Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization
Abstract
:1. Introduction
2. Results and Discussion
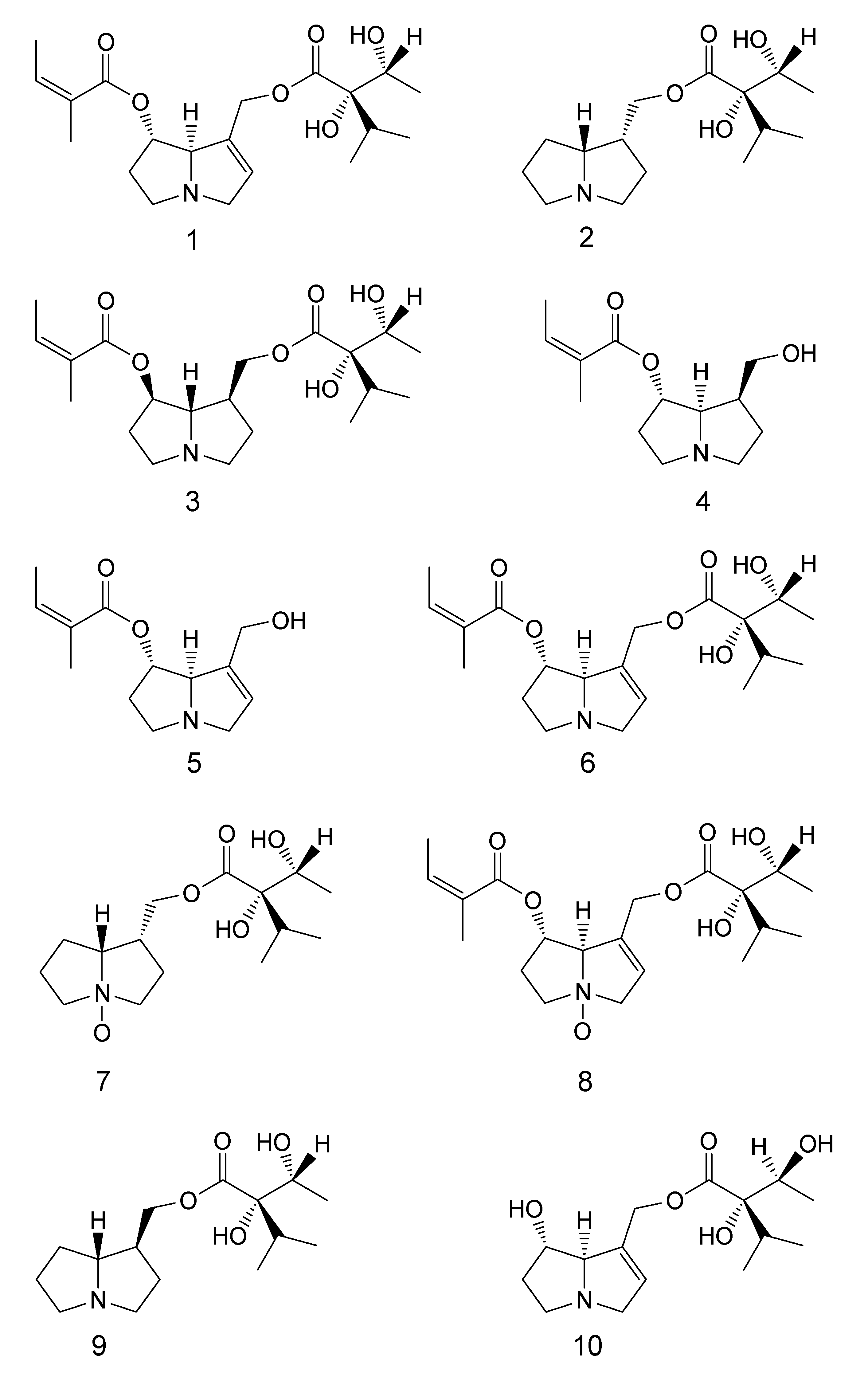
| Compound number | IUPAC name | Common name |
|---|---|---|
| 1 | (Z)-((1S,7aR)-7-(((2S,3R)-2,3-dihydroxy-2-isopropylbutanoyloxy)methyl)-2,3,5,7a-tetrahydro-1H-pyrrolizin-1-yl)2-methylbut-2-enoate | 7-Angeloyl-9-(+)-trachelanthylheliotridine |
| 2 | (2S,3R)-((1R,7aR)-hexahydro-1H-pyrrolizin-1-yl)methyl2,3-dihydroxy-2-isopropylbutanoate | Lindelofine |
| 3 | (Z)-((1R,7S,7aS)-7-(((2S,3R)-2,3-dihydroxy-2-isopropylbutanoyloxy)methyl)hexahydro-1H-pyrrolizin-1-yl) 2-methylbut-2-enoate | Punctanecine |
| 4 | (Z)-((1S,7S,7aR)-7-(hydroxymethyl)hexahydro-1H-pyrrolizin-1-yl) 2-methylbut-2-enoate | 7-Angeloyl heliotridane |
| 5 | (Z)-((1S,7aR)-7-(hydroxymethyl)-2,3,5,7a-tetrahydro-1H-pyrrolizin-1-yl) 2-methylbut-2-enoate | 7-Angeloyl heliotridine |
| 6 | (Z)-((1S,7aR)-7-(((R)-2,3-dihydroxy-2-((R)-1-hydroxyethyl)-3-methylbutanoyloxy)methyl)-2,3,5,7a-tetrahydro-1H-pyrrolizin-1-yl) 2-methylbut-2-enoate | Heliosupine |
| 7 | (2S,3R)-((1R,7aR)-hexahydro-1H-pyrrolizin-1-yl)methyl2,3-dihydroxy-2-isopropylbutanoate-N-oxide | Lindelofine- N-oxide |
| 8 | (Z)-((1S,7aR)-7-(((R)-2,3-dihydroxy-2-((R)-1-hydroxyethyl)-3-methylbutanoyloxy)methyl)-2,3,5,7a-tetrahydro-1H-pyrrolizin-1-yl) 2-methylbut-2-enoate-N-oxide | Heliosupine- N-oxide |
| 9 | (2S,3R)-((1S,7aR)-hexahydro-1H-pyrrolizin-1-yl)methyl 2,3-dihydroxy-2-isopropylbutanoate | 9-(+)-Trachelanthyl-laburnine |
| 10 | (2S,3R)-((1S,7aR)-1-hydroxy-2,3,5,7a-tetrahydro-1H-pyrrolizin-7-yl)methyl 2,3-dihydroxy-2-isopropylbutanoate | Echinatine |
| PA | Jun 2007 dry aerial parts | May 2008 dry aerial parts | May 2008 dry roots | July 2009 dry seeds |
|---|---|---|---|---|
| 1 | 37.2 | |||
| 2 | 233.3 | 141.6 | 66.9 | |
| 3 | 7.1 | |||
| 4 | 30.6 | |||
| 5 | 5.7 | |||
| 6 | 143.8 | 110 | 233.8 | |
| 7 | 2051.0 | 113.2 | 94.4 | |
| 8 | 238.6 | 270.2 | 130.3 | 62.5 |
| 9 | 91.9 | |||
| 10 | 63.8 |
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation of PAs
3.3.1. Harvest I (June 2007)
3.3.2. Harvest II (May 2008)
3.3.3. Harvest III (July 2009)
3.4. Compound Characterization
3.4.1. Compound 1
3.4.2. Compound 2
3.4.3. Compound 3
3.4.4. Compound 4
3.4.5. Compound 5
3.4.6. Compound 6
3.4.7. Compound 7
3.4.8. Compound 8
3.4.9. Compound 9
3.4.10. Compound 10
3.5. Extraction of Fatty Acids and GC-MS/FID Analyses
3.6. Tubulin Polymerization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Langel, D.; Ober, D.; Pelser, P. The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochem. Rev. 2011, 10, 3–74. [Google Scholar] [CrossRef]
- Mandic, B.; Godevac, D.; Beskoski, V.; Simic, M.; Trifunovic, S.; Tesevic, V.; Vajs, V.; Milosavljevic, S. Pyrrolizidine alkaloids from seven Senecio species wild-growing in Serbia and Montenegro. J. Serbian Chem. Soc. 2009, 74, 27–34. [Google Scholar] [CrossRef]
- Mandic, B.; Godevac, D.; Vujsic, L.J.; Trifunovic, S.; Tesevic, V.; Vajs, V.; Milosavljevic, S. Semiquinol and phenol compounds from seven Senecio species. Chem. Papers 2011, 65, 90–92. [Google Scholar] [CrossRef]
- Christov, V.; Kostova, N.; Evstatieva, L. 6-Angeloylplatynecine: A new alkaloid from Senecio nemorensis subsp. fuchsii (C.C. Gmelin) celak. Nat. Prod. Res. 2005, 19, 389–392. [Google Scholar]
- Kostova, N.; Christov, V.; Cholakova, M.; Nikolova, E.; Evstatieva, L. Pyrrolizidine alkaloids from Bulgarian species of the genus Senecio. J. Serbian Chem. Soc. 2006, 71, 1275–1280. [Google Scholar] [CrossRef]
- Dreger, M.; Stanisławska, M.; Krajewska-Patan, M.; Mielcarek, S.; Łukasz Mikołajczak, P.; Buchwald, W. Pyrrolizidine alkaloids—Chemistry, biosynthesis, pathway, toxicity, safety and perspectives of medicinal usage. Herba Pol. 2009, 55, 127–147. [Google Scholar]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar]
- Roeder, E. Analysis of pyrrolizidine alkaloids. Curr. Org. Chem. 1999, 3, 557–576. [Google Scholar] [CrossRef]
- Ivanova, A.; Serly, J.; Christov, V.; Stamboliyska, B.; Molnar, J. Alkaloids derived from genus Veratrum and Peganum of Mongolian origin as multidrug resistance inhibitors of cancer cells. Fitoterapia 2011, 82, 570–575. [Google Scholar] [CrossRef]
- Cheeke, P.R. Alkaloids. In Toxicants of Plant Origin; CRC Press: Boca Raton, FL, USA, 1989; Volume 1. [Google Scholar]
- Bigazzi, M.; Nardi, E.; Selvi, F. Palynological contribution to the systematics of Rindera and the allied genera Paracaryum and Solenanthus (Boraginaceae-Cynoglosseae). Willdenowia 2006, 36, 37–46. [Google Scholar] [CrossRef]
- Smith, L.W.; Culvenor, C.J. Plant sources of hepatoxicpyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Singh, P.; Rathinasamy, K.; Mohan, R.; Panda, D. Microtubule assembly dynamics: An attractive target for anticancer drugs. IUBMB Life 2008, 60, 368–375. [Google Scholar] [CrossRef]
- Kingston, D.G.I. Tubulin-interactive natural products as anticancer agents. J. Nat. Prod. 2009, 72, 507–515. [Google Scholar] [CrossRef]
- Yuldasheva, N.K.; Ul’chenko, N.T.; Glushenkova, A.I. Lipids from fruit of Rindera oblongifolia. Chem. Nat. Compd. 2012, 47, 981–982. [Google Scholar] [CrossRef]
- Velasco, L.; Goffman, F.D. Chemotaxonomic significance of fatty acids and tocopherols in Boraginaceae. Phytochemistry 1999, 52, 423–426. [Google Scholar] [CrossRef]
- Taghreed, A.I. Chemical composition and biological activity of extracts from Salvia bicolor Desf. growing in Egypt. Molecules 2012, 17, 11315–11334. [Google Scholar] [CrossRef]
- Mandana, B.; Russly, A.R.; Farah, S.T.; Noranizan, M.A.; Md. Zaidul, I.S.; Ali, G. Supercritical carbon dioxide extraction of eeed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules 2013, 18, 997–1014. [Google Scholar] [CrossRef]
- Sousa, A.; Casal, S.; Bento, A.; Malheiro, R.; Oliveira, M.B.; Pereira, J.A. Chemical characterization of “Alcaparras” stoned table olives from northeast Portugal. Molecules 2011, 16, 9025–9040. [Google Scholar] [CrossRef]
- Logie, C.; Grue, M.; Liddel, J. Proton NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1994, 37, 43–109. [Google Scholar] [CrossRef]
- Roeder, E. Carbon-13 NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1990, 29, 11–29. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine Alkaloids—Genotoxicity, metabolism; enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar]
- Saburo, K.; Sei-ichi, U. A new unsaturated fat acid, C10H18O2, present in the oil of Rindera obtusiloda. Bull. Chem. Soc. Jpn. 1937, 12, 226. [Google Scholar] [CrossRef]
- Miller, R.W.; Earle, F.R.; Wolff, I.A.; Barclay, A.S. Search for new seed oils. XV. Oils of Boraginaceae. Lipids 1968, 3, 443–445. [Google Scholar]
- Gaskin, F.; Cantor, C.R.; Shelanski, M.L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J. Mol. Biol. 1974, 89, 737–755. [Google Scholar] [CrossRef]
- Pesic, M.; Brankovic, J.; Aljancic, I.; Todorovic, N.; Jadranin, M.; Vajs, V.; Tesevic, V.; Vuckovic, I.; Momcilovic, M.; Markovic, I.; et al. New anti-cancer characteristics of jatrophane diterpenes from Euphorbia dendroides. Food Chem. Toxicol. 2011, 49, 3165–3173. [Google Scholar] [CrossRef]
- Shelanski, M.L.; Gaskin, F.; Cantor, C.R. Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA 1973, 70, 765–768. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mandić, B.M.; Simić, M.R.; Vučković, I.M.; Vujisić, L.V.; Novaković, M.M.; Trifunović, S.S.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; Milosavljević, S.M. Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization. Molecules 2013, 18, 10694-10706. https://doi.org/10.3390/molecules180910694
Mandić BM, Simić MR, Vučković IM, Vujisić LV, Novaković MM, Trifunović SS, Nikolić-Mandić SD, Tešević VV, Vajs VV, Milosavljević SM. Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization. Molecules. 2013; 18(9):10694-10706. https://doi.org/10.3390/molecules180910694
Chicago/Turabian StyleMandić, Boris M., Milena R. Simić, Ivan M. Vučković, Ljubodrag V. Vujisić, Miroslav M. Novaković, Snežana S. Trifunović, Snežana D. Nikolić-Mandić, Vele V. Tešević, Vlatka V. Vajs, and Slobodan M. Milosavljević. 2013. "Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization" Molecules 18, no. 9: 10694-10706. https://doi.org/10.3390/molecules180910694
APA StyleMandić, B. M., Simić, M. R., Vučković, I. M., Vujisić, L. V., Novaković, M. M., Trifunović, S. S., Nikolić-Mandić, S. D., Tešević, V. V., Vajs, V. V., & Milosavljević, S. M. (2013). Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization. Molecules, 18(9), 10694-10706. https://doi.org/10.3390/molecules180910694






