Antiplasmodial Alkaloids from the Bark of Cryptocarya nigra (Lauraceae)
Abstract
:1. Introduction
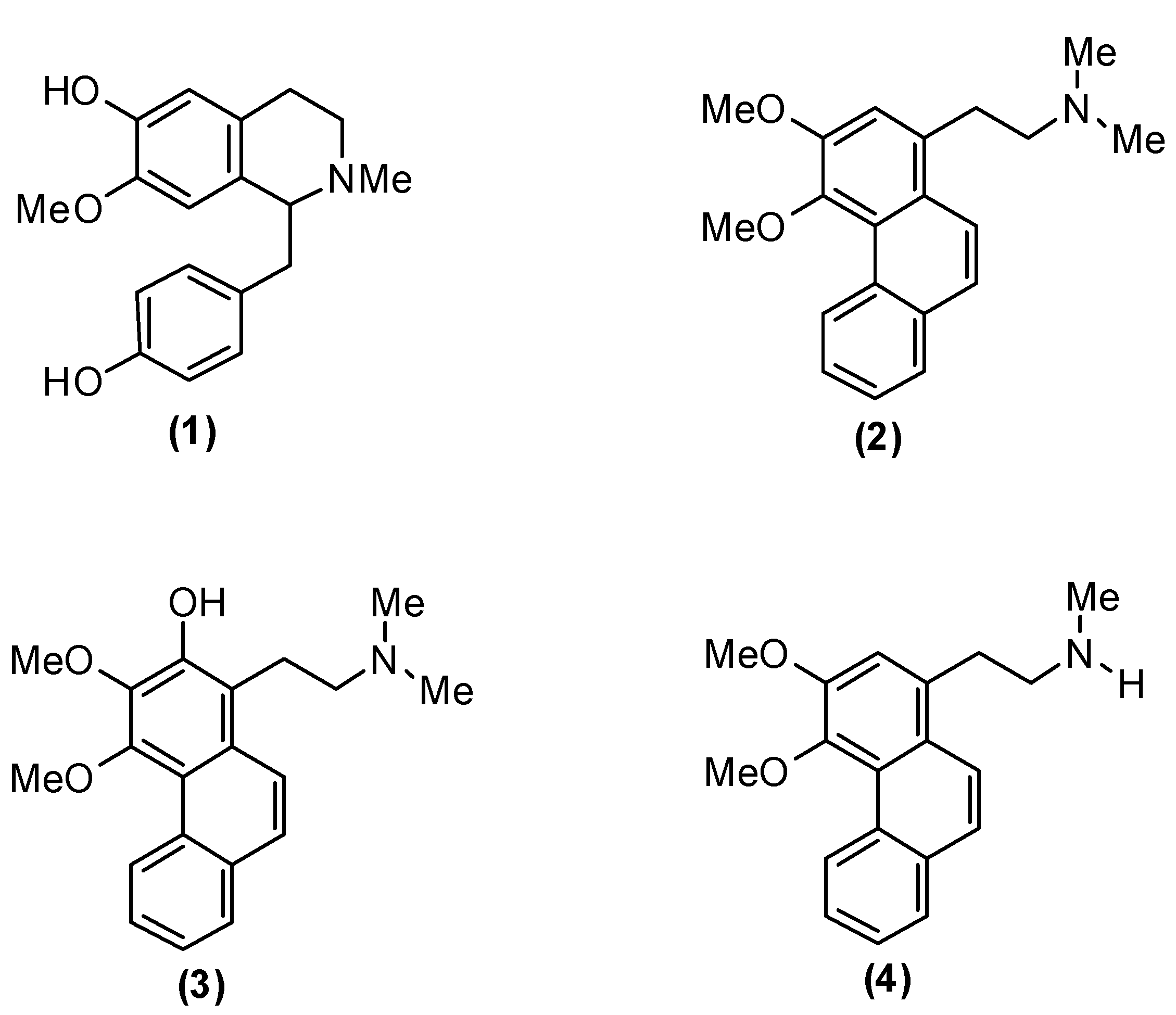
2. Results and Discussion
2.1. Antiplasmodial Activity
| Sample | Antiplasmodial Activity * The % of growth inhibition or IC50 | |
|---|---|---|
| μg/mL | μM | |
| 1 | 1.62 | 5.40 |
| 2 | 1.80 | 5.80 |
| 3 | 0.25 | 0.75 |
| 4 | nt † | nt † |
| Chloroquine | 90.39 ± 28.85 ** | |
| Artemisinin | 2.42 ± 1.06 ** | |
2.2. Antioxidant Activity
| Compound Name | IC50 DPPH Activity (ug/mL) | % FRAP | IC50 Metal Chelating Activity (ug/mL) |
|---|---|---|---|
| N-Methylisococlaurine (1) | 29.56 | 78.54 | 50.08 |
| Atherosperminine (2) | 54.53 | 70.66 | 42.87 |
| Ascorbic acid (Standard) | 13.69 | ||
| EDTA (Standard) | 83.74 | ||
| BHA (Standard) | 19.60 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Determination of Antiplasmodial Activity
3.5. Determination of Antioxidant Assay
3.5.1. DPPH Assay
3.5.2. Ferric Reducing Power Assay (FRAP)
3.5.3. Metal Chelating Activity Assay
4. Conclusion
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2012—Full Report. Available online: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_full_report.pdf (accessed on 16 February 2013).
- World Health Organization. World Malaria Report 2012—Fact Sheet. Available online: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_factsheet.pdf (accessed on 16 February 2013).
- Kritsiriwuthinan, K.; Chaotheing, S.; Shaw, P.J.; Wongsombat, C.; Chavalitshewinkoon-Petmitr, P.; Kamchonwongpaisan, S. Global gene expression profiling of Plasmodium falciparum in response to the anti-malarial drug pyronaridine. Malar. J. 2011, 10, 242. [Google Scholar] [CrossRef]
- Ali, A.M.; El-Sharkawy, S.H.; Hamid, J.H.; Nur-Hediani, I.; Lajis, N.H. Antimicrobial activity of some Malaysian plants. Pertanika J.Trop.Agric.Sci. 1995, 18, 49–52. [Google Scholar]
- Nugroho, A.E.; Sugai, M.; Hirasawa, Y.; Hosoya, T.; Awang, K.; Hadi, A.H.A.; Ekasari, W.; Widyawaruyanti, A.; Morita, H. New antiplasmodial indole alkaloids from Hunteria zeylanica. Bioorg. Med. Chem. Lett. 2011, 21, 3417–3419. [Google Scholar] [CrossRef]
- Awang, K.; Mukhtar, M.R.; Hadi, A.H.A.; Litaudon, M.; Latip, J.; Abdullah, N.R. New alkaloids from Phoebe grandis (Nees) Merr. Nat. Prod. Res. 2006, 20, 567–572. [Google Scholar] [CrossRef]
- Oshimi, S.; Takasaki, A.; Hirasawa, Y.; Hosoya, T.; Awang, K.; Hadi, A.H.A.; Ekasari, W.; Widyawaruyanti, A.; Morita, H. Delaumonones A and B, new antiplasmodial quassinoids from Laumoniera bruceadelpha. Chem. Pharm. Bull. 2009, 57, 867–869. [Google Scholar] [CrossRef]
- Hadi, A.H.A.; Mukhtar, M.R.; Wee, K.C.; Abd, A.S.S.S.; Awang, K. Alkaloids isolated from Dehaasia candolleana (Meisn.) Kosterm. Malays. J. Sci. 2008, 27, 115–121. [Google Scholar]
- Osman, C.P.; Ismail, N.H.; Ahmad, R.; Ahmat, N.; Awang, K.; Mohd, J.F. Anthraquinones with antiplasmodial activity from the roots of Rennellia elliptica Korth. (Rubiaceae). Molecules 2010, 15, 7218–7226. [Google Scholar] [CrossRef]
- Mohamad, K.; Hirasawa, Y.; Litaudon, M.; Awang, K.; Hadi, A.H.A.; Takeya, K.; Ekasari, W.; Widyawaruyanti, A.; Zaini, N.C.; Morita, H. Ceramicines B-D, new antiplasmodial limonoids from Chisocheton ceramicus. Bioorg. Med. Chem. 2009, 17, 727–730. [Google Scholar] [CrossRef]
- Slik, J.W.F. Trees of Sungai Wain. Available online: http://www.nationaalherbarium.nl/sungaiwain (accessed on 28 January 2013).
- Whitmore, T.C.; Ng, F.S.P. Tree Flora of Malaya: a Manual for Foresters; Longman: New York, NY, USA, 1989. [Google Scholar]
- Corner, E.J.H. Wayside Trees of Malaya, 4th ed.; United Selangor Press: Kuala Lumpur, Malaysia, 1997. [Google Scholar]
- Nehme, C.J.; de Moraes, P.L.R.; Tininis, A.G.; Cavalheiro, A.J. Intraspecific variability of flavonoid glycosides and styrylpyrones from leaves of Cryptocarya mandioccana Meisner (Lauraceae). Biochem. Syst. Ecol. 2008, 36, 602–611. [Google Scholar] [CrossRef]
- Wu, T.S.; Lin, F.W. Alkaloids of the wood of Cryptocarya chinensis. J. Nat. Prod. 2001, 64, 1404–1407. [Google Scholar] [CrossRef]
- Cave, A.; Leboeuf, M.; Moskowitz, H.; Ranaivo, A.; Bick, I.R.C.; Sinchai, W.; Nieto, M.; Sevenet, T.; Cabalion, P. Alkaloids of Cryptocarya phyllostemon. Aust. J. Chem. 1989, 42, 2243–2263. [Google Scholar] [CrossRef]
- Toribio, A.; Bonfils, A.; Delannay, E.; Prost, E.; Harakat, D.; Henon, E.; Richard, B.; Litaudon, M.; Nuzillard, J.M.; Renault, J.H. Novel seco-Dibenzopyrrocoline Alkaloid from Cryptocarya oubatchensis. Org. Lett. 2006, 8, 3825–3828. [Google Scholar] [CrossRef]
- Lin, F.W.; Wu, P.L.; Wu, T.S. Alkaloids from the leaves of Cryptocarya chinensis HEMSL. Chem. Pharm. Bull. 2001, 49, 1292–1294. [Google Scholar] [CrossRef]
- Chang, W.T.; Lee, S.S.; Chueh, F.S.; Liu, K.C.S. Formation of pavine alkaloids by callus culture of Cryptocarya chinensis. Phytochemistry 1998, 48, 119–124. [Google Scholar]
- Lin, F.W.; Wang, J.J.; Wu, T.S. New pavine N-oxide alkaloids from the stem bark of Cryptocarya chinensis Hemsl. Chem. Pharm. Bull. 2002, 50, 157–159. [Google Scholar] [CrossRef]
- Lee, S.S.; Lin, Y.J.; Chen, C.K.; Liu, K.C.S.; Chen, C.H. Quaternary alkaloids from Litsea cubeba and Cryptocarya konishii. J. Nat. Prod. 1993, 56, 1971–1976. [Google Scholar] [CrossRef]
- Saidi, N.; Hadi, A.H. A.; Awang, K.; Mukhtar, M.R. Aporphine Alkaloids from Bark of Cryptocarya ferra. Indo. J. Chem 2009, 9, 461–465. [Google Scholar]
- Saidi, N. Two Oxoaporphine Alkloids from Bark of Cryptocarya rugulosa. Jur. Nat. 2011, 11, 48–51. [Google Scholar]
- Awang, K.; Hadi, A.H.A.; Saidi, N.; Mukhtar, M.R.; Morita, H.; Litaudon, M. New phenantrene alkaloids from Cryptocarya crassinervia. Fitoterapia 2008, 79, 308–310. [Google Scholar] [CrossRef]
- Yen, K.H.; Din, L. B.; Syah, Y.M.; Zakaria, Z.; Ismail, N.H.; Hakim, E.H. Coumarins and flavonoids from leaves of Cryptocarya nigra (Lauraceae) and their cytotoxic activity against murine leukemia P-388 cells. ACGC Chem. Res. Commun. 2008, 22, 57–60. [Google Scholar]
- Kunitomo, J.; Yoshikawa, Y.; Tanaka, S.; Imori, Y.; Isoi, K.; Masada, Y.; Hashimoto, K.; Inoue, T. Alkaloids of Nelumbo nucifera XVI. Phytochemistry 1973, 12, 699–701. [Google Scholar] [CrossRef]
- Okwu, D.E.; Nnamdi, F.U. A novel antimicrobial phenanthrene alkaloid from Bryopyllum pinnatum. J. Chem. 2011, 8, 1456–1461. [Google Scholar]
- Castedo, L.; Tojo, G. Chapter 3 Phenanthrene Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Arnold, B., Ed.; Academic Press: Burlington, MA, USA, 1990; Volume 39, pp. 99–138. [Google Scholar]
- Cordell, G.A. The Alkaloids: Chemistry and Physiology; Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Li, S.; Han, L.; Sun, L.; Zheng, D.; Liu, J.; Fu, Y.; Huang, X.; Wang, Z. Synthesis and antitumor activities of phenanthrene-based alkaloids. Molecules 2009, 14, 5042–5053. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Bose, R.; Ghosh, P.; Tripathi, V.J.; Ray, A.B.; Dasgupta, B. Psychopharmacological studies on (−)-nuciferine and its Hofmann degradation product atherosperminine. Psychopharmacology (Berlin) 1978, 59, 29–33. [Google Scholar] [CrossRef]
- Jee, J.H.; Kang, J.C. Biochemical changes of enzymatic defense system after phenanthrene exposure in olive flounder, Paralichthys olivaceus. Physiol. Res. (Prague, Czech Repub.) 2005, 54, 585–591. [Google Scholar]
- Egan, T.J.; Combrinck, J.M.; Egan, J.; Hearne, G.R.; Marques, H.M.; Ntenteni, S.; Sewell, B.T.; Smith, P.J.; Taylor, D.; van, S.D.A.; et al. Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem. J. 2002, 365, 343–347. [Google Scholar] [CrossRef]
- Pradines, B.; Ramiandrasoa, F.; Basco, L.K.; Bricard, L.; Kunesch, G.; Le, B.J. In vitro activities of novel catecholate siderophores against Plasmodium falciparum. Antimicrob. Agents Chemother. 1996, 40, 2094–2098. [Google Scholar]
- Pradines, B.; Rolain, J.M.; Ramiandrasoa, F.; Fusai, T.; Mosnier, J.; Rogier, C.; Daries, W.; Baret, E.; Kunesch, G.; Le, B.J.; et al. Iron chelators as antimalarial agents: in vitro activity of dicatecholate against Plasmodium falciparum. J. Antimicrob. Chemother. 2002, 50, 177–187. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, X.; Cheng, L.; Bu, Y.; Liu, G.; Yi, F.; Yang, Z.; Song, F. Evaluation of antioxidant and immune activity of Phellinus ribis glucan in mice. Food Chem. 2009, 115, 581–584. [Google Scholar] [CrossRef]
- Reis, P.A.; Comim, C.M.; Hermani, F.; Silva, B.; Barichello, T.; Portella, A.C.; Gomes, F.C.A.; Sab, I.M.; Frutuoso, V.S.; Oliveira, M.F.; et al. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog. 2010, 6, e1000963. [Google Scholar] [CrossRef]
- Percario, S.; Moreira, D.R.; Gomes, B.A.Q.; Ferreira, M.E.S.; Goncalves, A.C.M.; Laurindo, P.S.O.C.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavenger. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nasrullah, A.A.; Zahari, A.; Mohamad, J.; Awang, K. Antiplasmodial Alkaloids from the Bark of Cryptocarya nigra (Lauraceae). Molecules 2013, 18, 8009-8017. https://doi.org/10.3390/molecules18078009
Nasrullah AA, Zahari A, Mohamad J, Awang K. Antiplasmodial Alkaloids from the Bark of Cryptocarya nigra (Lauraceae). Molecules. 2013; 18(7):8009-8017. https://doi.org/10.3390/molecules18078009
Chicago/Turabian StyleNasrullah, Ayu Afiqah, Azeana Zahari, Jamaludin Mohamad, and Khalijah Awang. 2013. "Antiplasmodial Alkaloids from the Bark of Cryptocarya nigra (Lauraceae)" Molecules 18, no. 7: 8009-8017. https://doi.org/10.3390/molecules18078009
APA StyleNasrullah, A. A., Zahari, A., Mohamad, J., & Awang, K. (2013). Antiplasmodial Alkaloids from the Bark of Cryptocarya nigra (Lauraceae). Molecules, 18(7), 8009-8017. https://doi.org/10.3390/molecules18078009





