH2CH2); 2.01, 2.09, 2.14, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 3.97 (m, 4 h, H6 and CH2CHSO2); 4.11 (m, 3H, H5 and CH2O); 4.64 (d, 1H, J = 1.2 Hz, H1); 4.74 (m, 2H, OCH2CH=CH); 5.11 (t, 1H, J = 10.2 Hz, H4); 5.20 (m, 1H, CHCHSO2); 5.30 (m, 2H, H2 and CH=CCH2S); 5.35 (dd, 1H, J = 10.4 Hz, J = 3.6 Hz, H3); 7.53 (m, 2H, CHAr); 7.64 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.2 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.11, 16.09 (2CH=CCH3); 20.58, 20.72, 20.79, 30.44 (4COCH3); 26.15, 39.05 (CH2CH2); 37.96 (CH2S); 55.53 (CHSO2); 55.96, 59.43 (C6 and CHCHSO2); 62.24 (CH2O); 63.25 (C5); 66.10 (C3); 66.82 (C4); 70.31 (C2); 98.34, 98.65 (OCH2CH=CH); 99.35 (C1); 110.57 (CHCHSO2); 127.73 (CH=CCH2S); 128.43 (CHAr); 128.91 (CHAr); 131.96 (CHAr); 133.49 (CHAr); 130.97, 138.61, 145.81 (2C=CH and CSO2); 169.63, 169.73, 170.78 ppm (4C=O); MS (ESI) m/z: 737.01 [M+H]+, 758.98 [M+Na]+.
 = +10.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.59 (2s, 6H, 2CH=CCH3); 2.00 (m, 4 h, CH2CH2); 2.01, 2.09, 2.14, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 3.97 (m, 4 h, H6 and CH2CHSO2); 4.11 (m, 3H, H5 and CH2O); 4.64 (d, 1H, J = 1.2 Hz, H1); 4.74 (m, 2H, OCH2CH=CH); 5.11 (t, 1H, J = 10.2 Hz, H4); 5.20 (m, 1H, CHCHSO2); 5.30 (m, 2H, H2 and CH=CCH2S); 5.35 (dd, 1H, J = 10.4 Hz, J = 3.6 Hz, H3); 7.53 (m, 2H, CHAr); 7.64 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.2 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.11, 16.09 (2CH=CCH3); 20.58, 20.72, 20.79, 30.44 (4COCH3); 26.15, 39.05 (CH2CH2); 37.96 (CH2S); 55.53 (CHSO2); 55.96, 59.43 (C6 and CHCHSO2); 62.24 (CH2O); 63.25 (C5); 66.10 (C3); 66.82 (C4); 70.31 (C2); 98.34, 98.65 (OCH2CH=CH); 99.35 (C1); 110.57 (CHCHSO2); 127.73 (CH=CCH2S); 128.43 (CHAr); 128.91 (CHAr); 131.96 (CHAr); 133.49 (CHAr); 130.97, 138.61, 145.81 (2C=CH and CSO2); 169.63, 169.73, 170.78 ppm (4C=O); MS (ESI) m/z: 737.01 [M+H]+, 758.98 [M+Na]+.
= +10.9 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.59 (2s, 6H, 2CH=CCH3); 2.00 (m, 4 h, CH2CH2); 2.01, 2.09, 2.14, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 3.97 (m, 4 h, H6 and CH2CHSO2); 4.11 (m, 3H, H5 and CH2O); 4.64 (d, 1H, J = 1.2 Hz, H1); 4.74 (m, 2H, OCH2CH=CH); 5.11 (t, 1H, J = 10.2 Hz, H4); 5.20 (m, 1H, CHCHSO2); 5.30 (m, 2H, H2 and CH=CCH2S); 5.35 (dd, 1H, J = 10.4 Hz, J = 3.6 Hz, H3); 7.53 (m, 2H, CHAr); 7.64 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.2 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.11, 16.09 (2CH=CCH3); 20.58, 20.72, 20.79, 30.44 (4COCH3); 26.15, 39.05 (CH2CH2); 37.96 (CH2S); 55.53 (CHSO2); 55.96, 59.43 (C6 and CHCHSO2); 62.24 (CH2O); 63.25 (C5); 66.10 (C3); 66.82 (C4); 70.31 (C2); 98.34, 98.65 (OCH2CH=CH); 99.35 (C1); 110.57 (CHCHSO2); 127.73 (CH=CCH2S); 128.43 (CHAr); 128.91 (CHAr); 131.96 (CHAr); 133.49 (CHAr); 130.97, 138.61, 145.81 (2C=CH and CSO2); 169.63, 169.73, 170.78 ppm (4C=O); MS (ESI) m/z: 737.01 [M+H]+, 758.98 [M+Na]+.(2Z,6E,10E)-12-(Acetylthio)-7,11-dimethyl-5-(phenylsulfonyl)dodeca-2,6,10-trien-1-yl 2,3,4-tri-O-acetyl-6-deoxy-α-d-heptomannopyranosiduronic acid (29): The procedure described for compound 28 was applied to 22 and 27 to give compound 29 as a beige oil (17%): Rf = 0.16 (EtOAc/petroleum ether 1:1);  = +9.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.58 (2s, 6H, 2CH=CCH3); 1.99 (m, 4 h, CH2CH2); 2.04, 2.07, 2.15, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 4.05 (m, 5 h, H5, CH2O and CH2CHSO2); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, CH=CH, 2CH=CCH3); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 (d, 1H, J = 1.6 Hz, H1); 7.52 (t, 2H, J = 7.6 Hz, CHAr); 7.63 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.6 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH=CCH3); 20.50, 20.60, 20.70 (4COCH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CSO2); 61.92 (C6, CH2O and CH2CHSO2); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=CCH3); 110.81 (CH=CH); 127.44–133.42 (5CHAr); 138.48, 145.70, 146.84 (2C=CH and CSO2); 169.36, 169.56, 168.80, 170.44 (4C=O); 195.37 ppm (CO2H); MS (ESI) m/z: 740.05 [M+H]+, 761.99 [M+Na]+.
= +9.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.58 (2s, 6H, 2CH=CCH3); 1.99 (m, 4 h, CH2CH2); 2.04, 2.07, 2.15, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 4.05 (m, 5 h, H5, CH2O and CH2CHSO2); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, CH=CH, 2CH=CCH3); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 (d, 1H, J = 1.6 Hz, H1); 7.52 (t, 2H, J = 7.6 Hz, CHAr); 7.63 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.6 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH=CCH3); 20.50, 20.60, 20.70 (4COCH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CSO2); 61.92 (C6, CH2O and CH2CHSO2); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=CCH3); 110.81 (CH=CH); 127.44–133.42 (5CHAr); 138.48, 145.70, 146.84 (2C=CH and CSO2); 169.36, 169.56, 168.80, 170.44 (4C=O); 195.37 ppm (CO2H); MS (ESI) m/z: 740.05 [M+H]+, 761.99 [M+Na]+.
 = +9.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.58 (2s, 6H, 2CH=CCH3); 1.99 (m, 4 h, CH2CH2); 2.04, 2.07, 2.15, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 4.05 (m, 5 h, H5, CH2O and CH2CHSO2); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, CH=CH, 2CH=CCH3); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 (d, 1H, J = 1.6 Hz, H1); 7.52 (t, 2H, J = 7.6 Hz, CHAr); 7.63 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.6 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH=CCH3); 20.50, 20.60, 20.70 (4COCH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CSO2); 61.92 (C6, CH2O and CH2CHSO2); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=CCH3); 110.81 (CH=CH); 127.44–133.42 (5CHAr); 138.48, 145.70, 146.84 (2C=CH and CSO2); 169.36, 169.56, 168.80, 170.44 (4C=O); 195.37 ppm (CO2H); MS (ESI) m/z: 740.05 [M+H]+, 761.99 [M+Na]+.
= +9.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.29, 1.58 (2s, 6H, 2CH=CCH3); 1.99 (m, 4 h, CH2CH2); 2.04, 2.07, 2.15, 2.31 (4s, 12H, 4COCH3); 3.50 (s, 2H, CH2S); 3.78 (m, 1H, CHSO2); 4.05 (m, 5 h, H5, CH2O and CH2CHSO2); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, CH=CH, 2CH=CCH3); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 (d, 1H, J = 1.6 Hz, H1); 7.52 (t, 2H, J = 7.6 Hz, CHAr); 7.63 (m, 1H, CHAr); 7.84 ppm (d, 2H, J = 7.6 Hz, CHAr); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH=CCH3); 20.50, 20.60, 20.70 (4COCH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CSO2); 61.92 (C6, CH2O and CH2CHSO2); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=CCH3); 110.81 (CH=CH); 127.44–133.42 (5CHAr); 138.48, 145.70, 146.84 (2C=CH and CSO2); 169.36, 169.56, 168.80, 170.44 (4C=O); 195.37 ppm (CO2H); MS (ESI) m/z: 740.05 [M+H]+, 761.99 [M+Na]+.(2Z,6E,10E)-12-Mercapto-7,11-dimethyl-5-(phenylsulfonyl)dodeca-2,6,10-trien-1-yl-6-deoxy-6-azido-α-D-mannopyranoside (30): The procedure described for compound 3 was applied to 2 to give compound 30 as a white oil (97%): Rf = 0.34 (CH2Cl2/MeOH 4:1); 1H-NMR (CDCl3): δ = 1.61, 1.66 (2s, 6H, 2CH3); 2.02, 2.11 (2m, 4 h, CH2CH2); 3.49 (m, 1H, H5); 3.61 (t, 1H, J = 9.4 Hz, H4); 3.67 (dd, 1H, J = 3.2 Hz, J = 9.2 Hz, H3); 3.72 (dd, 1H, J = 5.6 Hz, J = 12.0 Hz, H6a); 3.79 (q, 1H, J = 1.6 Hz, H2); 3.83 (dd, 1H, J = 2.4 Hz, J = 12.0 Hz, H6b); 4.08 (d, 4 h, J = 6.4 Hz, CH2O and CH2S); 4.64 (d, 1H, J = 1.6 Hz, H1); 5.11 (m, 3H, 3CH=C); 5.35 ppm (m, 3H, 3CH=C); 13C-NMR (CDCl3): δ = 16.27, 25.89 (2CH3); 27.52 (CH2CH2); 40.72 (CH2CH2); 59.45 (CH2O and CH2S); 62.83 (C6); 68.57 (C4); 72.09 (C2); 72.59 (C3); 74.42 (C5); 102.71 (C1); 124.92, 125.14, 129.95, 130.07, 133.05, 133.15 (6CH=C); 132.44, 139.40 ppm (2C=CH); MS (ESI) m/z: 426.12 [M+H]+, 448.24 [M+Na]+.
(2Z,6E,10E)-12-Mercapto-7,11-dimethyl-5-(phenylsulfonyl)dodeca-2,6,10-trien-1-yl-6-deoxy-α-d-heptomannopyranosiduronic acid (31): The procedure described for compound 3 was applied to 29 to give compound 31 as a white oil (97%): Rf = 0.50 (i-PrOH/NH4OH 1:1);  = +4.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.30, 1.55 (2s, 6H, 2CH3); 1.99 (m, 4 h, CH2CH2); 3.50 (s, 2H, CH2S); 3.76 (m, 1H, CH=CH); 4.03 (m, 5 h, H5, CH2O and CH=CH); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, 2CH=CH, 2CH=C); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CH=CH); 61.92 (C6, CH2O andCH=CH); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=C); 110.81 (2CH=CH); 138.48, 145.70 (2C=CH); 195.37 ppm (CO2H); MS (ESI) m/z: 471.76 [M+H]+, 493.78 [M+Na]+.
= +4.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.30, 1.55 (2s, 6H, 2CH3); 1.99 (m, 4 h, CH2CH2); 3.50 (s, 2H, CH2S); 3.76 (m, 1H, CH=CH); 4.03 (m, 5 h, H5, CH2O and CH=CH); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, 2CH=CH, 2CH=C); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CH=CH); 61.92 (C6, CH2O andCH=CH); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=C); 110.81 (2CH=CH); 138.48, 145.70 (2C=CH); 195.37 ppm (CO2H); MS (ESI) m/z: 471.76 [M+H]+, 493.78 [M+Na]+.
 = +4.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.30, 1.55 (2s, 6H, 2CH3); 1.99 (m, 4 h, CH2CH2); 3.50 (s, 2H, CH2S); 3.76 (m, 1H, CH=CH); 4.03 (m, 5 h, H5, CH2O and CH=CH); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, 2CH=CH, 2CH=C); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CH=CH); 61.92 (C6, CH2O andCH=CH); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=C); 110.81 (2CH=CH); 138.48, 145.70 (2C=CH); 195.37 ppm (CO2H); MS (ESI) m/z: 471.76 [M+H]+, 493.78 [M+Na]+.
= +4.4 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.30, 1.55 (2s, 6H, 2CH3); 1.99 (m, 4 h, CH2CH2); 3.50 (s, 2H, CH2S); 3.76 (m, 1H, CH=CH); 4.03 (m, 5 h, H5, CH2O and CH=CH); 4.12 (dd, 1H, J = 2.4 Hz, J = 14.4 Hz, H6a); 4.27 (dd, 1H, J = 4.8 Hz, J = 12.4 Hz, H6b); 5.15 (t, 4 h, J = 8.0 Hz, 2CH=CH, 2CH=C); 5.25 (m, 1H, H2); 5.32 (m, 2H, H3 and H4); 6.08 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CDCl3): δ = 15.02, 15.99 (2CH3); 26.05, 38.95 (CH2CH2); 37.86 (CH2S); 55.85 (CH=CH); 61.92 (C6, CH2O andCH=CH); 65.33, 68.57 (C3 and C4); 68.16 (C2); 70.42 (C5); 90.41 (C1); 110.49, 126.98 (2CH=C); 110.81 (2CH=CH); 138.48, 145.70 (2C=CH); 195.37 ppm (CO2H); MS (ESI) m/z: 471.76 [M+H]+, 493.78 [M+Na]+.6-Deoxy-6-azido-2,3,4-tri-O-acetyl-α-d-mannopyranosyl bromide (32): A solution of hydrobromic acid (5.7 M in acetic acid, 2.35 mL,13.40 mmol, 25 eq.) was added to a solution of acetic anhydride (1 mL) and compound 17 (200 mg, 0.54 mmol, 1 eq.). After 16 h at RT, the mixture was diluted in CH2Cl2 and washed with a saturated solution of NaHCO3 until basic pH. The aqueous phase was extracted with CH2Cl2 (3 times). The organic layers were assembled, then washed with a saturated solution of NaCl, dried, filtered and concentrated in vacuo. The obtained colourless oil was used without purification (quantitative): Rf = 0.56 (petroleum ether/Et2O 3:7);  = +96.1 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 2.01, 2.10, 2.17 (3s, 9H, 3CH3); 3.21 (dd, 1H, J = 6.6 Hz, J = 11.4 Hz, H6a); 3.35 (dd, 1H, J = 2.9 Hz, J = 11.4 Hz, H6b); 3.94–3.99 (m, 1H, H5); 5.27 (t, 1H, J = 10.0 Hz, H4); 5.41 (dd, 1H, J = 1.4 Hz, J = 3.3 Hz, H2); 5.71 (dd, 1H, J = 3.4 Hz, J = 10.0 Hz, H3); 6.3 ppm (d, 1H, J = 1.1 Hz, H1); 13C-NMR (CDCl3): δ = 2.46 (C6); 20.60, 20.73, 20.78 (3CH3); 67.66 (C3); 69.49 (C4); 72.21 (C2); 73.45 (C5); 82.46 (C1); 169.58, 169.64, 169.72 (3C=O); MS (ESI) m/z: 393.98, 395.40 [M+H]+.
= +96.1 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 2.01, 2.10, 2.17 (3s, 9H, 3CH3); 3.21 (dd, 1H, J = 6.6 Hz, J = 11.4 Hz, H6a); 3.35 (dd, 1H, J = 2.9 Hz, J = 11.4 Hz, H6b); 3.94–3.99 (m, 1H, H5); 5.27 (t, 1H, J = 10.0 Hz, H4); 5.41 (dd, 1H, J = 1.4 Hz, J = 3.3 Hz, H2); 5.71 (dd, 1H, J = 3.4 Hz, J = 10.0 Hz, H3); 6.3 ppm (d, 1H, J = 1.1 Hz, H1); 13C-NMR (CDCl3): δ = 2.46 (C6); 20.60, 20.73, 20.78 (3CH3); 67.66 (C3); 69.49 (C4); 72.21 (C2); 73.45 (C5); 82.46 (C1); 169.58, 169.64, 169.72 (3C=O); MS (ESI) m/z: 393.98, 395.40 [M+H]+.
 = +96.1 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 2.01, 2.10, 2.17 (3s, 9H, 3CH3); 3.21 (dd, 1H, J = 6.6 Hz, J = 11.4 Hz, H6a); 3.35 (dd, 1H, J = 2.9 Hz, J = 11.4 Hz, H6b); 3.94–3.99 (m, 1H, H5); 5.27 (t, 1H, J = 10.0 Hz, H4); 5.41 (dd, 1H, J = 1.4 Hz, J = 3.3 Hz, H2); 5.71 (dd, 1H, J = 3.4 Hz, J = 10.0 Hz, H3); 6.3 ppm (d, 1H, J = 1.1 Hz, H1); 13C-NMR (CDCl3): δ = 2.46 (C6); 20.60, 20.73, 20.78 (3CH3); 67.66 (C3); 69.49 (C4); 72.21 (C2); 73.45 (C5); 82.46 (C1); 169.58, 169.64, 169.72 (3C=O); MS (ESI) m/z: 393.98, 395.40 [M+H]+.
= +96.1 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 2.01, 2.10, 2.17 (3s, 9H, 3CH3); 3.21 (dd, 1H, J = 6.6 Hz, J = 11.4 Hz, H6a); 3.35 (dd, 1H, J = 2.9 Hz, J = 11.4 Hz, H6b); 3.94–3.99 (m, 1H, H5); 5.27 (t, 1H, J = 10.0 Hz, H4); 5.41 (dd, 1H, J = 1.4 Hz, J = 3.3 Hz, H2); 5.71 (dd, 1H, J = 3.4 Hz, J = 10.0 Hz, H3); 6.3 ppm (d, 1H, J = 1.1 Hz, H1); 13C-NMR (CDCl3): δ = 2.46 (C6); 20.60, 20.73, 20.78 (3CH3); 67.66 (C3); 69.49 (C4); 72.21 (C2); 73.45 (C5); 82.46 (C1); 169.58, 169.64, 169.72 (3C=O); MS (ESI) m/z: 393.98, 395.40 [M+H]+.6-Deoxy-6-azido-2,3,4-tri-O-acetyl-1-thio-β-d-mannopyranose (34): A solution of compound 32 (100 mg, 0.25 mmol, 1 eq.) and thiourea (25 mg, 0.33 mmol) in acetone (2 mL) was stirred under reflux for 20 h. The reaction was cooled to room temperature. The solvent was removed under reduced pressure to give the isothiouronium salt as a white solid. K2S2O5 (85 mg, 0.38 mmol) was added to a suspension of this salt in CHCl3/H2O (1/1 v/v) (3 mL). After stirring under reflux for 5 h, the solution was cooled to RT, the CHCl3 layer was separated and the aqueous layer was extracted with CH2Cl2. The combined organic layers were dried and concentrated under reduced pressure. Purification by chromatography onsilica gel (EtOAc/petroleum ether 1:1) gave a colourless oil (50%): Rf = 0.56 (CH2Cl2/Et2O 8:2); 1H- NMR (CDCl3): δ = 2.00, 2.06, 2.19 (3s, 9H, 3CH3); 3.32 (m, 2H, H6); 4.17 (ddd, 1H, J = 4.1 Hz, J = 5.4 Hz, J = 9.5 Hz, H5); 4.56 (d, 1H, J = 4.1 Hz, H1); 5.22 (m, 2H, H2 et H4); 5.39 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.6, 20.6, 20.8 (3CH3); 51.1 (C6); 67.2 (C4); 68.6 (C3); 69.5 (C5); 70.1 (C2); 91.8 (C1); 169.9, 170.1, 170.3 (3C=O); MS (ESI) m/z: 370.44 [M+Na]+.
6-Deoxy-2,3,4-tri-O-acetyl-α-d-heptomannopyranuronosyl bromide (33): The procedure described for compound 32 was applied to 21 to give compound 33 as a white oil (quant): Rf = 0.22 (petroleum ether/EtOAc 1:1); 1H-NMR (CDCl3): δ = 1.97, 2.05, 2.12 (3s, 9H, CH3); 1.96–2.21 (m, 2H, H6); 3.91 (td, 1H, J = 2.6 Hz, J = 10.0 Hz, H5); 5.13 (t, 1H, J = 10.0 Hz, H4); 5.20 (dd, 1H, J = 1.9 Hz, J = 3.5 Hz, H2); 5.27 (dd,1H, J = 3.5 Hz, J = 10.0 Hz, H3); 5.94 ppm (d, 1H, J = 1.8 Hz, H1); 13C-NMR (CDCl3): δ = 20.60, 20.75 (3CH3); 30.02 (C6); 68.47 (C2); 68.74 (C3); 68.97 (C4); 69.24 (C5); 90.11 (C1); 168.09, 169.00, 169.33, 169.75 (3C=O et CO2H); MS (ESI) m/z: 419.67, 421.28 [M+Na]+.
6-Deoxy-2,3,4-tri-O-acetyl-1-thio-β-d-heptomannopyranuronic acid (35): The procedure described for compound 34 was applied to 33 to give compound 35 as a white oil (48%): Rf = 0.20 (CH2Cl2/MeOH 4:1); 1H-NMR (CDCl3): δ = 1.99, 2.06, 2.14 (3s, 9H, CH3); 1.95–2.26 (m, 2H, H6); 4.01 (td, 1H, J = 2.6 Hz, J = 10.0 Hz, H5); 5.11 (t, 1H, J = 10.0 Hz, H4); 5.12 (d, 1H, J = 3.2 Hz, H1); 5.23 (dd, 1H, J = 1.7 Hz, J = 3.4 Hz, H2); 5.35 ppm (dd,1H, J = 3.4 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.72, 20.88, 20.93 (3CH3); 30.32 (C6); 67.46 (C5); 68.88 (C3); 69.46 (C4); 70.11 (C2); 92.03 (C1); 169.40, 167.55, 170.08, 170.13 (3C=O et CO2H); MS (ESI) m/z: 373.28 [M+Na]+.
2-{2-[2-(Allyloxy)ethoxy]ethoxy}ethanol (36): The procedure described for compound 6 was applied to triethylene glycol and 3-bromopropene to give compound 36 as a red-orange oil (85%): Rf = 0.34 (CH2Cl2/MeOH 9:1); 1H-NMR (CDCl3): δ = 3.57–3.71 (m, 12H, 6CH2O); 4.00 (m, 2H, CH2CH=CH2); 5.16 (dd, 1H, J = 1.6 Hz, J = 10.4 Hz, CH2 = CH); 5.25 (dd, 1H, J = 1.6 Hz, J = 17.2 Hz, CH2=CH); 5.89 ppm (m, 1H, CH=CH2); 13C-NMR (CDCl3): δ = 61.66–72.57 (7CH2O); 117.30 (CH2=CH); 134.59 ppm (CH=CH2); MS (ESI) m/z: 213.22 [M+Na]+, 229.17 [M+K]+.
3-{2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy}propyl2,3,4-tri-O-acetyl-6-deoxy-6-azido-1-thio-β-d-manno-pyranoside (37): Under argon, compound 36 (36 mg, 0.19 mmol) and AIBN (0.29 mmol, 1.5 eq.) were added to a solution of compound 34 (200 mg, 0.58 mmol) in dioxane degassed under argon (12 mL). After 3 h under ultrasound (amplitude of 20%, pulse on 0.2 s, pulse off 0.2 s), the mixture was reduced under pressure then purified by chromatography on silica gel (EtOAc/petroleum ether 7:3 to 10:0) to give a colourless oil (79%) Rf = 0.38 (EtOAc);  = +43.8 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.98 (s, 3H, CH3); 2.03 (m, 5 h, CH2CH2S and CH3); 2.14 (s, 3H, CH3); 3.27 (m, 5 h, H6a, CH2S and CH2(CH2)2S); 3.35 (dd, 1H, J = 6.4 Hz, J = 13.2 Hz, H6b); 3.59–3.72 (m, 12H, 6CH2O); 4.01 (m, 1H, H5); 4.87 (d, 1H, J = 7.4 Hz, H1); 5.22 (t, 1H, J = 10.0 Hz, H4); 5.25 (dd, 1H, J = 1.6 Hz, J = 3.6 Hz, H2); 5.35 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.65, 20.69, 20.83 (3CH3); 27.98 (CH2S); 35.59 (CH2CH2S); 58.73 (CH2(CH2)2S); 51.05 (C6); 61.62–72.51 (CH2O); 67.16 (C4); 68.80 (C3); 69.48 (C2); 69.93 (C5); 97.42 (C1); 169.82, 169.95, 170.08 ppm (3C=O); MS(ESI) m/z: 560.12 [M+Na]+.
= +43.8 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.98 (s, 3H, CH3); 2.03 (m, 5 h, CH2CH2S and CH3); 2.14 (s, 3H, CH3); 3.27 (m, 5 h, H6a, CH2S and CH2(CH2)2S); 3.35 (dd, 1H, J = 6.4 Hz, J = 13.2 Hz, H6b); 3.59–3.72 (m, 12H, 6CH2O); 4.01 (m, 1H, H5); 4.87 (d, 1H, J = 7.4 Hz, H1); 5.22 (t, 1H, J = 10.0 Hz, H4); 5.25 (dd, 1H, J = 1.6 Hz, J = 3.6 Hz, H2); 5.35 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.65, 20.69, 20.83 (3CH3); 27.98 (CH2S); 35.59 (CH2CH2S); 58.73 (CH2(CH2)2S); 51.05 (C6); 61.62–72.51 (CH2O); 67.16 (C4); 68.80 (C3); 69.48 (C2); 69.93 (C5); 97.42 (C1); 169.82, 169.95, 170.08 ppm (3C=O); MS(ESI) m/z: 560.12 [M+Na]+.
 = +43.8 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.98 (s, 3H, CH3); 2.03 (m, 5 h, CH2CH2S and CH3); 2.14 (s, 3H, CH3); 3.27 (m, 5 h, H6a, CH2S and CH2(CH2)2S); 3.35 (dd, 1H, J = 6.4 Hz, J = 13.2 Hz, H6b); 3.59–3.72 (m, 12H, 6CH2O); 4.01 (m, 1H, H5); 4.87 (d, 1H, J = 7.4 Hz, H1); 5.22 (t, 1H, J = 10.0 Hz, H4); 5.25 (dd, 1H, J = 1.6 Hz, J = 3.6 Hz, H2); 5.35 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.65, 20.69, 20.83 (3CH3); 27.98 (CH2S); 35.59 (CH2CH2S); 58.73 (CH2(CH2)2S); 51.05 (C6); 61.62–72.51 (CH2O); 67.16 (C4); 68.80 (C3); 69.48 (C2); 69.93 (C5); 97.42 (C1); 169.82, 169.95, 170.08 ppm (3C=O); MS(ESI) m/z: 560.12 [M+Na]+.
= +43.8 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.98 (s, 3H, CH3); 2.03 (m, 5 h, CH2CH2S and CH3); 2.14 (s, 3H, CH3); 3.27 (m, 5 h, H6a, CH2S and CH2(CH2)2S); 3.35 (dd, 1H, J = 6.4 Hz, J = 13.2 Hz, H6b); 3.59–3.72 (m, 12H, 6CH2O); 4.01 (m, 1H, H5); 4.87 (d, 1H, J = 7.4 Hz, H1); 5.22 (t, 1H, J = 10.0 Hz, H4); 5.25 (dd, 1H, J = 1.6 Hz, J = 3.6 Hz, H2); 5.35 ppm (dd, 1H, J = 3.2 Hz, J = 10.0 Hz, H3); 13C-NMR (CDCl3): δ = 20.65, 20.69, 20.83 (3CH3); 27.98 (CH2S); 35.59 (CH2CH2S); 58.73 (CH2(CH2)2S); 51.05 (C6); 61.62–72.51 (CH2O); 67.16 (C4); 68.80 (C3); 69.48 (C2); 69.93 (C5); 97.42 (C1); 169.82, 169.95, 170.08 ppm (3C=O); MS(ESI) m/z: 560.12 [M+Na]+.3-{2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy}propyl2,3,4-tri-O-ace-tyl-6-deoxy-6-azido-1-thio-β-d-hepto-mannopyranosiduronic acid (38): The procedure described for compound 37 was applied to 35 and 36 to give compound 38 as a beige oil (80%): Rf = 0.08 (EtOAc);  = +40.0 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.87 (qt, 2H, J = 6.8 Hz, CH2CH2S); 1.95, 2.06, 2.12 (3s, 12H, 4CH3); 2.69 (m, 2H, CH2S); 3.51 (t, 2H, J = 6.0 Hz, CH2(CH2)2S); 3.54–3.69 (m, 12H, 6CH2O); 4.05 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.27 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.34 (m, 1H, H5); 4.80 (d, 1H, J = 7.4 Hz, H1); 5.23 (m, 2H, H3 et H4); 5.30 ppm (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CDCl3): δ = 19.62, 19.69, 19.91 (3CH3); 27.19 (CH2S); 28.41 (CH2CH2S); 60.69 (CH2(CH2)2S); 61.41 (C6); 65.31 (C3); 67.96 (C5); 68.45 (C4); 68.26–71.53 (7CH2O); 70.14 (C2); 81.64 (C1); 168.72, 168.80, 168.99, 169.63 ppm (3C=O et CO2H); MS (ESI) m/z: 563.76 [M+Na]+.
= +40.0 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.87 (qt, 2H, J = 6.8 Hz, CH2CH2S); 1.95, 2.06, 2.12 (3s, 12H, 4CH3); 2.69 (m, 2H, CH2S); 3.51 (t, 2H, J = 6.0 Hz, CH2(CH2)2S); 3.54–3.69 (m, 12H, 6CH2O); 4.05 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.27 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.34 (m, 1H, H5); 4.80 (d, 1H, J = 7.4 Hz, H1); 5.23 (m, 2H, H3 et H4); 5.30 ppm (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CDCl3): δ = 19.62, 19.69, 19.91 (3CH3); 27.19 (CH2S); 28.41 (CH2CH2S); 60.69 (CH2(CH2)2S); 61.41 (C6); 65.31 (C3); 67.96 (C5); 68.45 (C4); 68.26–71.53 (7CH2O); 70.14 (C2); 81.64 (C1); 168.72, 168.80, 168.99, 169.63 ppm (3C=O et CO2H); MS (ESI) m/z: 563.76 [M+Na]+.
 = +40.0 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.87 (qt, 2H, J = 6.8 Hz, CH2CH2S); 1.95, 2.06, 2.12 (3s, 12H, 4CH3); 2.69 (m, 2H, CH2S); 3.51 (t, 2H, J = 6.0 Hz, CH2(CH2)2S); 3.54–3.69 (m, 12H, 6CH2O); 4.05 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.27 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.34 (m, 1H, H5); 4.80 (d, 1H, J = 7.4 Hz, H1); 5.23 (m, 2H, H3 et H4); 5.30 ppm (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CDCl3): δ = 19.62, 19.69, 19.91 (3CH3); 27.19 (CH2S); 28.41 (CH2CH2S); 60.69 (CH2(CH2)2S); 61.41 (C6); 65.31 (C3); 67.96 (C5); 68.45 (C4); 68.26–71.53 (7CH2O); 70.14 (C2); 81.64 (C1); 168.72, 168.80, 168.99, 169.63 ppm (3C=O et CO2H); MS (ESI) m/z: 563.76 [M+Na]+.
= +40.0 (c = 1.00 in chloroform); 1H-NMR (CDCl3): δ = 1.87 (qt, 2H, J = 6.8 Hz, CH2CH2S); 1.95, 2.06, 2.12 (3s, 12H, 4CH3); 2.69 (m, 2H, CH2S); 3.51 (t, 2H, J = 6.0 Hz, CH2(CH2)2S); 3.54–3.69 (m, 12H, 6CH2O); 4.05 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.27 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.34 (m, 1H, H5); 4.80 (d, 1H, J = 7.4 Hz, H1); 5.23 (m, 2H, H3 et H4); 5.30 ppm (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CDCl3): δ = 19.62, 19.69, 19.91 (3CH3); 27.19 (CH2S); 28.41 (CH2CH2S); 60.69 (CH2(CH2)2S); 61.41 (C6); 65.31 (C3); 67.96 (C5); 68.45 (C4); 68.26–71.53 (7CH2O); 70.14 (C2); 81.64 (C1); 168.72, 168.80, 168.99, 169.63 ppm (3C=O et CO2H); MS (ESI) m/z: 563.76 [M+Na]+.3-{2-[2-(2-Mercaptoethoxy)ethoxy]ethoxy}propyl 6-deoxy-6-azido-1-thio-β-d-mannopyranoside (39): The procedures described for compounds 27 then 3 were applied to 37 to give compound 39 as a beige oil: Rf = 0.21 (CH2Cl2/MeOH 4:1);  = +40.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.95 (m, 2H, CH2CH2SC); 2.88 (t, 2H, J = 6.6 Hz, CH2SH); 3.26 (m, 4 h, CH2SC and CH2(CH2)2SC); 3.40 (dd, 1H, J = 7.0 Hz, J = 13.0 Hz, H6a); 3.55 (m, 2H, H5 and H6a); 3.58–3.69 (m, 10H, H3,4 and 4CH2O); 3.72 (t, 2H, J = 6.4 Hz, CH2CH2SH); 3.80 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 4.77 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CD3OD): δ = 28.02 (CH2SC); 37.42 (CH2CH2SC); 39.51 (CH2SH); 57.92 (CH2(CH2)2SC); 53.02 (C6); 62.55–71.65 (5CH2O); 69.52, 72.01, 72.36 (C3, C4 and C5); 73.86 (C2); 101.87 ppm (C1); MS (ESI) m/z: 428.73 [M+H]+, 460.57 [M+Na]+.
= +40.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.95 (m, 2H, CH2CH2SC); 2.88 (t, 2H, J = 6.6 Hz, CH2SH); 3.26 (m, 4 h, CH2SC and CH2(CH2)2SC); 3.40 (dd, 1H, J = 7.0 Hz, J = 13.0 Hz, H6a); 3.55 (m, 2H, H5 and H6a); 3.58–3.69 (m, 10H, H3,4 and 4CH2O); 3.72 (t, 2H, J = 6.4 Hz, CH2CH2SH); 3.80 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 4.77 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CD3OD): δ = 28.02 (CH2SC); 37.42 (CH2CH2SC); 39.51 (CH2SH); 57.92 (CH2(CH2)2SC); 53.02 (C6); 62.55–71.65 (5CH2O); 69.52, 72.01, 72.36 (C3, C4 and C5); 73.86 (C2); 101.87 ppm (C1); MS (ESI) m/z: 428.73 [M+H]+, 460.57 [M+Na]+.
 = +40.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.95 (m, 2H, CH2CH2SC); 2.88 (t, 2H, J = 6.6 Hz, CH2SH); 3.26 (m, 4 h, CH2SC and CH2(CH2)2SC); 3.40 (dd, 1H, J = 7.0 Hz, J = 13.0 Hz, H6a); 3.55 (m, 2H, H5 and H6a); 3.58–3.69 (m, 10H, H3,4 and 4CH2O); 3.72 (t, 2H, J = 6.4 Hz, CH2CH2SH); 3.80 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 4.77 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CD3OD): δ = 28.02 (CH2SC); 37.42 (CH2CH2SC); 39.51 (CH2SH); 57.92 (CH2(CH2)2SC); 53.02 (C6); 62.55–71.65 (5CH2O); 69.52, 72.01, 72.36 (C3, C4 and C5); 73.86 (C2); 101.87 ppm (C1); MS (ESI) m/z: 428.73 [M+H]+, 460.57 [M+Na]+.
= +40.0 (c = 1.00 in chloroform); 1H-NMR (CD3OD): δ = 1.95 (m, 2H, CH2CH2SC); 2.88 (t, 2H, J = 6.6 Hz, CH2SH); 3.26 (m, 4 h, CH2SC and CH2(CH2)2SC); 3.40 (dd, 1H, J = 7.0 Hz, J = 13.0 Hz, H6a); 3.55 (m, 2H, H5 and H6a); 3.58–3.69 (m, 10H, H3,4 and 4CH2O); 3.72 (t, 2H, J = 6.4 Hz, CH2CH2SH); 3.80 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 4.77 ppm (d, 1H, J = 1.6 Hz, H1); 13C-NMR (CD3OD): δ = 28.02 (CH2SC); 37.42 (CH2CH2SC); 39.51 (CH2SH); 57.92 (CH2(CH2)2SC); 53.02 (C6); 62.55–71.65 (5CH2O); 69.52, 72.01, 72.36 (C3, C4 and C5); 73.86 (C2); 101.87 ppm (C1); MS (ESI) m/z: 428.73 [M+H]+, 460.57 [M+Na]+.3-{2-[2-(2-Mercaptoethoxy)ethoxy]ethoxy}propyl 6-deoxy-1-thio-β-d-heptomannopyranosiduronic acid (40): The procedures described for compounds 27 then 3 were applied to 38 to give compound 40 as a beige oil: Rf = 0.18 (i-PrOH/NH4OH 1:1);  = +32.0 (c = 1.05 in chloroform); 1H-NMR (CD3OD): δ = 1.92 (qt, 2H, J = 6.4 Hz, CH2CH2SC); 2.73 (m, 2H, CH2SC); 3.09 (t, 2H, J = 6.6 Hz, CH2(CH2)2SC); 3.53 (t, 2H, J = 6.0 Hz, CH2SH); 3.55–3.62 (m, 10H, 5CH2O); 4.08 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.30 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.37 (m, 1H, H5); 5.21 (m, 2H, H1, H3 and H4); 5.31 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CD3OD): δ = 27.22 (CH2SC); 27.82 (CH2CH2SC); 28.48 (CH2(CH2)2SC); 61.41 (C6); 65.32 (C3); 67.96 (C5); 68.44 (C4); 68.28–69.59 (5CH2O and CH2SH); 70.14 (C2); 81.65 (C1);168.90 (CO2H); MS (ESI) m/z: 431.59 [M+H]+, 463.64 [M+Na]+.
= +32.0 (c = 1.05 in chloroform); 1H-NMR (CD3OD): δ = 1.92 (qt, 2H, J = 6.4 Hz, CH2CH2SC); 2.73 (m, 2H, CH2SC); 3.09 (t, 2H, J = 6.6 Hz, CH2(CH2)2SC); 3.53 (t, 2H, J = 6.0 Hz, CH2SH); 3.55–3.62 (m, 10H, 5CH2O); 4.08 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.30 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.37 (m, 1H, H5); 5.21 (m, 2H, H1, H3 and H4); 5.31 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CD3OD): δ = 27.22 (CH2SC); 27.82 (CH2CH2SC); 28.48 (CH2(CH2)2SC); 61.41 (C6); 65.32 (C3); 67.96 (C5); 68.44 (C4); 68.28–69.59 (5CH2O and CH2SH); 70.14 (C2); 81.65 (C1);168.90 (CO2H); MS (ESI) m/z: 431.59 [M+H]+, 463.64 [M+Na]+.
 = +32.0 (c = 1.05 in chloroform); 1H-NMR (CD3OD): δ = 1.92 (qt, 2H, J = 6.4 Hz, CH2CH2SC); 2.73 (m, 2H, CH2SC); 3.09 (t, 2H, J = 6.6 Hz, CH2(CH2)2SC); 3.53 (t, 2H, J = 6.0 Hz, CH2SH); 3.55–3.62 (m, 10H, 5CH2O); 4.08 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.30 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.37 (m, 1H, H5); 5.21 (m, 2H, H1, H3 and H4); 5.31 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CD3OD): δ = 27.22 (CH2SC); 27.82 (CH2CH2SC); 28.48 (CH2(CH2)2SC); 61.41 (C6); 65.32 (C3); 67.96 (C5); 68.44 (C4); 68.28–69.59 (5CH2O and CH2SH); 70.14 (C2); 81.65 (C1);168.90 (CO2H); MS (ESI) m/z: 431.59 [M+H]+, 463.64 [M+Na]+.
= +32.0 (c = 1.05 in chloroform); 1H-NMR (CD3OD): δ = 1.92 (qt, 2H, J = 6.4 Hz, CH2CH2SC); 2.73 (m, 2H, CH2SC); 3.09 (t, 2H, J = 6.6 Hz, CH2(CH2)2SC); 3.53 (t, 2H, J = 6.0 Hz, CH2SH); 3.55–3.62 (m, 10H, 5CH2O); 4.08 (dd, 1H, J = 2.2 Hz, J = 12.2 Hz, H6a); 4.30 (dd, 1H, J = 5.4 Hz, J = 12.2 Hz, H6b); 4.37 (m, 1H, H5); 5.21 (m, 2H, H1, H3 and H4); 5.31 (dd, 1H, J = 1.6 Hz, J = 3.2 Hz, H2); 13C-NMR (CD3OD): δ = 27.22 (CH2SC); 27.82 (CH2CH2SC); 28.48 (CH2(CH2)2SC); 61.41 (C6); 65.32 (C3); 67.96 (C5); 68.44 (C4); 68.28–69.59 (5CH2O and CH2SH); 70.14 (C2); 81.65 (C1);168.90 (CO2H); MS (ESI) m/z: 431.59 [M+H]+, 463.64 [M+Na]+.3.2. Preparation of Citrate-Reduced Gold Nanoparticles
Hydrogen tetrachloroaurate trihydrate (60 mg, 0.15 mmol) was dissolved in water (250 mL) to give a pale-yellow solution. A second solution with sodium citrate (150 mg, 0.58 mmol) dissolved in water (10 mL) was prepared. Both solutions were heated to 60 °C for 10 min then the sodium citrate solution was rapidly added to the gold solution. The temperature was then increased to 120 °C with continuous stirring for 2 h 30 min. A deep-red solution was formed. The solution was warmed to RT and each thiol-derivatized carbohydrate (50 mg) dissolved in methanol (1 mL) was added to freshly prepared citrate-reduced gold nanoparticles. Self-assembly was facilitated by leaving the solution under stirring for 48 h. To remove any unbound carbohydrate, the solution was diluted with brine to precipitate nano-objects and left to rest over night. Then the supernatant was removed and nanoparticles were resuspended in water. They were then centrifuged for 30 min at 14,000 rpm. The centrifugation step was repeated three times to ensure complete removal of any unbound carbohydrate.
3.3. CAM Assays
Paper discs were saturated with a phosphate buffer saline dispersion of coated AuNPs (60 mg/mL) in PBS or a control (phosphate buffer saline) and then deposited on chorioallantoic membranes of 7-day-old chicken embryos for 4 days in ovo at 38 °C. Sutent® (sunitinib, a non-proteic inhibitor) and ECGS (endothelial cell growth supplement) were used at 60 mg/mL as negative and positive stimuli, respectively. Quantification of the angiogenic response was carried out by measuring the area of neo-vascularization on each particular membrane (Figure 2). The vascularization was evaluated using Image J software, and are given in pixels compared to phosphate buffer saline (PBS, control).
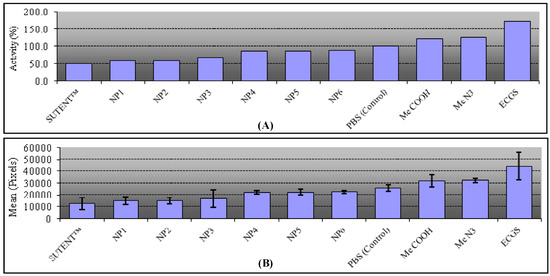
Figure 2.
(A) CAM activity in percentage compared to PBS (control); (B) CAM activity in pixels (Image J) compared to PBS (control).
4. Conclusions
We have reported the synthesis of a series of gold glyconanoparticles bearing diverse M6P neoglycoconjugates. These M6P analogues were synthesized either by Huisgen cycloaddition, by the Julia olefination, or by thiol-ene coupling. The thiol-ene reaction strategy under ultrasound activation proved to be the most efficient in terms of yields and ease of implementation. The angiogenic activities of the AuNPs have been tested by the CAM assay, and all possess angiogenic activities via the M6P receptor with no apparent toxicity. The results of this study have allowed us: (a) to demonstrate that the activity is not dependent of the structure of the linker between the nanoparticles and the carbohydrate and (b) to identify the inhibitory multivalent effect of M6P derivatives on gold surfaces compared with the corresponding monomeric activators. Although our biological results are obviously in a preliminary stage, the work described herein is valuable in that it provides synthetic access to some potentially useful multi-functional and biologically active systems.
Acknowledgments
This work was supported by grants from the Ministère de la Recherche et de l’Enseignement Supérieur and by the Ligue contre le Cancer Comité du Languedoc Roussillon. We thank also Mélanie Sabadotto (Laboratoires Pierre Fabre Dermocosmetique, Toulouse) for useful discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kornfeld, S. Structure and function of the mannose 6-phosphate insulin-like growth factor-ii receptors. Annu. Rev. Biochem. 1992, 61, 307–330. [Google Scholar] [CrossRef]
- Kang, J.K.; Li, Y.; Leaf, A. Retinoic acid alters the intracellular trafficking of the mannose-6-phosphate insulin-like growth factor II receptor and lysosomal enzymes. Proc. Natl. Acad. Sci. USA 1998, 95, 13671–13676. [Google Scholar]
- Volpert, A.; Jackson, D.; Bouck, N.; Linzer, D.I.H. The insulin-like growth factor II mannose 6-phosphate receptor is required for proliferin-induced angiogenesis. Endocrinology 1996, 137, 3871–3876. [Google Scholar]
- Barragan-Montero, V.; Awwad, A.; Combemale, S.; de Santa Barbara, P.; Jover, B.; Molès, J.-P.; Montero, J.-L. Synthesis of Mannose-6-Phosphate analogues and their utility as angiogenesis regulators. Chem. Med. Chem. 2011, 6, 1771–1774. [Google Scholar] [CrossRef]
- Clavel, C.; de Santa Barbara, P.; Jover, B.; Moles, J.-P.; Montero, J.-L.; Montero, V. Novel Uses of D-Mannopyranose Derivatives. U.S. Patent WO 2009138601 A2, 19 November 2009. [Google Scholar]
- Awwad, A.; Combemale, S.; de Santa Barbara, P.; Jover, B.; Molès, J.-P.; Montero, J.-L.; Barragan-Montero, V. Nouveaux Derives de Mannopyranoside Ayant une Activite Anticancereuse. EP 2499150 A1, 19 September 2012. [Google Scholar]
- De Santa Barbara, P.; Jover, B.; Moles, J.-P.; Montero, J.-L.; Montero, V. Nouvelles Utilisations de Derives de D-Mannopyranose Activateurs de l’angiogenese. EP 2285817 A2, 23 February 2011. [Google Scholar]
- Kitov, P.I.; Bundle, D.R. Carbohydrate-Based Drug Discovery; Wong, C.-H., Ed.; Wiley-VCH: Weinheim, Germany, 2003; Volume 2, pp. 541–574. [Google Scholar]
- Kiessling, L.L.; Pontrello, J.K.; Schuster, M.C. Carbohydrate-Based Drug Discovery; Wong, C.-H., Ed.; Wiley-VCH: Weinheim, Germany, 2003; Volume 2, pp. 575–608. [Google Scholar]
- Kiessling, L.; Gestwicki, J.E.; Strong, L.E. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed. 2006, 45, 2348–2368. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.R.T. Synthetic multivalent ligands as probes of signal transduction. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The cluster glycoside effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef]
- De La Fuente, J.M.; Barrientos, A.G.; Rojas, T.C.; Rojo, J.; Cañada, J.; Fernández, A.; Penadés, S. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew. Chem. Int. Ed. 2001, 40, 2257–2261. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley-VCH: Weinheim, Germany, 1984; Volume 1, pp. 1–176. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Li, N.; Binder, W.H. Click-chemistry for nanoparticle-modification. Mater. Chem. 2011, 21, 16717–16734. [Google Scholar] [CrossRef]
- Bastide, J.; Henri-Rousseau, O. The Chemistry of the Carbon-Carbon Triple Bond; Patai, S., Ed.; Interscience Publishers: London, UK, 1978; pp. 447–552. [Google Scholar]
- Tornøe, W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Davis, M. Chemotherapy of schistosomiasis. V. Cholesteryl and choloyl derivatives of 4-amino-2-methoxyphenyl ethers. J. Chem. Soc. 1962, 1962, 178–181. [Google Scholar] [CrossRef]
- Zemplén, G. Decomposition of reducing disaccharides, VII Determination of the constitution of maltose. Berich. Deutschen Chem. Gesellsch. 1927, 7, 1555–1564. [Google Scholar] [CrossRef]
- Khanjin, N.A.; Montero, J.-L. Synthesis of mannose-6-phosphate analogs: Large-scale preparation of isosteric mannose-6-phosphonate via cyclic sulfate precursor. Tetrahedron Lett. 2002, 43, 4017–4020. [Google Scholar] [CrossRef]
- Kim, B.M.; Sharpless, K.B. Cyclic sulfates containing acid-sensitive groups and chemoselective hydrolysis of sulfate esters. Tetrahedron Lett. 1989, 30, 655–658. [Google Scholar] [CrossRef]
- Gibson, T. Phase-transfer synthesis of mono-alkyl ethers of oligoethylene glycols. J. Org. Chem. 1980, 45, 1095–1098. [Google Scholar] [CrossRef]
- Cunneen, J.I. The addition of thio-compounds to olefins. II. Reactions of thiolacetic and mono-chlorothiolacetic, di-chlorothiolacetic, and tri-chlorothiolacetic acids. J. Chem. Soc. 1947, 1947, 134–141. [Google Scholar] [CrossRef]
- Ciavatta, L.; Ferri, D.; Palombari, R. On the equilibrium Cu2++Cu(s) reversible 2Cu+. J. Inorg. Nucl. Chem. 1980, 42, 593–598. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-Catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. Cu-I-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. [Google Scholar]
- Rodionov, V.O.; Fokin, V.V.; Finn, M.G. Mechanism of the ligand-free CuI-catalyzed azide-alkyne cycloaddition reaction. Angew. Chem. Int. Ed. 2005, 44, 2210–2215. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Bertrand, P.; Gesson, J.-P. Click chemistry with O-dimethylpropargylcarbamate for preparation of pH-sensitive functional groups. A case study. J. Org. Chem. 2007, 72, 3596–3599. [Google Scholar]
- Orgueira, H.A.; Fokas, D.; Isome, Y.; Chan, P.C.; Baldino, C.M. Regioselective synthesis of [1,2,3]-triazoles catalyzed by Cu(I) generated in situ from Cu(0) nanosize activated powder and amine hydrochloride salts. Tetrahedron Lett. 2005, 46, 2911–2914. [Google Scholar] [CrossRef]
- Smith, G.F.; Sullivan, V.R. Frank, hexanitrato ammonium cerate as a proposed reference standard in oxidimetry G. Ind. Eng. Chem. Anal. Ed. 1936, 8, 449–451. [Google Scholar] [CrossRef]
- Jarowicki, K.; Kocienski, P. Protecting groups. J. Chem. Soc. Perkin Trans. 2001, 18, 2109–2135. [Google Scholar] [CrossRef]
- Wittig, G.; Geissler, G. Zur reaktionsweise des pentaphenyl-phosphors und einiger derivate. Liebigs Ann. Chem. 1953, 580, 44–57. [Google Scholar] [CrossRef]
- Wittig, G.; Schöllkopf, U. Uber triphenyl-phosphinmethylene als olefinbildende reagenzien. Chem. Ber. 1954, 87, 1318–1330. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; Reitz, A.B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions-stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Horner, L.; Hoffmann, H.; Wippel, H.G. Phosphororganische Verbindungen, XII. Phosphinoxyde als Olefinierungsreagenzien. Chem. Ber. 1958, 91, 61–63. [Google Scholar] [CrossRef]
- Horner, L.; Hoffmann, H.; Wippel, H.G.; Klahre, G. Phosphororganische verbindungen, XX. phosphinoxyde als olefinierungsreagenzien. Chem. Ber. 1959, 92, 2499–2505. [Google Scholar] [CrossRef]
- Wadsworth, W.S.; Emmons, W.D. Utility of phosphonate carbanions in olefin synthesis. J. Am. Chem. Soc. 1961, 83, 1733–1738. [Google Scholar] [CrossRef]
- Wadsworth, W.S. Synthetic applications ofphosphoryl-stabilized anions. Org. React. 1977, 25, 73–253. [Google Scholar]
- Peterson, D.J. A carbonyl olefination reaction using silyl-subsituted organometallic compounds. J. Org. Chem. 1968, 33, 780–784. [Google Scholar] [CrossRef]
- Van Staden, L.F.; Gravestock, D.; Ager, D.J. New developments in the Peterson olefination reaction. Chem. Soc. Rev. 2002, 31, 195–200. [Google Scholar] [CrossRef]
- Ager, D.J. The Peterson olefination reaction. Org. React. 1990, 38, 1–223. [Google Scholar]
- Johnson, C.R.; Shanklin, J.R.; Kirchhoff, R.A. Olefin synthesis by reductive elimination of β-hydroxysulfoximines-methylenation of carbonyl-compounds. J. Am. Chem. Soc. 1973, 95, 6462–6463. [Google Scholar] [CrossRef]
- Julia, M.; Paris, J.-M. Syntheses using sulfones V(+). Method for general synthesis of double bonds. Tetrahedron Lett. 1973, 14, 4833–4836. [Google Scholar] [CrossRef]
- Baudin, J.B.; Hareau, G.; Julia, S.A.; Ruel, O. A direct synthesis of olefins by reaction of carbonyl-compounds with lithio derivatives of 2-[alkyl-sulfonyl or (2'-alkenyl)-sulfonyl or benzyl-sulfonyl]-benzothiazoles. Tetrahedron Lett. 1991, 32, 1175–1178. [Google Scholar] [CrossRef]
- Baudin, J.B.; Hareau, G.; Julia, S.A.; Lorne, R.; Ruel, O. Stereochemistry of the olefin formation from anti and syn heterocyclic beta-hydroxy-sulfones. Bull. Soc. Chim. Fr. 1993, 130, 336–357. [Google Scholar]
- Blakemore, P.R. The modified Julia olefination: Alkene synthesis via the condensation of metallated heteroarylalkylsulfones with carbonyl compounds. J. Chem. Soc. Perkin Trans. 2002, 2002, 2563–2585. [Google Scholar] [CrossRef]
- Umbreit, M.A.; Sharpless, K.B. Allylic oxidation of olefins by catalytic and stoichiometric selenium dioxide with tert-butyl hydroperoxide. J. Am. Chem. Soc. 1977, 99, 5526–5528. [Google Scholar] [CrossRef]
- Stevens, M.P. Polymer Chemistry, 3rd ed.; Oxford University Press: Oxford, NY, USA, 1999; pp. 265–267. [Google Scholar]
- Mayo, F.R.; Walling, C. The peroxide effect in the addition of reagents to unsaturated compounds and in rearrangement reactions. Chem. Rev. 1940, 27, 351–412. [Google Scholar] [CrossRef]
- Zard, S.Z. Radical Reactions in Organic Synthesis, 1st ed.; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Posner, T. Information on unsaturated compounds II. The addition of mercaptan to unsaturated hydrocarbon. Berich. Deutschen Chem. Gesellsch. 1905, 38, 646–657. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J.A. A study of the nucleation and growth processes in the synthesis of colloidal gold. Disc. Far. Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Slot, J.W.; Gueuze, H.J. Sizing of protein a-colloidal gold probes for immunoelectron microscopy. J. Cell Biol. 1981, 90, 533–536. [Google Scholar] [CrossRef]
- Chow, M.K.; Zukoski, C.F. Gold sol formation mechanisms-role of colloidal stability. J. Colloid Interf. Sci. 1994, 165, 97–109. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar]
- Ribatti, D. Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int. Rev. Cell Mol. Biol. 2008, 270, 181–224. [Google Scholar] [CrossRef]
- Richardson, M.; Singh, G. Observations on the use of the avian chorioallantoic membrane (CAM) model in investigations into angiogenesis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003, 3, 155–185. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) Assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Pergamon Press: Oxford, UK, 1998. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).