Harvestman Phenols and Benzoquinones: Characterisation and Biosynthetic Pathway
Abstract
:1. Introduction
2. Results and Discussion
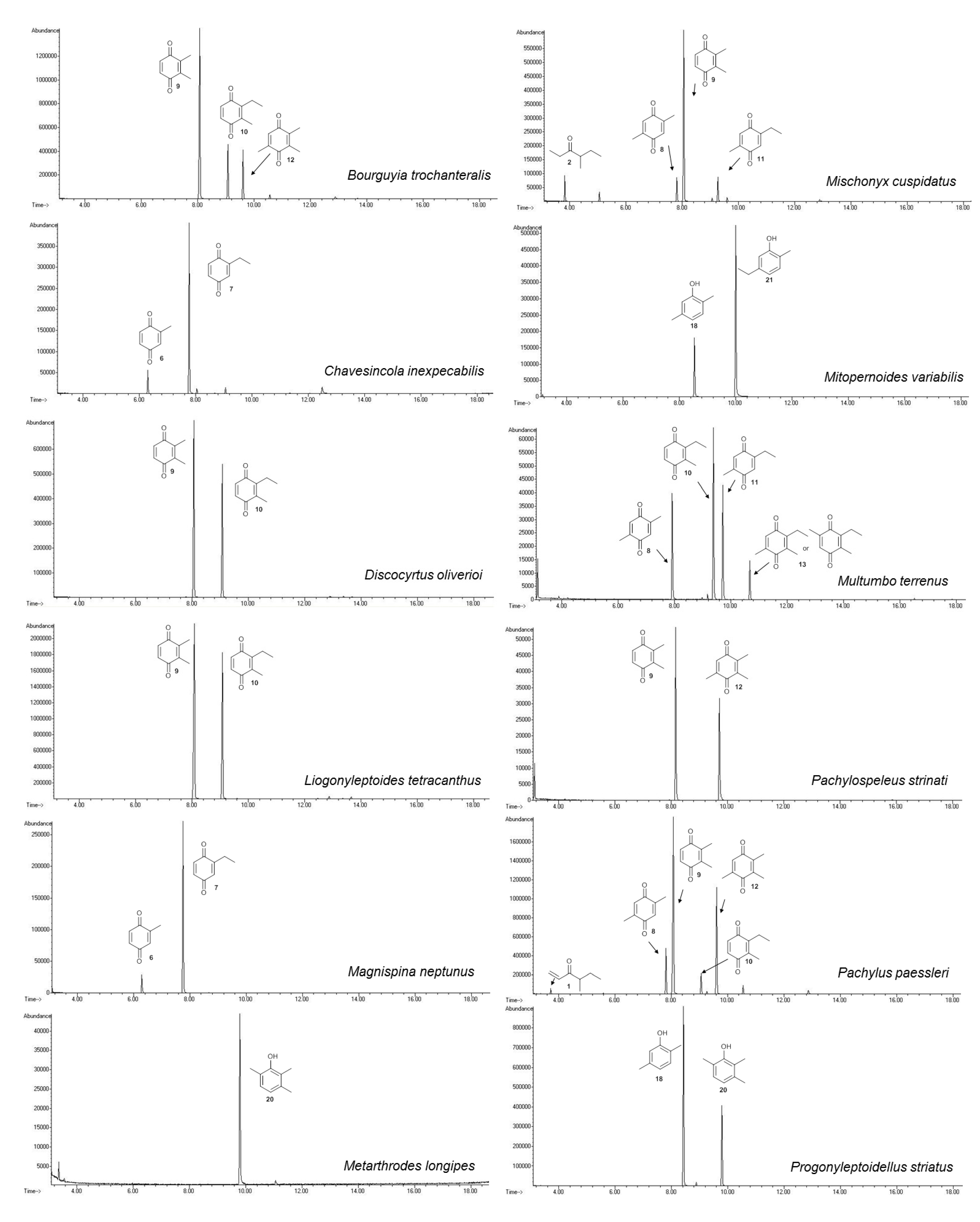
2.1. Chemical Profile of Gonyleptid Exudates
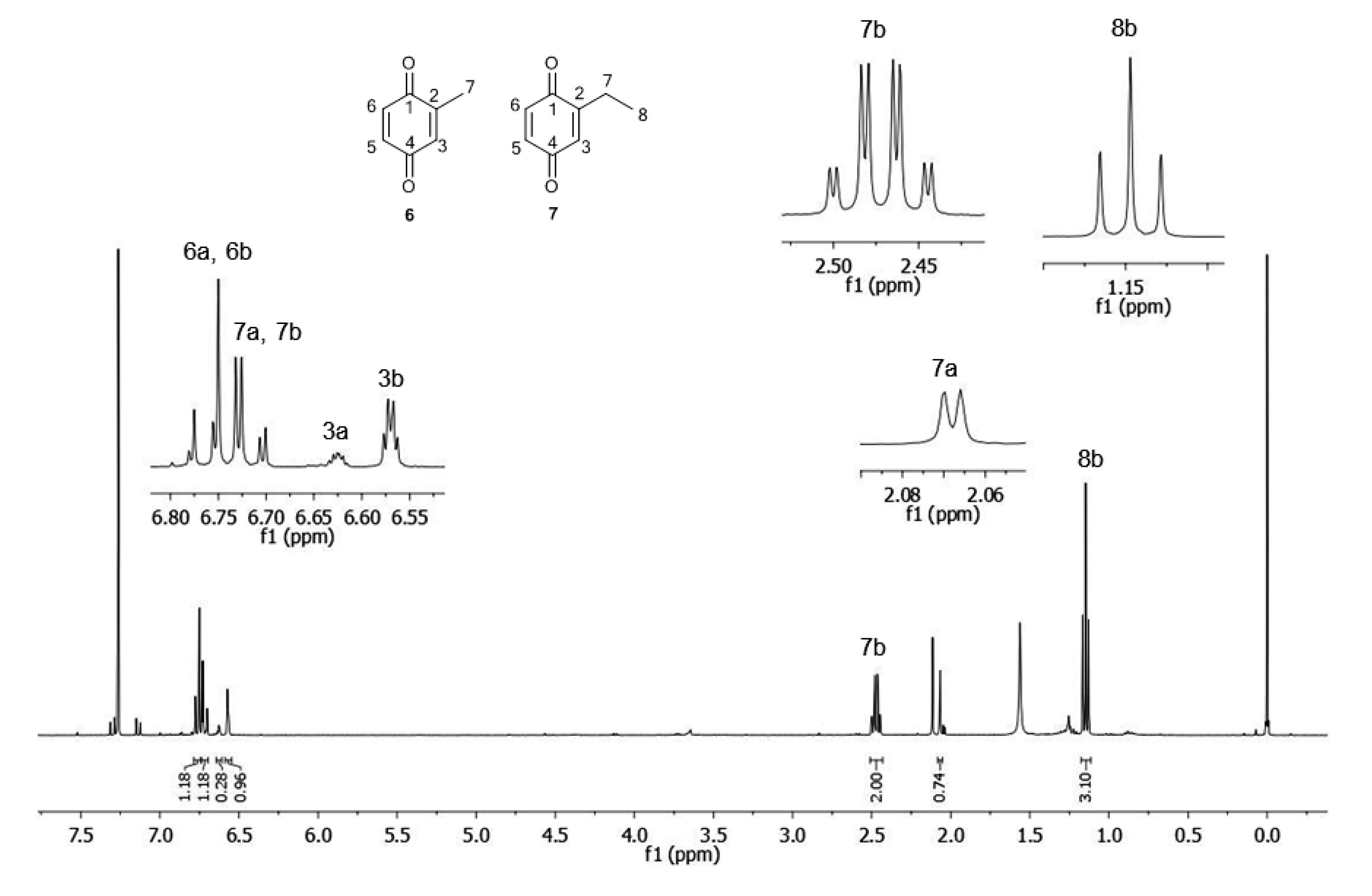
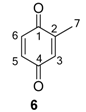
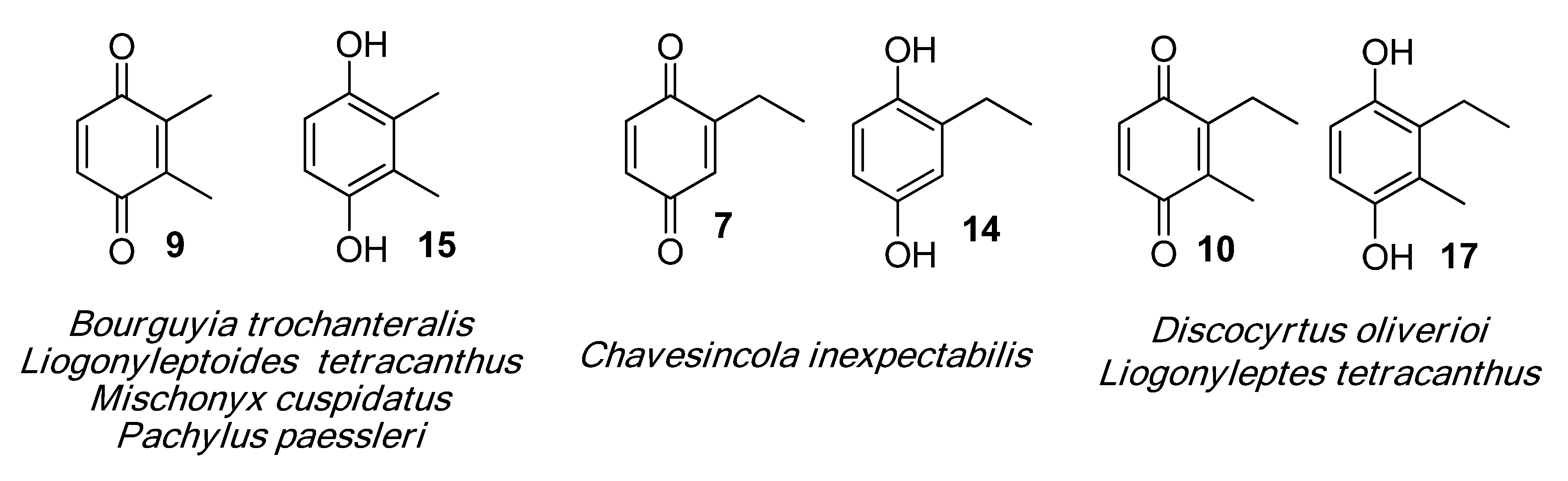
2.1.1. Benzoquinones Identification
| Structure | RI | Characteristic ions [m/z (abundance)] | Species | Relative abundance |
|---|---|---|---|---|
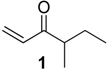 | 831 | 112(M+,15), 97(12), 84(35), 83(12), 69(12), 58(28), 56(23), 55(100), 41(29) | Pachylus paessleri | 0.9% |
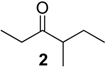 | 835 | 114(M+,14), 85(10), 57(100), 41(14) | Mischonyx cuspidatus | 7.0% |
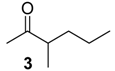 | 845 * | 72(64), 71(19), 70(17), 57(19), 55(14), 43(100), 41(21) | Mischonyx cuspidatus | 0.2% |
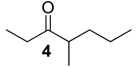 | 929 | 128(M+, 3), 99(11), 86(60), 71(82), 57(100), 55(13), 43(64), 41(16) | Mischonyx cuspidatus | 2.8% |
 | 956 * | 126(M+,26), 111(49), 97(100), 83(21), 69(43), 67(21), 56(26), 55(63), 43(73), 41(78) | Pachylus paessleri | 0.1% |
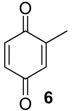 | 1010 | 124(45), 123(27), 122(M+,100), 94(64), 82(55), 68(31), 66(46), 54(55), 40(24) | Chavesincola inexpectabilis Magnispina neptunus | 10.1% 9.2% |
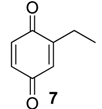 | 1103 | 136(M+,67), 123(16), 108(100), 107(42), 82(42), 80(18), 79(73), 77(15), 54(52), 53(30) | Magnispina neptunus Chavesincola inexpectabilis | 90.8% 80.2% |
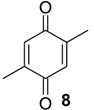 | 1104 | 138(14), 137(13), 136(M+,100), 108(25), 96(20), 80(19), 79(37), 68(67) | Multumbo terrenus Pachylus paessleri Mischonyx cuspidatus | 24.2 %10.3% 8.7% |
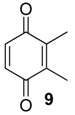 | 1119 | 136(M+,100), 108(47), 107(47), 82(39), 80(17), 79(41), 54(37), 53(16) | Mischonyx cuspidatus Bourguyia trochanteralis Pachylospeleus strinati Liogonyleptoides tetracanthus Discocyrtus oliverioi Pachylus paessleri Chavesincola inexpectabilis | 68.3% 65.0% 60.3% 58.6% 57.4% 53.2% 2.4% |
 | 1182 | 150(M+,100), 135(10), 122(31), 121(16), 107(69), 82(20), 79(32), 77(16), 67(10), 54(18), 53(11) | Discocyrtus oliverioi Liogonyleptoides tetracanthus Bourguyia trochanteralis Pachylus paessleri Chavesincola inexpectabilis Mischonyx cuspidatus Multumbo terrenus | 41.4% 39.9% 17.1% 4.6% 2.7% 1.3% 0.9% |
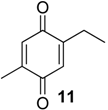 | 1197 | 150(M+,100), 137(14), 122(45), 121(14), 107(41), 82(13), 79(54), 77(17), 68(24), 54(13), 53(19) | Multumbo terrenus Mischonyx cuspidatus Pachylus paessleri | 38.4% 9.3% 0.6% |
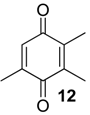 | 1216 | 150(M+,100), 122(35), 121(19), 107(55), 96(11), 79(39), 77(13), 68(29), 54(16), 53(14) | Pachylospeleus strinati Multumbo terrenus Pachylus paessleri Bourguyia trochanteralis Mischonyx cuspidatus Liogonyleptoides tetracanthus | 39.7% 27.6% 27.2% 15.8% 1.5% 0.1% |
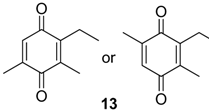 | 1280 | 164(M+,100), 136(23), 135(13), 121(82), 93(24), 91(15), 77(13), 68(18), 67(13) | Multumbo terrenus Pachylus paessleri Bourguyia trochanteralis | 8.9% 2.0% 1.3% |
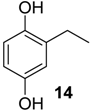 | 1409 * | 138(M+,58), 123(100), 107(4), 95(6), 67(10) | Chavesincola inexpectabilis | 4.6% |
 | 1433 * | 138(M+,100), 137(29), 123(50), 95(12), 91(13) | Pachylus paessleri Bourguyia trochanteralis Liogonyleptoides tetracanthus Mischonyx cuspidatus Discocyrtus oliverioi | 1.1% 0.8% 0.8% 0.9% 0.5% |
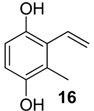 | 1467 * | 150(M+,100), 149(21), 121(17), 107(37), 77(15) | Discocyrtus oliverioi Liogonyleptoides tetracanthus | 0.4% 0.1% |
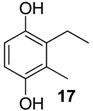 | 1487 * | 152(M+,53), 151(11), 138(9), 137(100), 107(8), 79(10), 77(8) | Liogonyleptoides tetracanthus Discocyrtus oliverioi | 0.5% 0.3% |
 | 1138 | 122(M+,100), 121(42), 107(96), 91(21), 79(15), 77(27) | Progonyleptoidellus striatus Mitopernoides variabilis | 67.4% 24.5% |
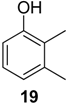 | 1172 * | 122(M+,91), 121(32), 107(100), 91(19), 79(16), 77(29) | Progonyleptoidellus striatus | 1.3% |
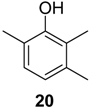 | 1220 | 136(M+,81), 135(20), 121(100), 91(26), 77(13) | Metarthrodes longipes Progonyleptoidellus striatus | 97.1% 31.3% |
 | 1230 | 136(M+,43), 121(100), 91(15), 77(13) | Mitopernoides variabilis | 75.5% |
| (C4H9)-phenol (22) | 1315 * | 150(M+,48), 135(100) | Metarthrodes longipes | 2.9% |
 |  | |||
|---|---|---|---|---|
| C | δH | δCa | δH | δCb |
| 1 | - | n.d. c (C) | - | 187.5 (C) |
| 2 | - | n.d. c (C) | - | 150.9 (C) |
| 3 | 6.62 (1H, m) | 133.4 (CH) | 6.57 (1H, m) | 131.7 (CH) |
| 4 | - | n.d. c (C) | - | 187.9 (C) |
| 5 | 6.71 (1H, dd, 3J = 10 Hz; 4J = 2.25 Hz) | 136.5 (CH) | 6.71 (1H, dd, 3J = 10 Hz; 4J = 2.25 Hz) | 136.3 (CH) |
| 6 | 6.77 (1H, d, 3J = 10 Hz) | 136.6 (CH) | 6.77 (1H, d, 3J = 10 Hz) | 136.8 (CH) |
| 7 | 2.07 (3H, d, 4J = 1,5 Hz) | 15.8 (CH3) | 2.47 (2H, qd, 3J = 7.5 Hz; 4J = 1.5 Hz) | 22.1 (CH2) |
| 8 | - | - | 1.15 (3H, t, 3J = 7.5 Hz) | 11.6 (CH3) |
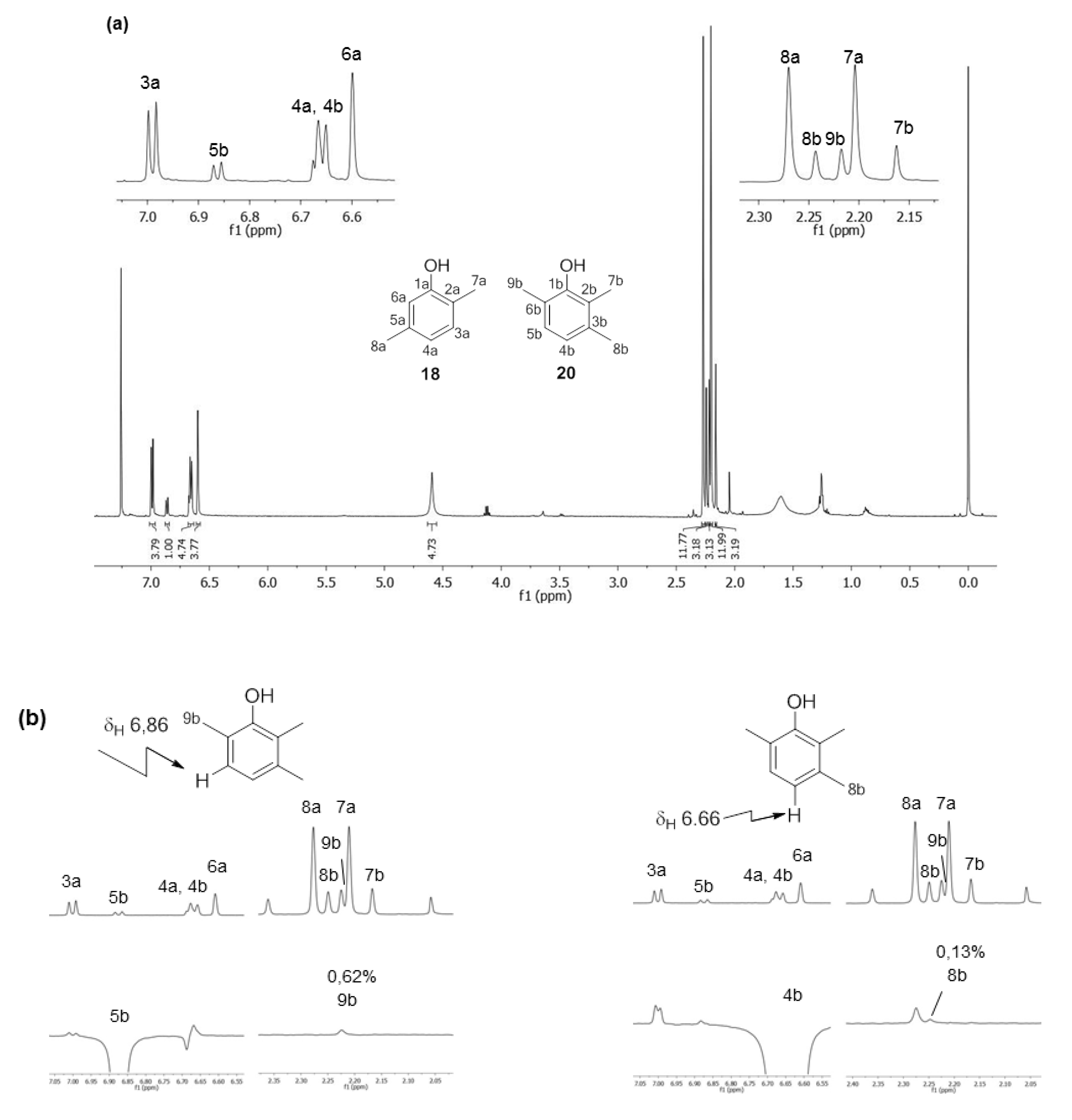
2.1.2. Phenols Identification

 |  | |||
|---|---|---|---|---|
| Х | δH | δCa | δH | δCa |
| 1 | - | 153.8 (C) | - | 152.1 (C) |
| 2 | - | 120.6 (C) | - | n.d. b |
| 3 | 6.99 (1H, d, 3J = 7.6 Hz) | 131.0 (CH) | - | n.d. b |
| 4 | 6.66 (1H, d, 3J = 7.6 Hz) | 121.6 (CH) | 6.66 (1H, d, 3J = 7.6 Hz) | 121.9 (CH) |
| 5 | - | 137.3 (C) | 6.86 (1H, d, 3J = 7.6 Hz) | 127.6 (CH) |
| 6 | 6.60 (1H, s) | 115.8 (CH) | - | n.d. b |
| 7 | 2.20 (3H, s) | 15.5 (CH3) | 2.16 (3H, s) | 11.9 (CH3) |
| 8 | 2.27 (3H, s) | 21.2 (CH3) | 2.24 (3H, s) | 20.2 (CH3) |
| 9 | - | - | 2.22 (3H, s) | 16.1 (CH3) |
2.2. Antimicrobial Activity of Harvestman Benzoquinones and Phenols
| Microorganism | MIC (µg/mL) | ||
|---|---|---|---|
 | 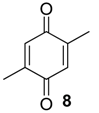 | 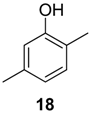 | |
| Bacillus pumilus | <125 | <125 | 250 |
| Pseudomonas aeruginosa | <125 | <125 | 1000 |
| Candida albicans | 125 | <82.5 | >500 |
| Rhodotorula glutinis | <82.5 | <82.5 | <82.5 |
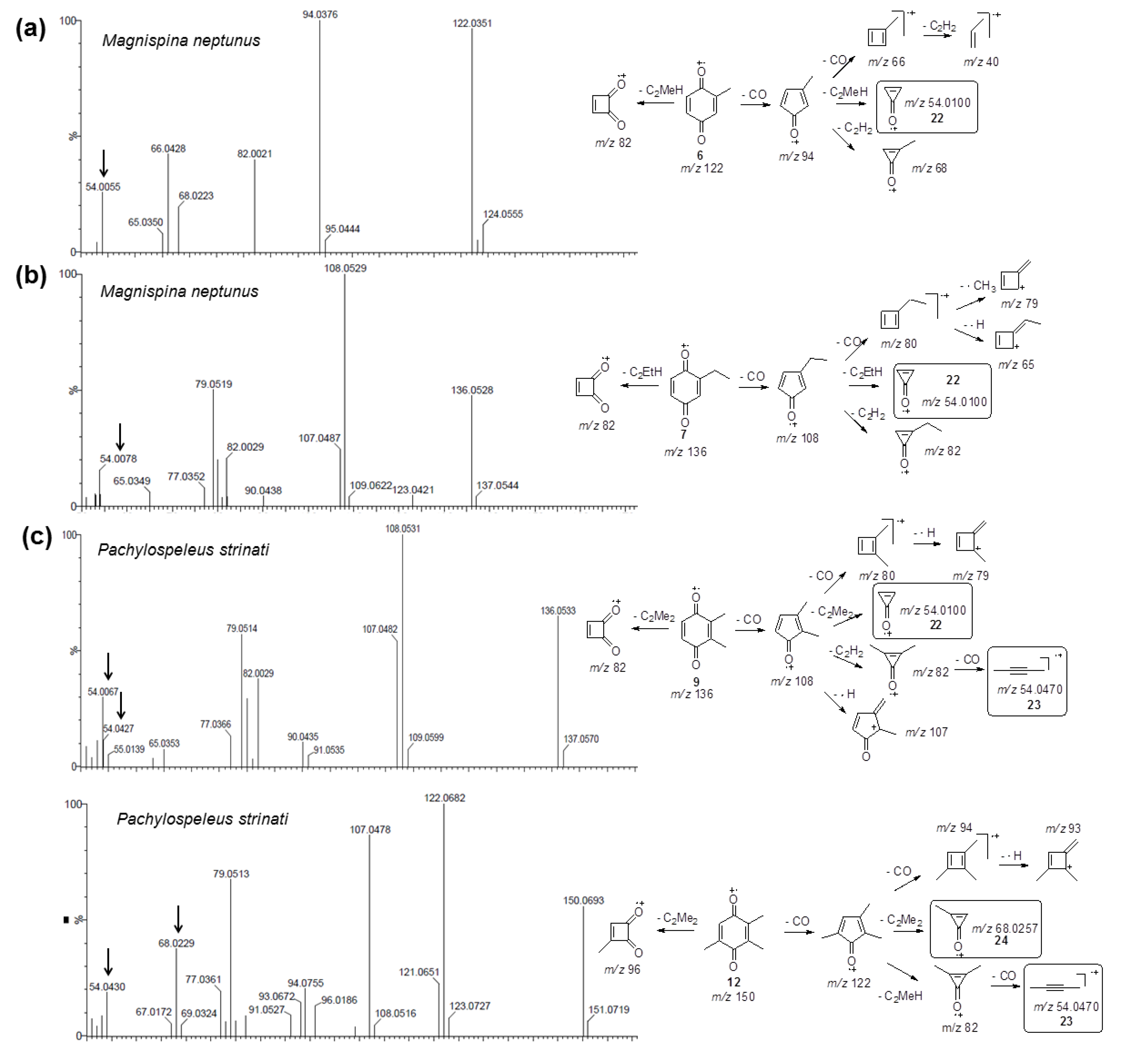
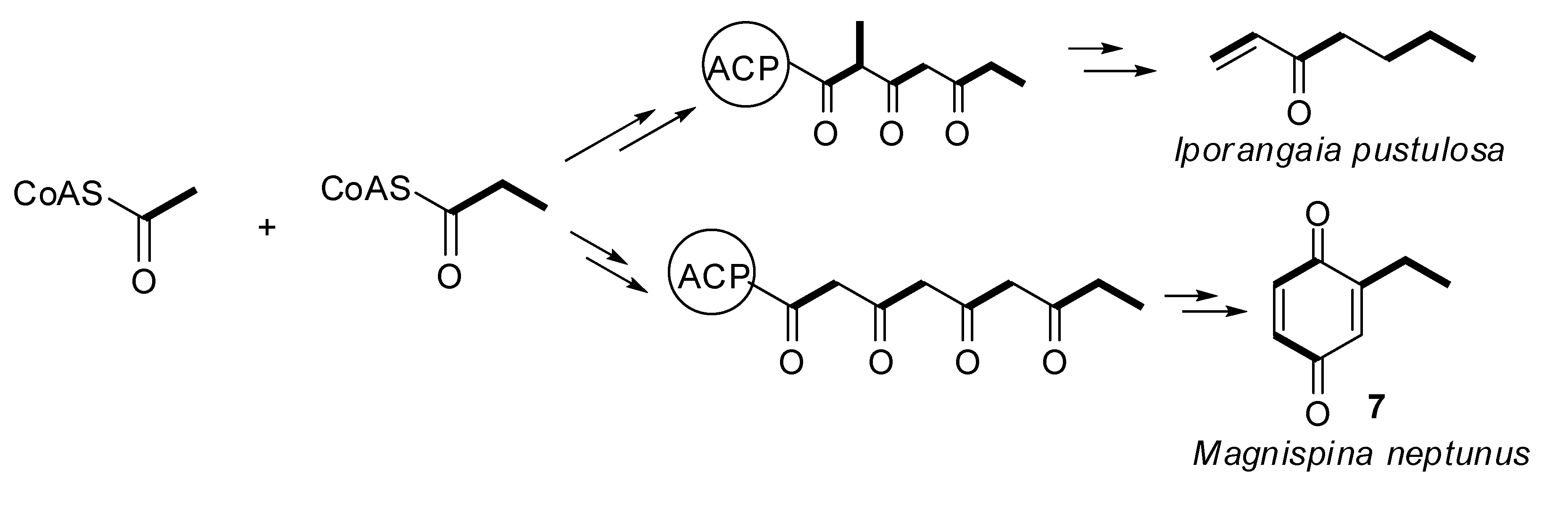
2.3. Biosynthetic Study of Magnispina neptunus Benzoquinone
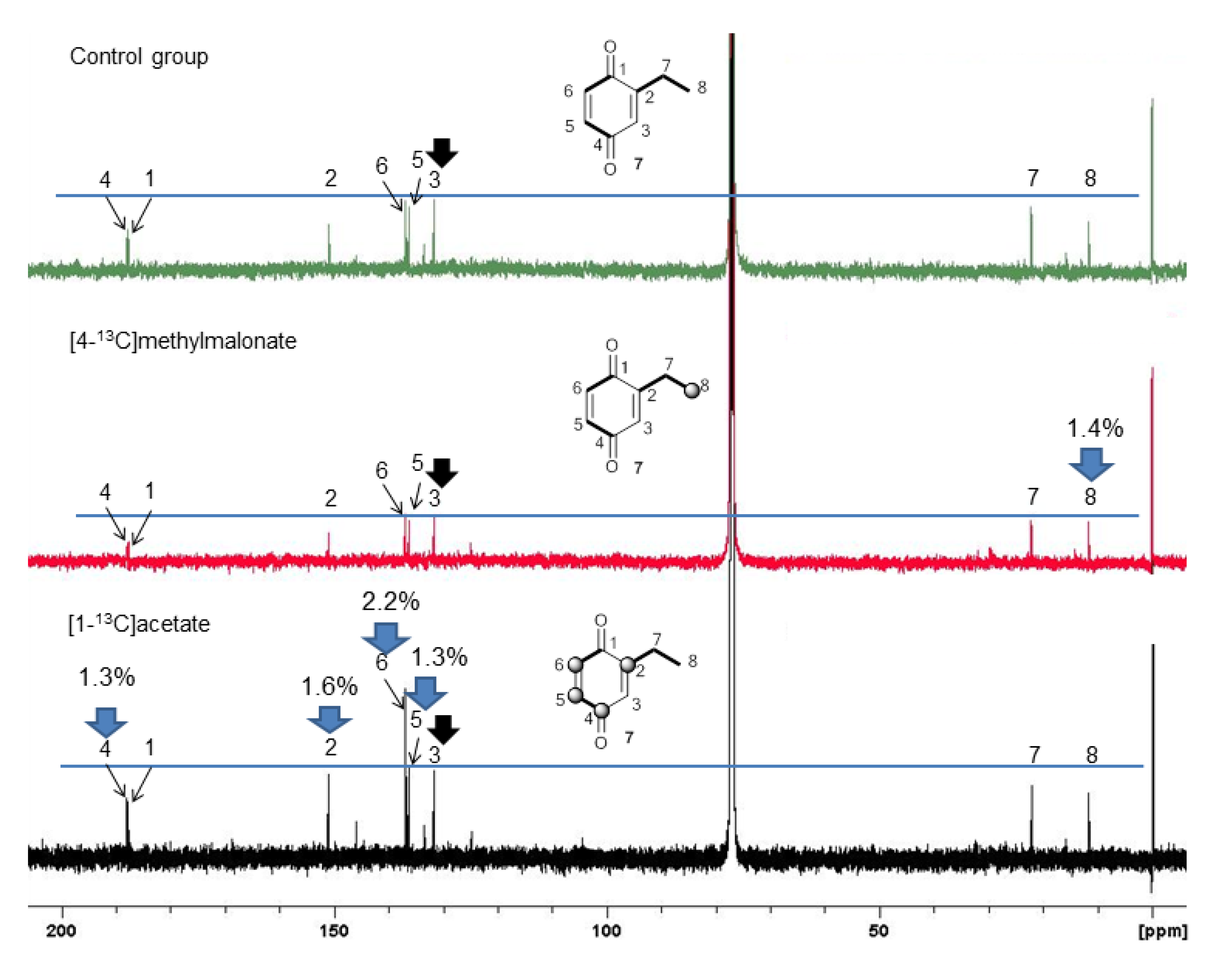
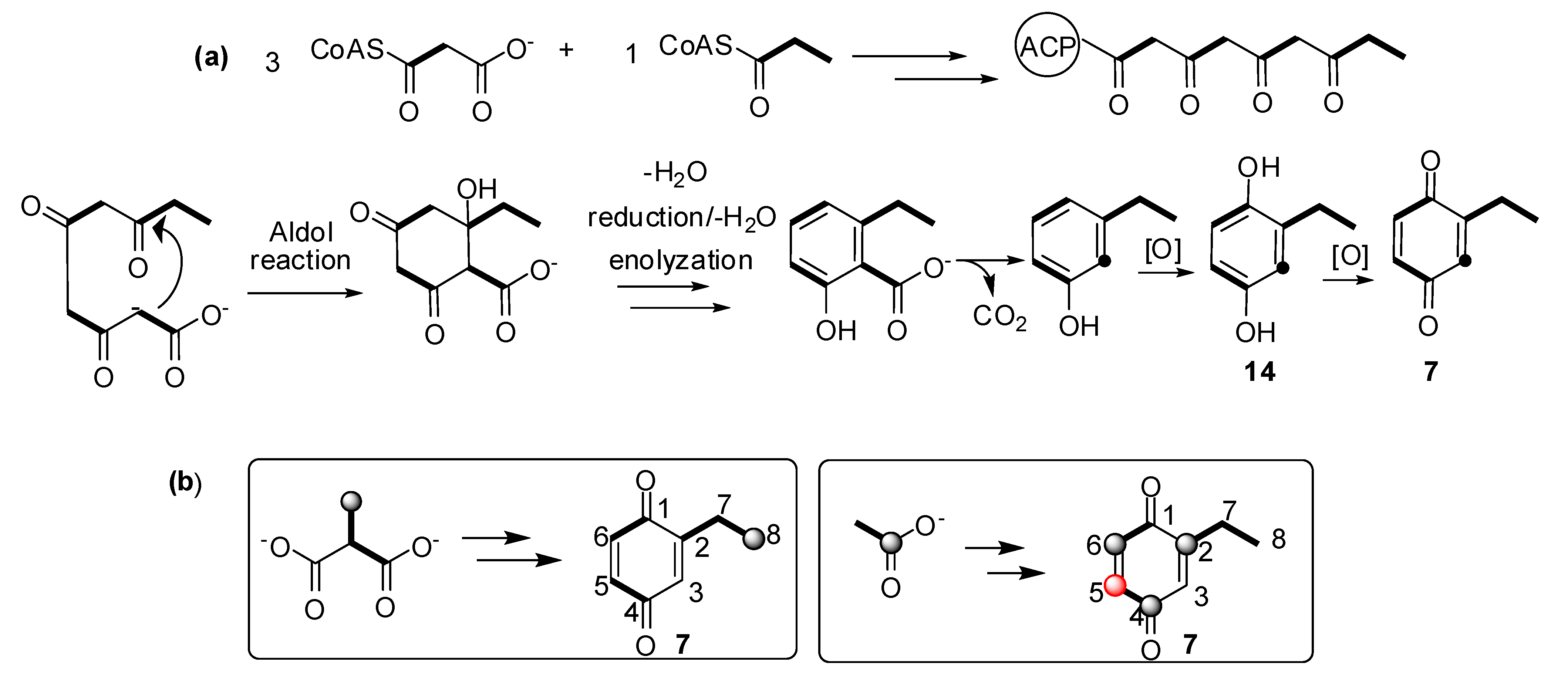
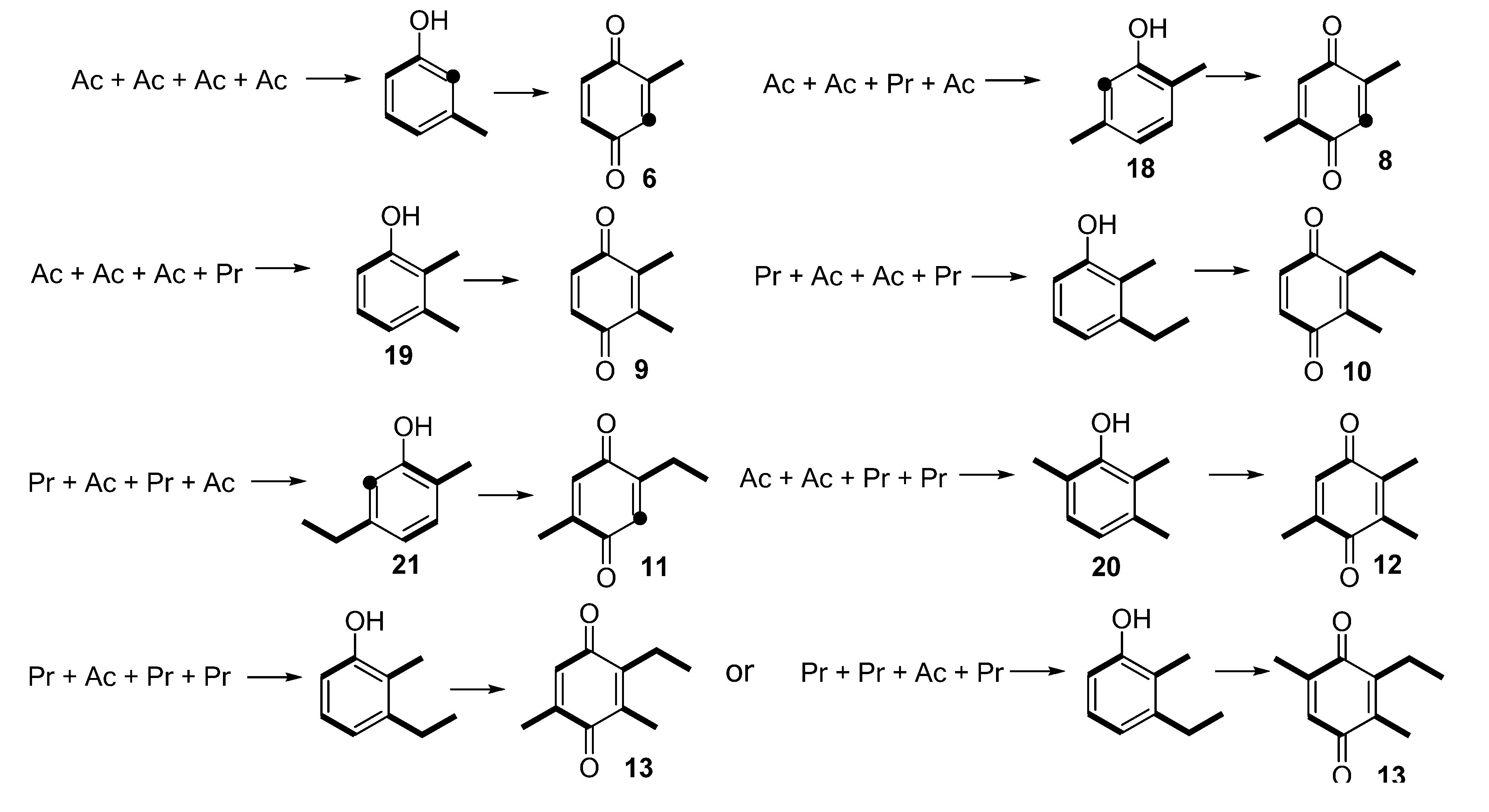
3. Experimental
3.1. Chemical Profile of Harvestman Exudates
3.1.1. General Methods
3.1.2. Collection of Individuals
| Species | Locality | Number of individuals |
|---|---|---|
| BOURGOUYINAE | ||
| Bourguyia trochanteralis | Cananéia, São Paulo, SE Brazil | 22 |
| CAELOPYGINAE | ||
| Metarthrodes longipes | Ubatuba, São Paulo, SE Brazil | 3 |
| GONYLEPTINAE | ||
| Liogonyleptoides tetracanthus | Linhares, Espírito Santo, SE Brazil | 9 |
| Mischonyx cuspidatus | Campinas, São Paulo, SE Brazil | 29 |
| HERNANDARIINAE | ||
| Multumbo terrenus | Teresópolis, Rio de Janeiro, SE Brazil | 30 |
| HETEROPACHYLINAE | ||
| Chavesincola inexpectabilis | Santa Tereza, Espírito Santo, SE Brazil | 31 |
| Magnispina neptunus | Arraial D’Ajuda, Bahia, NE Brazil | 20 |
| PACHYLINAE | ||
| Discocyrtus oliverioi | Campinas, São Paulo, SE Brazil | 11 |
| Pachylus paessleri | San Carlos de Apoquindo, Santiago, Chile | 24 |
| PACHYLOSPELEINAE | ||
| Pachylospeleus strinati | Iporanga, São Paulo, SE Brazil | 34 |
| PROGONYLEPTOIDELINAE | ||
| Mitopernoides variabilis | Ubatuba, São Paulo, SE Brazil | 9 |
| Progonyleptoidellus striatus | Santo André, São Paulo, SE Brazil | 10 |
3.2. Antimicrobial Activity
3.3. Biosynthetic Study of Magnispina neptunus
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Machado, G.; Pinto-da-Rocha, R.; Giribet, G. What are Harvestmen. In Harvestmen: The Biology of Opiliones; Pinto-Da-Rocha, R., Machado, G., Giribet, G., Eds.; Harvard University Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Raspotnig, G. Scent gland chemistry and chemosystematics in harvestmen. Biol. Serbica 2012, 34, 5–18. [Google Scholar]
- Hara, M.R.; Cavalheiro, A.J.; Gnaspini, P.; Santos, D.Y.A.C. A comparative analysis of the chemical nature of defensive secretions of Gonyleptidae (Arachnida: Opiliones: Laniatores). Biochem. Syst. Ecol. 2005, 33, 1210–1225. [Google Scholar] [CrossRef]
- Rocha, D.F.O.; Hamilton, K.; Gonçalves, C.C.S.; Machado, G.; Marsaioli, A.J. 6-Alkyl-3,4-dihydro-2H-pyrans: Chemical secretion compounds in neotropical harvestmen. J. Nat. Prod. 2011, 74, 658–663. [Google Scholar] [CrossRef]
- Wouters, F.C.; Rocha, D.F.O.; Gonçalves, C.C.S.; Machado, G.; Marsaioli, A.J. Additional vinyl ketones and their pyranyl ketones in gonyleptid harvestmen (Arachnida: Opiliones) suggest that the hetero-Diels-Alder reaction is widespread in this family. J. Nat. Prod. 2013. [Google Scholar] [CrossRef]
- Eisner, T.; Jones, T.H.; Hicks, K.; Silberglied, R.E.; Meinwald, J. Quinones and phenols in the defensive secretions of neotropical opilionids. J. Chem. Ecol. 1977, 3, 321–329. [Google Scholar] [CrossRef]
- Duffield, R.M.; Olubajo, O.; Wheeler, J.W.; Shear, W.A. Alkylphenols in the defensive secretion of the nearctic opilionid, Stygnomma spinifera (Arachnida: Opiliones). J. Chem. Ecol. 1981, 7, 445–452. [Google Scholar] [CrossRef]
- Acosta, L.E.; Poretti, T.I.; Mascarelli, P.E. The defensive secretions of Pachyloidellus goliath (Opiliones, Laniatores, Gonyleptidae). Bonn. Zool. Beitr. 1993, 44, 19–31. [Google Scholar]
- Machado, G.; Pomini, A.M. Chemical and behavioral defenses of the neotropical harvestman Camarana flavipalpi (Arachnida: Opiliones). Biochem. Syst. Ecol. 2008, 36, 369–376. [Google Scholar] [CrossRef]
- Pomini, A.M.; Machado, G.; Pinto-da-Rocha, R.; Macías-Ordóñez, R.; Marsaioli, A.J. Lines of defense in the harvestman Hoplobunus mexicanus (Arachnida: Opiliones): Aposematism, stridulation, thanatosis, and irritant chemicals. Biochem. Syst. Ecol. 2010, 38, 300–308. [Google Scholar] [CrossRef]
- Estable, C.; Ardao, M.I.; Brasil, N.P.; Fieser, L.F. Gonyleptidine. J. Am. Chem. Soc. 1955, 77, 4942. [Google Scholar]
- Eisner, T.; Rossini, C.; Gonzalez, A.; Eisner, M. Chemical defense of an opilionid (Acanthopachylus aculeatus). J. Exp. Biol. 2004, 207, 1313–1321. [Google Scholar] [CrossRef]
- Machado, G.; Carrera, P.C.; Pomini, A.M.; Marsaioli, A.J. Chemical defense in harvestmen (Arachnida, Opiliones): Do benzoquinone secretions deter invertebrate and vertebrate predators? J. Chem. Ecol. 2005, 31, 2519–2539. [Google Scholar] [CrossRef]
- Föttinger, P.; Acosta, L.E.; Leis, H.; Raspotnig, G. Benzoquinone-rich exudates from the harvestman Pachylus paessleri (Opiliones: Gonyleptidae: Pachylinae). J. Arachnol. 2010, 38, 584–587. [Google Scholar] [CrossRef]
- Blum, M.S. Biosynthesis of arthropods exocrine compounds. Ann. Rev. Entomol. 1987, 32, 381–413. [Google Scholar] [CrossRef]
- Abraham, I.; Joshi, R.; Pardasani, P.; Pardasani, R.T. Recent advances in 1,4-benzoquinone chemistry. J. Braz. Chem. Soc. 2011, 22, 385–421. [Google Scholar] [CrossRef]
- Schildknecht, H.; Holoubek, K. Die bombardierkafer und ihre explosionschemie. Angew. Chem. 1961, 73, 1–7. [Google Scholar] [CrossRef]
- Eisner, T.; Jones, T.H.; Aneshansley, D.J.; Tschinkel, V.R.; Silberglied, R.E.; Meinwald, J. Chemistry of defensive secretions of bombardier beetles (Brachinini, Metriini, Ozaenini, Paussini). J. Insect Physiol. 1977, 23, 1383–1386. [Google Scholar] [CrossRef]
- Happ, G.M. Quinone and hydrocarbon production in the defensive glands of Eleodes longicollis and Tribolium castaneum (Coleoptera, Tenebrioidae). J. Insect Physiol. 1968, 14, 1821–1837. [Google Scholar] [CrossRef]
- Ikanl, R.; Cohen, E.; Shulov, A. Benzo- and hydroquinones in the defense secretions of Blaps sulcata and Blaps wiedemanni. J. Insect Physiol. 1970, 16, 2201–2206. [Google Scholar] [CrossRef]
- Eisner, T.; Rossini, C.; Eisner, M. Chemical defense of an earwig (Doru taeniatum). Chemoecology 2000, 10, 81–87. [Google Scholar] [CrossRef]
- Olagbemiro, T.O.; Lajide, L.; Sani, K.M.; Staddon, B.W. 2-Hydroxy-5-methyl-l,4-benzoquinone from the salivary gland of the soldier termites Odontotermes magdalenae. Experientia 1988, 44, 1022–1024. [Google Scholar] [CrossRef]
- Ruther, J.; Podsiadlowski, L.; Hilker, M. Quinones in cockchafers: Additional function of a sex attractant as an antimicrobial agent. Chemoecology 2001, 11, 225–229. [Google Scholar] [CrossRef]
- Rocha, D.F.O.; Wouters, F.C.; Machado, G.; Marsaioli, A.J. Alternative sources of propionate and methylmalonate in the biosynthesis of a vinyl ketone in the defensive secretion of an arachnid. Sci. Reports 2013. submitted for publication. [Google Scholar]
- Pankewitz, F.; Hilker, M. Polyketides in insects: Ecological role of these widespread chemicals and evolutionary aspects of their biogenesis. Biol. Rev. 2008, 83, 209–226. [Google Scholar] [CrossRef]
- Gross, J.H. Mass Spectrometry—A Textbook, 2nd ed.; Springer-Verlag: Heidelberg, Germany, 2011. [Google Scholar]
- El-Najar, N.; Gali-Muhtasib, H.; Ketola, R.A.; Vuorela, P.; Urtti, A.; Vuorela, H. The chemical and biological activities of quinones: Overview and implications in analytical detection. Phytochem. Rev. 2011, 10, 353–370. [Google Scholar] [CrossRef]
- Cole, L.K.; Blum, M.S.; Roncadori, R.W. Antifungal properties of the insect alarm pheromones, citral, 2-heptanone, and 4-methyl-3-heptanone. Mycologia 1975, 67, 701–708. [Google Scholar]
- Shapiro, S.; Guggenheim, B. The action of thymol on oral bacteria. Oral Microbiol. Immunol. 1995, 10, 241–246. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction, 10th ed.; Pearson Benjamin Cummings: San Francisco, CA, USA, 2010. [Google Scholar]
- Greenberg, M.; Dodds, M.; Tian, M. Naturally occurring phenolic antibacterial compounds show effectiveness against oral bacteria by a quantitative structure-activity relationship study. J. Agric. Food Chem. 2008, 56, 11151–11156. [Google Scholar] [CrossRef]
- Morgan, E.D. Biosynthesis in Insects, Advanced ed.; RSC: Cambridge, UK, 2010. [Google Scholar]
- Halarnkar, P.P.; Chambers, J.D.; Blomquist, G.J. Metabolism of propionate to acetate in nine insect species. Comp. Biochem. Physiol. 1986, 84, 469–472. [Google Scholar]
- Meinwald, J.; Koch, K.F.; Rogers, J.E., Jr.; Eisner, T. Biosynthesis of arthropod secretions. III. Synthesis of simple p-benzoquinones in a beetle (Eleodes 1ongicollis). J. Am. Chem. Soc 1996, 88, 1590–1592. [Google Scholar]
- Sun, C.M.; Toia, R.F. Biosynthetic studies on ant metabolites of Mellein and 2,4-dihydroxyacetophenone from [1,2–13C2] acetate. J. Nat. Prod. 1993, 56, 953–956. [Google Scholar] [CrossRef]
- Holliday, A.E.; Walker, F.M.; Brodie, E.D., III; Formica, V.A. Differences in defensive volatiles of the forked fungus beetle, Bolitotherus cornutus, living on two species of fungus. J. Chem. Ecol. 2009, 35, 1302–1308. [Google Scholar]
- Eisner, T. The protective role of the spray mechanism of the Bombardier beetle. J. Insect Physiol. 1958, 2, 215–220. [Google Scholar] [CrossRef]
- Eisner, T.; Alsop, D.; Hicks, K.; Meinwald, J. Defensive Secretions of Millipeds. In Handbook of Experimental Pharmacology; Bettini, S., Ed.; Springer: Berlin, Germany, 1978; Volume 48, pp. 41–72. [Google Scholar]
- Deml, R.; Huth, A. Benzoquinones and hydroquinones in defensive secretions of tropical millipedes. Naturwissenschaften 2000, 87, 80–82. [Google Scholar] [CrossRef]
- Wu, X.; Buden, D.W.; Attygalle, A.B. Hydroquinones from defensive secretion of a giant Pacific millipede, Acladocricus setigerus (Diplopoda: Spirobolida). Chemoecology 2007, 17, 131–138. [Google Scholar] [CrossRef]
- Vujisić, L.V.; Makarov, S.E.; Ćurčić, B.P.M.; Ilić, B.S.; Tešević, V.V.; Gođevac, D.M.; Vučković, I.M.; Ćurčić, S.B.; Mitić, B.M. Composition of the defensive secretion in three species of european millipedes. J. Chem. Ecol. 2011, 37, 1358–1364. [Google Scholar]
- Halarnkar, P.P.; Chambers, J.D.; Wakayama, E.J.; Blomquist, G.J. Vitamin B12 levels and propionate metabolism in selected non-insect arthropods and other invertebrates. Comp. Biochem. Physiol. 1987, 88, 869–873. [Google Scholar]
- Chu, A.J.; Blomquist, G.J. Biosynthesis of hydrocarbons in insects: Succinate is a precursor of the methyl branched alkanes. Arch. Biochem. Biophys. 1980, 201, 304–312. [Google Scholar]
- Wakayama, E.D.; Dillwith, J.W.; Howard, R.W.; Blomquist, G.J. Vitamin B12 levels in selected insects. Insect Biochem. 1984, 14, 175–179. [Google Scholar]
- Halarnkar, P.P.; Blomquist, G.J. Comparative aspects of propionate metabolism. Comp. Biochem. Physiol. 1989, 92B, 227–231. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar]
- Schneider, B. Nuclear magnetic resonance spectroscopy in biosynthetic studies. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 51, 155–198. [Google Scholar]
- Maier, W.; Shneider, B.; Strack, D. Biosynthesis of sesquiterpenoid cyclohexenone derivatives in mycorrhizal barley roots proceeds via glyceraldehyde 3-phosphate/pyruvate pathway. Tetrahedron Lett. 1998, 39, 521–524. [Google Scholar] [CrossRef]
- Caetano, D.S.; Machado, G. The ecological tale of Gonyleptidae (Arachnida, Opiliones) evolution: Phylogeny of a Neotropical lineage of armoured harvestmen using ecological, behavioural and chemical characters. Cladistics 2013. [Google Scholar] [CrossRef]
- Cokendolpher, J.C.; Mitov, P.G. Natural Enemies. In Harvestmen: The Biology of Opiliones; Pinto-Da-Rocha, R., Machado, G., Giribet, G., Eds.; Harvard University Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Sample Availability: Samples of the compounds 6 and 8 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rocha, D.F.O.; Wouters, F.C.; Zampieri, D.S.; Brocksom, T.J.; Machado, G.; Marsaioli, A.J. Harvestman Phenols and Benzoquinones: Characterisation and Biosynthetic Pathway. Molecules 2013, 18, 11429-11451. https://doi.org/10.3390/molecules180911429
Rocha DFO, Wouters FC, Zampieri DS, Brocksom TJ, Machado G, Marsaioli AJ. Harvestman Phenols and Benzoquinones: Characterisation and Biosynthetic Pathway. Molecules. 2013; 18(9):11429-11451. https://doi.org/10.3390/molecules180911429
Chicago/Turabian StyleRocha, Daniele F. O., Felipe C. Wouters, Dávila S. Zampieri, Timothy J. Brocksom, Glauco Machado, and Anita J. Marsaioli. 2013. "Harvestman Phenols and Benzoquinones: Characterisation and Biosynthetic Pathway" Molecules 18, no. 9: 11429-11451. https://doi.org/10.3390/molecules180911429
APA StyleRocha, D. F. O., Wouters, F. C., Zampieri, D. S., Brocksom, T. J., Machado, G., & Marsaioli, A. J. (2013). Harvestman Phenols and Benzoquinones: Characterisation and Biosynthetic Pathway. Molecules, 18(9), 11429-11451. https://doi.org/10.3390/molecules180911429




