Differential Growth Inhibitory Effects of Highly Oxygenated Guaianolides Isolated from the Middle Eastern Indigenous Plant Achillea falcata in HCT-116 Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Achillea falcata Extract Rich in Sesquiterpene Lactones Decreases the Growth of HCT-116 Colorectal Cancer Cells
| HCT-116 | HaCaT II4 | HL-60 | Jurkat | |
|---|---|---|---|---|
| Average IC50 I.2 Extract (μg/mL) ± SE | 21.0 ± 2.08 | 11.7 ± 0.54 | 4.6 ± 0.34 | 4.0 ± 0.54 |
2.2. The Bioactive I.2 Sub-Fractions are Rich in Sesquiterpene Lactones
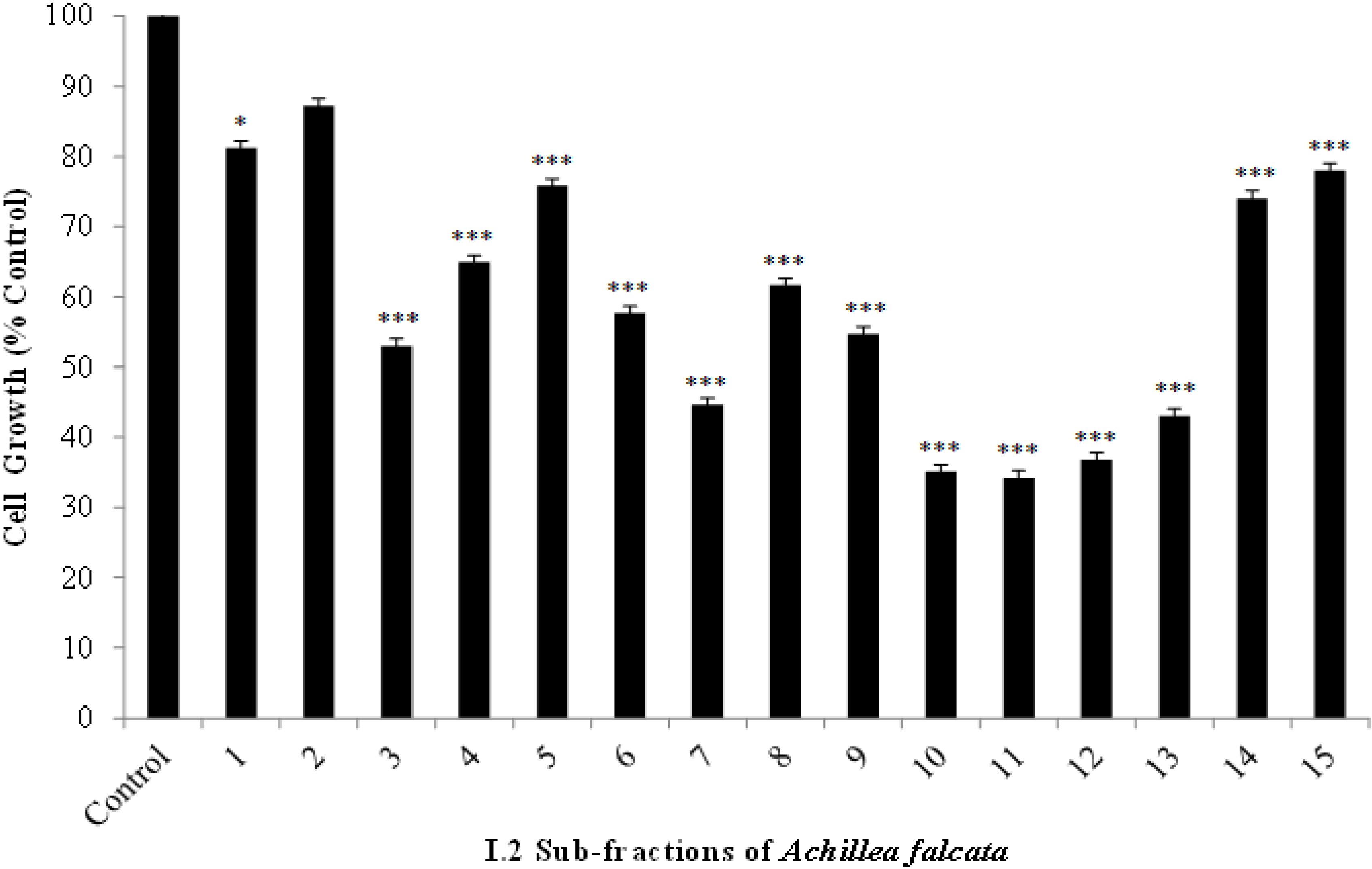
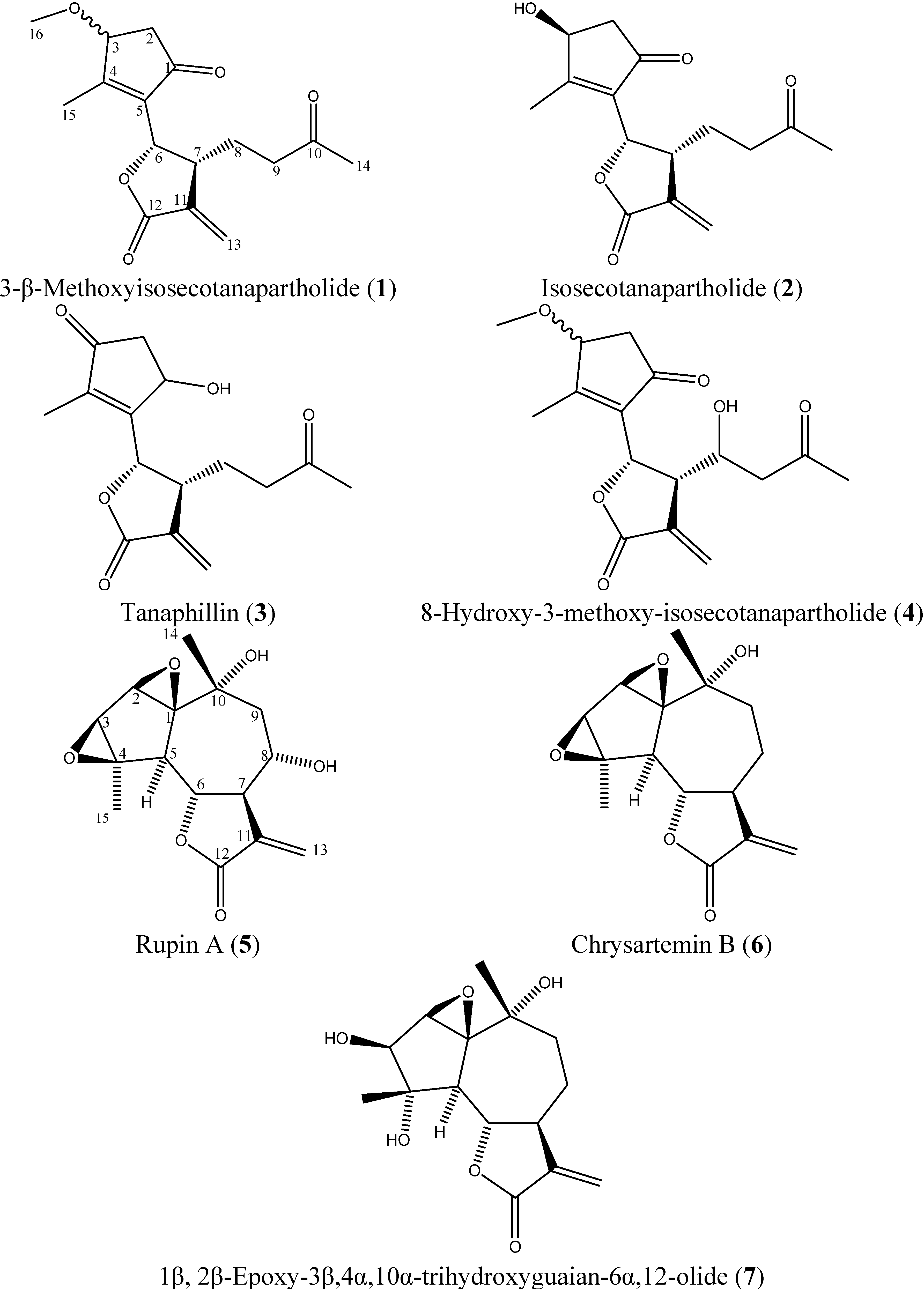
2.3. Isolated Sesquiterpene Lactones from Achillea falcata Exhibit Dose-Dependent Growth Inhibitory Effects on HCT-116 Cancer Cells
| Sesquiterpene Lactone Concentrations (μg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | 0 | 5 | 10 | 15 | 20 | 25 | 50 | 75 | 100 | |
| 1 | 0 ± 0.5 | 0 ± 0.9 | 0 ± 0.7 | 0 ± 0.7 | 0 ± 1.9 | 0 ± 0.8 | 0 ± 1.0 | 3 ± 2.7 | 0 ± 2.1 | |
| 2 | 0 ± 0.5 | 0 ± 0.5 | 0 ± 0.3 | 0 ± 2.1 | 0 ± 0.3 | 0 ± 0.5 | 0 ± 1.7 | 0 ± 0.6 | 0 ± 1.3 | |
| 5 | 0 ± 0.5 | 0 ± 0.2 | 0 ± 0.3 | 0 ± 0.8 | 0 ± 0.7 | 2 ± 0.3 | 16 ± 0.7 | 16 ± 0.4 | 38 ± 0.2 | |
| 6 | 0 ± 0.6 | 0 ± 1.4 | 0 ± 1.7 | 0 ± 2.2 | 0 ± 2.5 | 0 ± 3.2 | 0 ± 2.4 | 0 ± 3.0 | 0 ± 3.8 | |
| 7 | 0 ± 0.6 | 0 ± 2.0 | 2 ± 1.4 | 1 ± 1.3 | 3 ± 1.0 | 2 ± 0.8 | 18 ± 4.0 | 24 ± 1.7 | 18 ± 4.2 | |
| Concentrations (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 100 | 1000 | |||
| Positive Control | Cytotoxicity at 6 h (% Control) ± SE | |||||||
| Triton X | 0 ± 1.0 | 1 ± 1.9 | 0 ± 0.9 | 3 ± 0.5 | 75 ± 3.5 | 74 ± 2.5 | ||
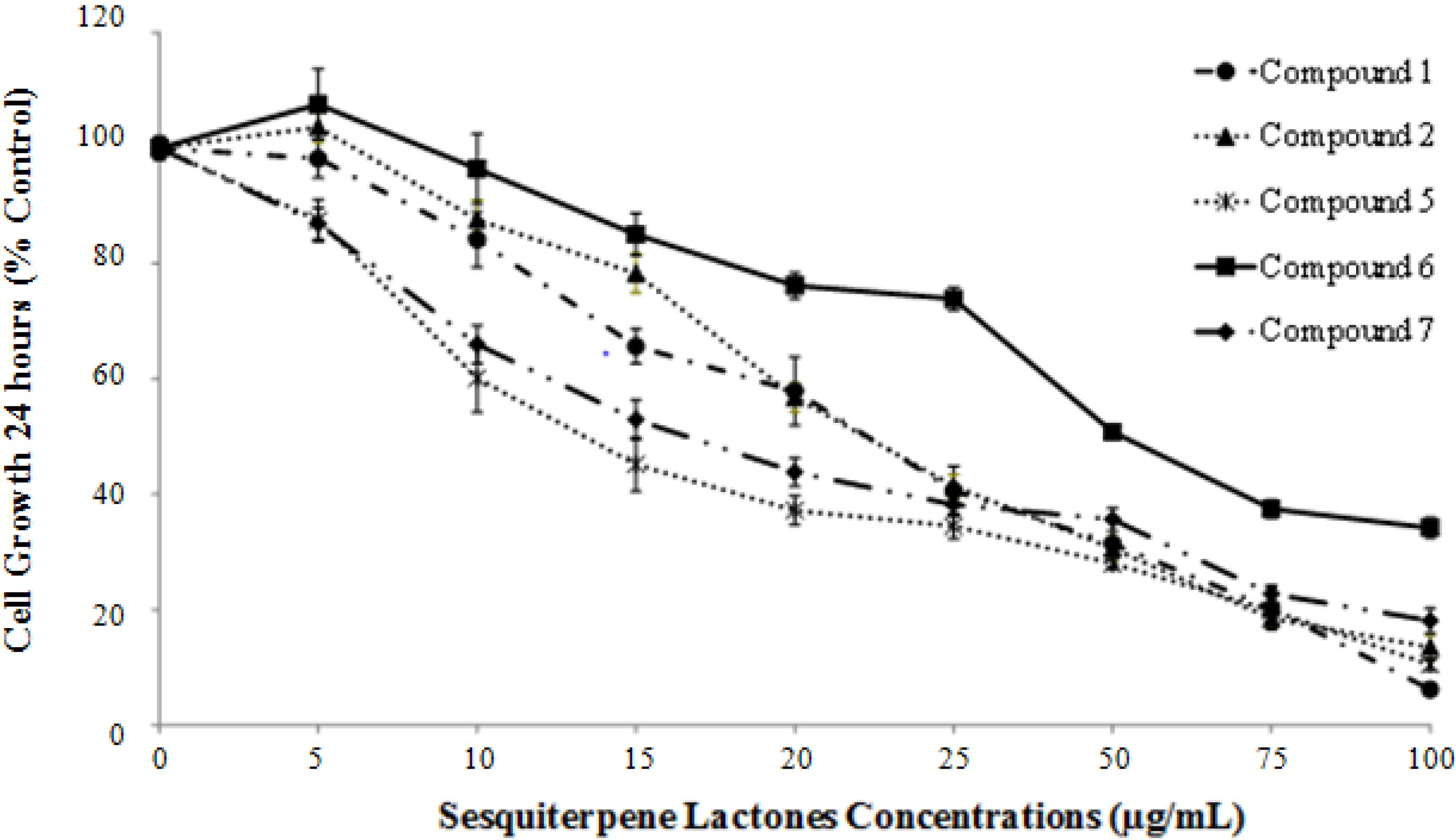
| Compounds | Average IC50 (µg/mL) | ±SE |
|---|---|---|
| Compound 1 | 25.6 | 1.52 |
| Compound 2 | 29.4 | 1.07 |
| Compound 5 | 15.2 | 0.93 |
| Compound 6 | 67.0 | 3.99 |
| Compound 7 | 19.3 | 1.02 |
3. Experimental
3.1. General
3.2. Harvesting and Processing of Achillea falcata
3.3. Extraction and Purification of Sesquiterpene Lactones
3.4. Structure Elucidation
3.5. Cell Culture
3.6. Cytotoxicity and Cell Growth Assays
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Wink, M. Biochemistry of Plant Secondary Metabolism; Sheffield Academic Press: Sheffield, England, 1999; Volume 2. [Google Scholar]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef]
- Ghantous, A.; Nasser, N.; Saab, I.; Darwiche, N.; Saliba, N.A. Structure-activity relationship of seco-tanapartholides isolated from Achillea falcata for inhibition of HaCaT cell growth. Eur. J. Med. Chem. 2009, 44, 3794–3797. [Google Scholar] [CrossRef]
- Merfort, I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets 2011, 12, 1560–1573. [Google Scholar] [CrossRef]
- Kreuger, M.R.; Grootjans, S.; Biavatti, M.W.; Vandenabeele, P.; D'Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anti-Cancer Drug. 2012, 23, 883–896. [Google Scholar]
- Kupchan, S.M.; Fessler, D.C.; Eakin, M.A.; Giacobbe, T.J. Reactions of alpha methylene lactone tumor inhibitors with model biological nucelophiles. Science 1970, 168, 376–378. [Google Scholar]
- Saikali, M.; Ghantous, A.; Halawi, R.; Talhouk, S.N.; Saliba, N.A.; Darwiche, N. Sesquiterpene lactones isolated from indigenous middle eastern plants inhibit tumor promoter-induced transformation of JB6 cells. BMC Complem. Altern. M. 2012, 12, 89. [Google Scholar] [CrossRef]
- Salla, M.; Fakhoury, I.; Saliba, N.; Darwiche, N.; Gali-Muhtasib, H. Synergistic anticancer activities of the plant-derived sesquiterpene lactones salograviolide A and iso-seco-tanapartholide. J. Nat. Med. 2013, 468–479. [Google Scholar]
- Adib, S.M.; Daniel, J. National cancer registry: Cancer in lebanon 2003; Republic of Lebanon Ministry of Public Health and World Health Organization: Beirut, Lebanon, 2003. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA: Cancer J. Clin. 2013, 63, 11–30. [Google Scholar]
- W.H.O. International Agency for Research on Cancer. Available online: http://www.iarc.fr/en/media-centre/iarcnews/2010/globocan2008.php (accessed on 28 April 2013).
- Ikediobi, O.N.; Davies, H.; Bignell, G.; Edkins, S.; Stevens, C.; O’Meara, S.; Santarius, T.; Avis, T.; Barthorpe, S.; Brackenbury, L.; et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 2006, 5, 2606–2612. [Google Scholar] [CrossRef]
- Yoshioka, H.; Mabry, T.J.; Irwin, M.A.; Geissman, T.A.; Samek, Z. The geminal coupling and paramagnetic shift of exomethylene protons in the α,β′-unsaturated γ-lactone group of sesquiterpene lactones containing c8-α-hydroxyl groups. Tetrahedron 1971, 27, 3317–3322. [Google Scholar] [CrossRef]
- Irwin, M.A.; Geissman, T.A. Rupicolin-A and -B, rupin-A and -B, and cumambrin-B oxide from Artemisia tripartita ssp. Rupicola. Phytochemistry 1973, 12, 863–869. [Google Scholar] [CrossRef]
- Jakupovic, J.; Boeker, R.; Grenz, M.; Paredes, L.; Bohlmann, F.; El-Din, A.S. Highly oxygenated guaianolides from Otanthus maritimus. Phytochemistry 1988, 27, 1135–1140. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Shafizadeh, F.; Campbell, J.A.; Craig, A.C.; Campana, C.F.; Craig, R.E.R. Canin from Artemisia cana Pursh ssp. cana. Crystal structure and identification of chrysartemin A. J. Org. Chem. 1983, 48, 125–127. [Google Scholar] [CrossRef]
- Milosavjević, S.; Macura, S.; Stefanović, M. Sesquiterpene lactones from Achillea crithmifolia. J. Nat. Prod. 1994, 57, 64–67. [Google Scholar] [CrossRef]
- Öksüz, S. Sesquiterpenoids and other constituents from Tanacetum cilicium. Phytochemistry 1990, 29, 887–890. [Google Scholar] [CrossRef]
- Romo, J.; Romo de Vivar, A.; Treviño, R.; Joseph-Nathan, P.; Díaz, E. Constituents of Artemisia and Chrysanthemum species-the structures of chrysartemins A and B. Phytochemistry 1970, 9, 1615–1621. [Google Scholar] [CrossRef]
- Jakupovic, J.; Tan, R.X.; Bohlmann, F.; Boldt, P.E.; Jia, Z.J. Sesquiterpene lactones from Artemisia ludoviciana. Phytochemistry 1991, 30, 1573–1577. [Google Scholar] [CrossRef]
- Yusupov, M.I.; Kasymov, S.Z.; Sidyakin, G.P.; Rakhmankulov, U. Sesquiterpene lactones from Ajania fastigiata. Chem. Nat. Compd. 1979, 15, 579–580. [Google Scholar]
- Yusupov, M.I.; Mallabaev, A.; Sidyakin, G.P. The lactones of Achillea biebersteinii. Chem. Nat. Compd. 1976, 12, 396–397. [Google Scholar]
- Milosavjević, S.; Aljančić, I.; Macura, S.; Milinkovic, D.; Stefanović, M. Sesquiterpene lactones from Achillea crithmifolia. Phytochemistry 1991, 30, 3464–3466. [Google Scholar] [CrossRef]
- Todorova, M.; Vogler, B.; Tsankova, E. Terpenoids from Achillea setacea. Zeitschrift fuer Naturforschung, C: J. Biosciences 2000, 55, 840–842. [Google Scholar]
- Ghavami, G.; Sardari, S.; Shokrgozar, M.A. Anticancerous potentials of Achillea species against selected cell lines. J. Med. Plants Res. 2010, 4, 2411–2417. [Google Scholar]
- Zakirov, S.K.; Kasymov, S.Z.; Abdusamatov, A.; Sidyakin, G.P. Canin and chrysartemin A from Artemesia aschurbajevii. Chem. Nat. Compd. 1985, 21, 542. [Google Scholar] [CrossRef]
- Ohno, N.; Gershenzon, J.; Roane, C.; Mabry, T.J. 11,13-dehydrodesacetylmatricarin and other sesquiterpene lactones from Artemisia ludoviciana var. Ludoviciana and the identity of artecanin and chyrsartemin B. Phytochemistry 1980, 19, 103–106. [Google Scholar]
- Ruiz-Cancino, A.; Cano, A.E.; Delgado, G. Sesquiterpene lactones and flavonoids from Artemisia ludoviciana ssp. Mexicana. Phytochemistry 1993, 33, 1113–1115. [Google Scholar] [CrossRef]
- Osawa, T.; Suzuki, A.; Tamura, S. Isolation of chrysartemins A and B as rooting cofactors in Chrysanthemum morifolium. Agric. Biol. Chem. 1971, 35, 1966–1972. [Google Scholar] [CrossRef]
- Bruno, M.; Herz, W. Guaianolides and other constituents of Achillea ligustica. Phytochemistry 1988, 27, 1871–1872. [Google Scholar] [CrossRef]
- Mallabaev, A.; Rakhmankulov, U.; Sidyakin, G.P. Lactones of Achillea santolina. Chem. Nat. Compd. 1978, 14, 457. [Google Scholar] [CrossRef]
- Tarasov, V.A.; Abdullaev, N.D.; Kasymov, S.Z.; Sidyakin, G.P. Chrysartemin B- a sesquiterpene lactone from Handelia trichophylla. Chem. Nat. Compd. 1976, 12, 601–602. [Google Scholar] [CrossRef]
- Zan, K.; Chen, X.-Q.; Fu, Q.; Zhou, S.-X.; Xiao, M.-T.; Wen, J.; Tu, P.F. Chemical ingredients isolated from the aerial parts of Artemisia anomala. J. Chinese Pharm. Sci. 2010, 19, 95–99. [Google Scholar]
- Marco, J.A.; Sanz-cervera, J.F.; Manglano, E.; Sancenon, F.; Rustaiyan, A.; Kardar, M. Sesquiterpene lactones from iranian Artemisia species. Phytochemistry 1993, 34, 1561–1564. [Google Scholar] [CrossRef]
- Meng, J.C.; Hu, Y.F.; Chen, J.H.; Tan, R.X. Antifungal highly oxygenated guaianolides and other constituents from Ajania fruticulosa. Phytochemistry 2001, 58, 1141–1145. [Google Scholar] [CrossRef]
- Guangrong, H.; Jiaxin, J.; Dehui, D. Antioxidative and antibacterial activity of the methanol extract of Artemisia anomala S. Moore. Afr. J. Biotechnol. 2008, 7, 1335–1338. [Google Scholar]
- Wen, J.; Shi, H.; Xu, Z.; Chang, H.; Jia, C.; Zan, K.; Jiang, Y.; Tu, P. Dimeric guaianolides and sesquiterpenoids from Artemisia anomala. J. Nat. Prod. 2010, 73, 67–70. [Google Scholar] [CrossRef]
- Sun, L.; Shah, F.; Helal, M.A.; Wu, Y.; Pedduri, Y.; Chittiboyina, A.G.; Gut, J.; Rosenthal, P.J.; Avery, M.A. Design, synthesis, and development of novel guaianolide-endoperoxides as potential antimalarial agents. J. Med. Chem. 2010, 53, 7864–7868. [Google Scholar] [CrossRef]
- Helal, M.A.; Avery, M.A. Combined receptor-based and ligand-based approach to delineate the mode of binding of guaianolide-endoperoxides to PfATP6. Bioorg. Med. Chem. Lett. 2012, 22, 5410–5414. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Standiford, M.; Johnson, D.A.; De Vos, R.C.H.; Todorović, S.; Banjanac, T.; Verpoorte, R.; Johnson, J.A. Activation of antioxidant response element in mouse primary cortical cultures with sesquiterpene lactones isolated from Tanacetum parthenium. Planta Med. 2012, 78, 1725–1730. [Google Scholar] [CrossRef]
- Neukirch, H.; Kaneider, N.C.; Wiedermann, C.J.; Guerriero, A.; D'Ambrosio, M. Parthenolide and its photochemically synthesized 1(10)z isomer: Chemical reactivity and structure–activity relationship studies in human leucocyte chemotaxis. Bioorg. Med. Chem. 2003, 11, 1503–1510. [Google Scholar] [CrossRef]
- Neukirch, H.; Guerriero, A.; D’Ambrosio, M. Transannular cyclization in cyclodecenes: The case study of melampolides. Eur. J. Org. Chem. 2003, 2003, 3969–3975. [Google Scholar]
- Dell’Agli, M.; Galli, G.V.; Bosisio, E.; D’Ambrosio, M. Inhibition of NF-κB and metalloproteinase-9 expression and secretion by parthenolide derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 1858–1860. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Eakin, M.A.; Thomas, A.M. Tumor inhibitors. 69. Structure-cytotoxicity relations among the sesquiterpene lactones. J. Med. Chem. 1971, 14, 1147–1152. [Google Scholar]
- Lee, K.H.; Meck, R.; Piantadosi, C.; Huang, E.S. Antitumor agents. 4. Cytotoxicity and in vivo activity of helenalin esters and related derivatives. J. Med. Chem. 1973, 16, 299–301. [Google Scholar] [CrossRef]
- Hall, I.H.; Lee, K.H.; Starenes, C.O.; Sumida, Y.; Wu, R.Y.; Waddell, T.G.; Cochran, J.W.; Gerhart, K.G. Anti-inflammatory activity of sesquiterpene lactones and related compounds. J. Pharm. Sci. 1979, 68, 537–542. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Scotti, M.T.; Ferreira, M.J.P.; Emerenciano, V.P. Use of self-organizing maps and molecular descriptors to predict the cytotoxic activity of sesquiterpene lactones. Eur. J. Med. Chem. 2008, 43, 2197–2205. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Saliba, N.A.; Dakdouki, S.; Homeidan, F.R.; Kogan, J.; Bouhadir, K.; Talhouk, S.; Talhouk, R. Bio-guided identification of an anti-inflammatory guaianolide from Centaurea ainetensis. Pharm. Biol. 2009, 47, 701–707. [Google Scholar]
- Sample Availability: Samples of the compounds rupin A and 1β, 2β-epoxy-3β,4α,10α-trihydroxyguaian- 6α,12-olide are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tohme, R.; Al Aaraj, L.; Ghaddar, T.; Gali-Muhtasib, H.; Saliba, N.A.; Darwiche, N. Differential Growth Inhibitory Effects of Highly Oxygenated Guaianolides Isolated from the Middle Eastern Indigenous Plant Achillea falcata in HCT-116 Colorectal Cancer Cells. Molecules 2013, 18, 8275-8288. https://doi.org/10.3390/molecules18078275
Tohme R, Al Aaraj L, Ghaddar T, Gali-Muhtasib H, Saliba NA, Darwiche N. Differential Growth Inhibitory Effects of Highly Oxygenated Guaianolides Isolated from the Middle Eastern Indigenous Plant Achillea falcata in HCT-116 Colorectal Cancer Cells. Molecules. 2013; 18(7):8275-8288. https://doi.org/10.3390/molecules18078275
Chicago/Turabian StyleTohme, Rita, Lamis Al Aaraj, Tarek Ghaddar, Hala Gali-Muhtasib, Najat A. Saliba, and Nadine Darwiche. 2013. "Differential Growth Inhibitory Effects of Highly Oxygenated Guaianolides Isolated from the Middle Eastern Indigenous Plant Achillea falcata in HCT-116 Colorectal Cancer Cells" Molecules 18, no. 7: 8275-8288. https://doi.org/10.3390/molecules18078275
APA StyleTohme, R., Al Aaraj, L., Ghaddar, T., Gali-Muhtasib, H., Saliba, N. A., & Darwiche, N. (2013). Differential Growth Inhibitory Effects of Highly Oxygenated Guaianolides Isolated from the Middle Eastern Indigenous Plant Achillea falcata in HCT-116 Colorectal Cancer Cells. Molecules, 18(7), 8275-8288. https://doi.org/10.3390/molecules18078275






