Phytochemical, Antimicrobial and Antiprotozoal Evaluation of Garcinia Mangostana Pericarp and α-Mangostin, Its Major Xanthone Derivative
Abstract
:1. Introduction
2. Results and Discussion
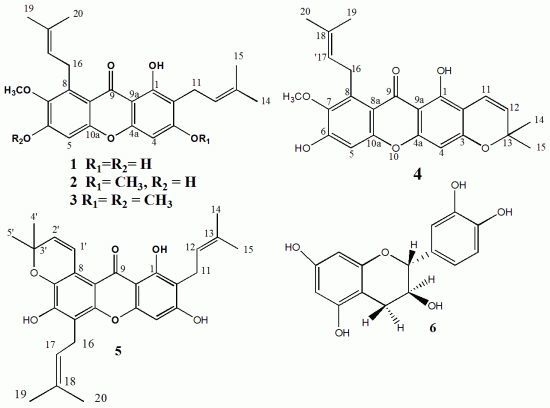
2.1. Phytochemical Study
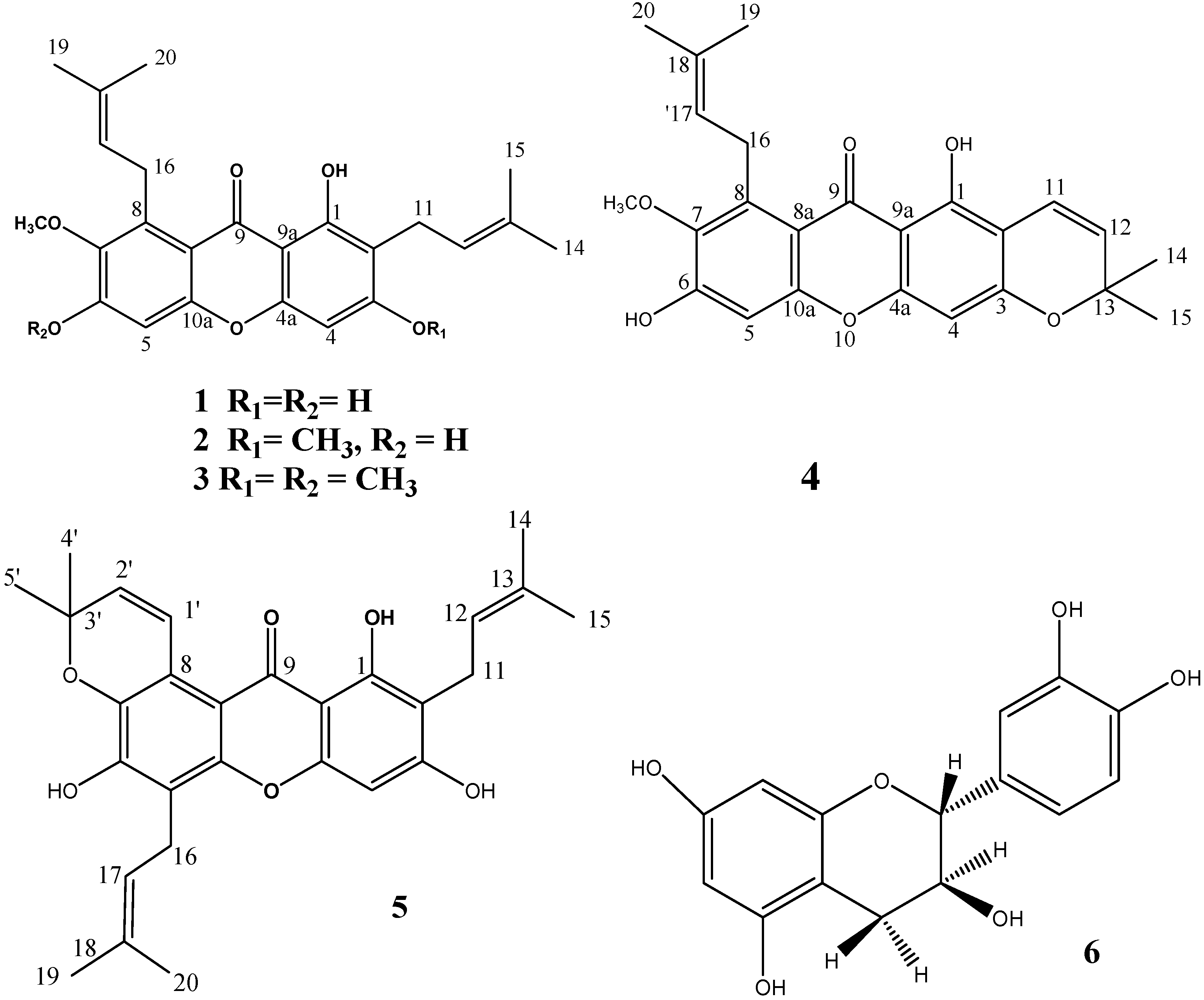
| Position | Compound 1 1 | Compound 2 2 | Compound 3 2 | Compound 4 2 | Compound 5 2 |
|---|---|---|---|---|---|
| 1 | 13.72, s | 13.42, s | 13.44, s | 13.72, s | 13.79, s |
| 4 | 6.28, s | 6.24, s | 6.30, s | 6.26, s | 6.37, s |
| 5 | 6.80, s | 6.74, s | 6.75, s | 6.85, s | − |
| 11 | 3.35, d (J = 7.3 Hz) | 3.37, d (J = 7.2 Hz) | 3.36, d (J = 7.1 Hz) | 6.74, d (J = 10.0 Hz) | 3.48, d (J = 6.0 Hz) |
| 12 | 5.17, t (J = 7.3 Hz) | 5.17, t (J = 7.2 Hz) | 5.26, t (J = 7.1 Hz) | 5.58, d (J = 10.0 Hz) | 5.31, t (J = 7.0 Hz) |
| 14 | 1.77, s | 1.75, s | 1.70, s | 1.27, s | 1.79, s |
| 15 | 1.63, s | 1.62, s | 1.71, s | 1.28, s | 1.71, s |
| 16 | 4.04, d (J = 7.0 Hz) | 4.09, d (J = 7.2 Hz) | 4.15, d (J = 7.2, Hz) | 4.10, d (J = 7.0 Hz) | 3.59, d (J = 6.0 Hz) |
| 17 | 5.17, t (J = 7.3 Hz) | 5.18, t (J = 7.2 Hz) | 5.26, t (J = 7.2 Hz) | 5.27, t (J = 7.3 Hz) | 5.31, t (J = 7.0 Hz) |
| 19 | 1.71, s | 1.61, s | 1.70, s | 1.71, s | 1.87, s |
| 20 | 1.73, s | 1.72, s | 1.82, s | 1.82, s | 1.89, s |
| 3-OMe | 3.82, s | 3.91, s | |||
| 6-OMe | 3.97, s | ||||
| 7-OMe | 3.71, s | 3.80, s | 3.82, s | 3.83, s | |
| 1' | 8.00, d (J = 10.0 Hz) | ||||
| 2' | 5.79, d (J = 10.0 Hz) | ||||
| 4' | 1.51, s | ||||
| 5' | 1.51, s |
| Position | Compound 1 1 | Compound 2 2 | Compound 3 2 | Compound 4 2 | Compound 5 2 |
|---|---|---|---|---|---|
| 1 | 159.9 | 159.7 | 157.9 | 157.8 | 160.44 |
| 2 | 109.9 | 111.5 | 109.6 | 104.4 | 108.4 |
| 3 | 162.3 | 163.5 | 161.5 | 159.8 | 161.6 |
| 4 | 92.3 | 88.8 | 86.7 | 94.0 | 93.4 |
| 4a | 154.5 | 154.4 | 153.4 | 156.1 | 155.3 |
| 5 | 101.8 | 101.5 | 96.3 | 101.6 | 115.2 |
| 6 | 156.6 | 155.6 | 156.1 | 154.5 | 151.0 |
| 7 | 143.4 | 142.5 | 142.1 | 142.7 | 135.8 |
| 8 | 136.3 | 137.0 | 135.2 | 136.9 | 136.5 |
| 8a | 112.2 | 112.3 | 112.9 | 112.1 | |
| 9 | 181.3 | 181.9 | 180.0 | 181.8 | 182.9 |
| 9a | 103.6 | 103.8 | 102.0 | 103.6 | 117.2 |
| 10a | 154.1 | 155.2 | 153.4 | 155.6 | |
| 11 | 21.3 | 21.3 | 20.4 | 115.6 | 21.4 |
| 12 | 122.4 | 122.3 | 121.6 | 126.9 | 121.4 |
| 13 | 130.3 | 132.0 | 129.6 | 77.8 | 132.6 |
| 14 | 25.5 | 25.8 | 25.8 | 28.3 | 25.8 |
| 15 | 17.9 | 18.2 | 16.3 | 25.6? | 17.9 |
| 16 | 25.6 | 31.2 | 24.1 | 26.5 | 22.6 |
| 17 | 123.7 | 123.2 | 120.5 | 123.1 | 121.0 |
| 18 | 130.3 | 131.7 | 129.7 | 131.8 | 131.3 |
| 19 | 17.7 | 17.8 | 15.8 | 18.1 | 17.9 |
| 20 | 25.7 | 26.7 | 24.1 | ? | 25.8 |
| 3-OMe | − | 55.8 | 54.9 | ||
| 6-OMe | − | − | 53.9 | ||
| 7-OMe | 60.1 | 62.0 | 60.9 | ||
| 8-OMe | 7-OMe | ||||
| 1' | 121.0 | ||||
| 2' | 131.3 | ||||
| 3' | 77.1 | ||||
| 4' | 27.4 | ||||
| 5' | 27.4 |
2.2. In Vitro Antiprotozoal and Antimicrobial Activity
| Sample | P. falciparum | L. infantum | T. cruzi | T. brucei | MRC-5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | |
| Dichloromethane extract | 2.7 µg | 3.5 | 7.5 µg | 3.5 | 7.6 µg | 1.2 | 0.5 µg | 18.8 | 9.4 µg |
| B. subtilis | C. albicans | E. coli | P. aeruginosa | S. aureus | Mycobacterium | ||||
|---|---|---|---|---|---|---|---|---|---|
| smegmatis | cheleneoi | xenopi | intracellulare | ||||||
| MIC (µM) | 3.9 | >200 | 7.8 | 3.7 | 3.7 | 3.7 | 3.7 | ||
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data (Table 1 and Table 2)
3.5. Reference Drugs
3.6. Biological Assays
3.6.1. Antiplasmodial Activity
3.6.2. Antileishmanial Activity
3.6.3. Antitrypanosomal Activity
3.6.4. Antimicrobial Activity
3.6.5. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of Mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef]
- Chin, Y.W.; Kinghorn, A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Min. Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Rajagopalan, K. Novel xanthones from Garcinia mangostana, structures of BR-xanthone-A and BR-xanthone-B. Phytochemistry 1988, 27, 1552–1554. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (Mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2004, 90, 161–166. [Google Scholar] [CrossRef]
- Kaomongkolgit, R.; Chaisomboon, N.; Pavasant, P. Apoptotic effect of alpha-mangostin on head and neck squamous carcinoma cells. Arch. Oral. Biol. 2011, 56, 483–490. [Google Scholar] [CrossRef]
- Yua, L.; Zhao, M.; Yang, B.; Bai, W. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 2009, 116, 969–973. [Google Scholar] [CrossRef]
- Ji, X.; Avula, B.; Khan, I.A. Quantitative and qualitative determination of six xanthones in Garcinia mangostana L. by LC-PDA and LC-ESI-MS. J. Pharm. Biomed. Anal. 2007, 43, 1270–1276. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J.; Faustino de Oliveira, F. Tetraoxygenated naturally occurring xantones. Phytochemistry 2000, 55, 683–710. [Google Scholar] [CrossRef]
- Williams, R.B.; Hoch, J.; Glass, T.E.; Evans, R.; Miller, J.S.; Wisse, J.H.; Kingston, D.G.I. A novel cytotoxic guttiferone analogue from Garcinia macrophylla from the Surinam rainforest. Planta Med. 2003, 69, 864–866. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef]
- Bennett, G.J.; Lee, H.H. Xanthones from Guttiferae. Phytochemistry 1989, 28, 967–998. [Google Scholar] [CrossRef]
- Sen, A.K.; Sarkar, K.K.; Mazumder, P.C.; Banerji, N.; Uusvuori, R.; Hase, T.A. The structure of garcinones A, B and C: Three new xanthones from Garcinia mangostana. Phytochemistry 1982, 21, 1747–1750. [Google Scholar]
- Ghazali, S.I.S.; Lian, G.E.; Abd Ghani, K.D. Chemical constituent from roots of Garcinia mangostana (Linn.). Int. J. Chem. 2010, 2, 134–142. [Google Scholar]
- Nilar; Harrison, L.J. Xanthones from the heartwood of Garcinia mangostana. Phytochemistry 2002, 60, 541–548. [Google Scholar] [CrossRef]
- Sen, A.K.; Sarkar, K.K.; Majumder, P.C.; Banerji, N. Minor xanthones of Garcinia mangostana. Phytochemistry 1981, 20, 183–185. [Google Scholar]
- Trisuwan, K.; Ritthiwigrom, T. Benzophenone and xanthone derivatives from the inflorescences of Garcinia cowa. Arch. Pharm. Res. 2012, 35, 1733–1738. [Google Scholar] [CrossRef]
- Antônio, A.A.L.; De Oliveira, W.G.; Taveira Neiva, R.M. Xanthones from Tovomita pyrifolium. Phytochemistry 1975, 14, 803–806. [Google Scholar] [CrossRef]
- Bennett, G.J.; Harrison, L.J.; Sia, G.L.; Sim, K.Y. Triterpenoids, tocotrienols and xanthones from the bark of Cratoxylum cochinchinense. Phytochemistry 1993, 32, 1245–1251. [Google Scholar] [CrossRef]
- Ejele, A.E.; Iwu, I.C.; Enenebeaku, C.K.; Ukiwe, L.N.; Okolue, B.N. Bioassay-guided isolation, purification and partial characterization of antimicrobial compound from basic metabolite of Garcinia Kola. J. Emerg. Trends Eng. Appl. Sci. 2012, 3, 668–672. [Google Scholar]
- Faizatun, S.; Rahayu, L. HPLC analysis and pharmacokinetic study of mangostin after orally administration in rats. Int. J. Pharm. Bio. Sci. 2009, 2, 43–49. [Google Scholar]
- Riscoe, M.; Kelly, J.X.; Winter, R. Xanthones as antimalarial agents: Discovery, mode of action, and optimization. Curr. Med. Chem. 2005, 12, 2539–2549. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Kuaha, K.; Wilairat, P.; Taylor, W.C. Prenylated xanthones as potential antiplasmodial substances. Planta Med. 2006, 72, 912–916. [Google Scholar]
- Priya, V.; Jainu, M.; Mohan, S.K.; Saraswathi, P.; Gopan, S.C. Antimicrobial activity of pericarp extract of Garcinia mangostana Linn. Int. J. Pharm. Sci. Res. 2010, 1, 278–281. [Google Scholar]
- Chomnawang, M.T.; Sakagami, S.S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 101, 330–333. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger In vitro proof-of-concept. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef]
- Raz, B.; Iten, M.; Grether-Buhler, Y.; Kaminsky, R.; Brun, R. The Alamiar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense, T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; la Flamme, A.C.; van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar]
- Ferraro, M.J. National committee for Clinical Laboratory Standards. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; NCCLS: Viallanova, PA, USA, 1997. [Google Scholar]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Massarani, S.M.; El Gamal, A.A.; Al-Musayeib, N.M.; Mothana, R.A.; Basudan, O.A.; Al-Rehaily, A.J.; Farag, M.; Assaf, M.H.; El Tahir, K.H.; Maes, L. Phytochemical, Antimicrobial and Antiprotozoal Evaluation of Garcinia Mangostana Pericarp and α-Mangostin, Its Major Xanthone Derivative. Molecules 2013, 18, 10599-10608. https://doi.org/10.3390/molecules180910599
Al-Massarani SM, El Gamal AA, Al-Musayeib NM, Mothana RA, Basudan OA, Al-Rehaily AJ, Farag M, Assaf MH, El Tahir KH, Maes L. Phytochemical, Antimicrobial and Antiprotozoal Evaluation of Garcinia Mangostana Pericarp and α-Mangostin, Its Major Xanthone Derivative. Molecules. 2013; 18(9):10599-10608. https://doi.org/10.3390/molecules180910599
Chicago/Turabian StyleAl-Massarani, Shaza M., Ali A. El Gamal, Nawal M. Al-Musayeib, Ramzi A. Mothana, Omer A. Basudan, Adnan J. Al-Rehaily, Mohamed Farag, Mahmoud H. Assaf, KamalEldin H. El Tahir, and Louis Maes. 2013. "Phytochemical, Antimicrobial and Antiprotozoal Evaluation of Garcinia Mangostana Pericarp and α-Mangostin, Its Major Xanthone Derivative" Molecules 18, no. 9: 10599-10608. https://doi.org/10.3390/molecules180910599
APA StyleAl-Massarani, S. M., El Gamal, A. A., Al-Musayeib, N. M., Mothana, R. A., Basudan, O. A., Al-Rehaily, A. J., Farag, M., Assaf, M. H., El Tahir, K. H., & Maes, L. (2013). Phytochemical, Antimicrobial and Antiprotozoal Evaluation of Garcinia Mangostana Pericarp and α-Mangostin, Its Major Xanthone Derivative. Molecules, 18(9), 10599-10608. https://doi.org/10.3390/molecules180910599