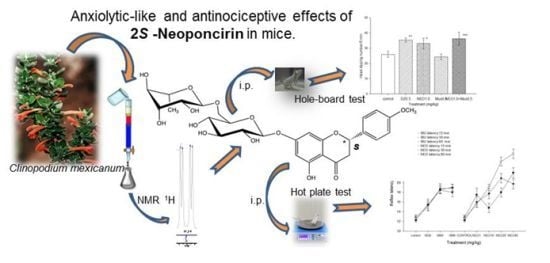
Anxiolytic-Like and Antinociceptive Effects of 2(S)-Neoponcirin in Mice
Abstract
:1. Introduction
- (1)
- The flavanone aglycone system has an asymmetric carbon in the position two, which can be in the S or R orientation. Though all natural flavanones have a thermodynamically favored conformation with the C-2 aryl group in an equatorial position, and this implies that all levorotatory flavanones possess a 2S configuration, the racemization of products (affording S/R mixtures) usually takes place during the isolation process. Thus, it is a fact that the S or R diasteroisomer has different chemical and physical properties, and thereby potentially different pharmacological actions.
- (2)
- The binding between sugar residues can take place in two different positions; 1→2 (glc→rha) or 1→6 (glc→rha), producing two regioisomers. Likewise, the impact of regioisomers in the pharmacological properties of drugs is well known, for example: hesperidin (1→2; glc→rha) [10] of bitter taste produces sedative effects that does not involve the GABAergic system, While, sweet tasting neohesperidin (1→6; glc→rha) of is a GABAergic modulator, without sedative effects per se.
2. Results and Discussion
2.1.Isolation and Identification of 2S-Neoponcirin
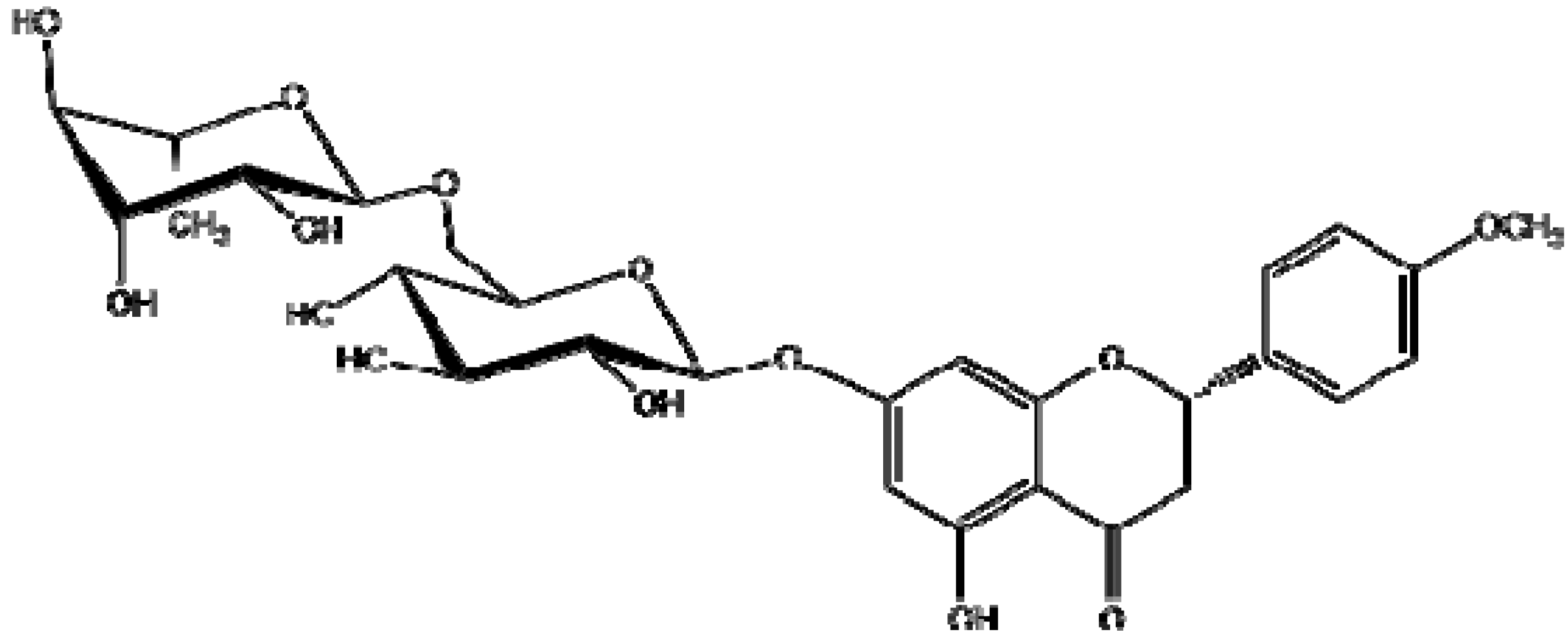
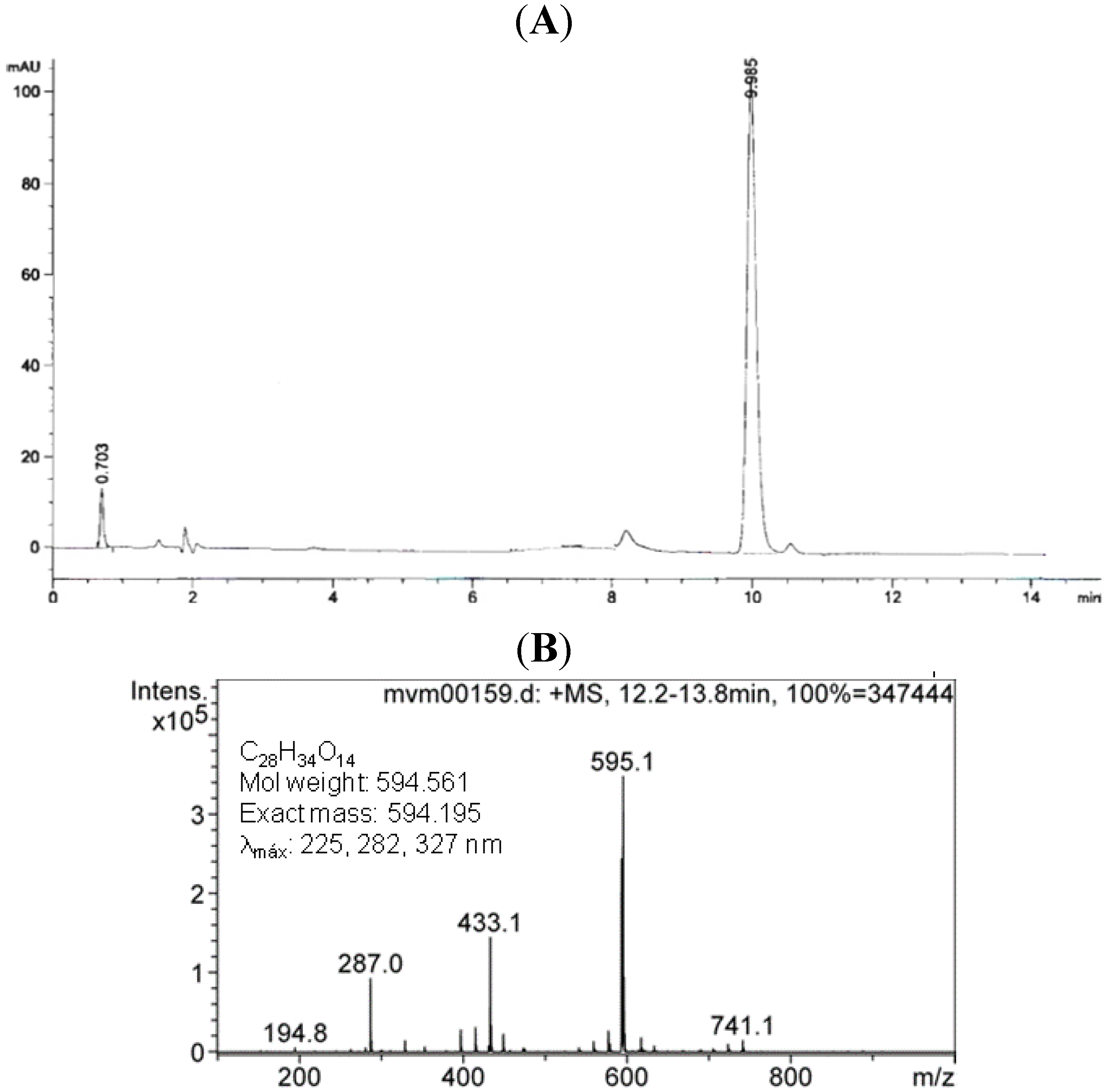
2.2. Pharmacological Evaluations
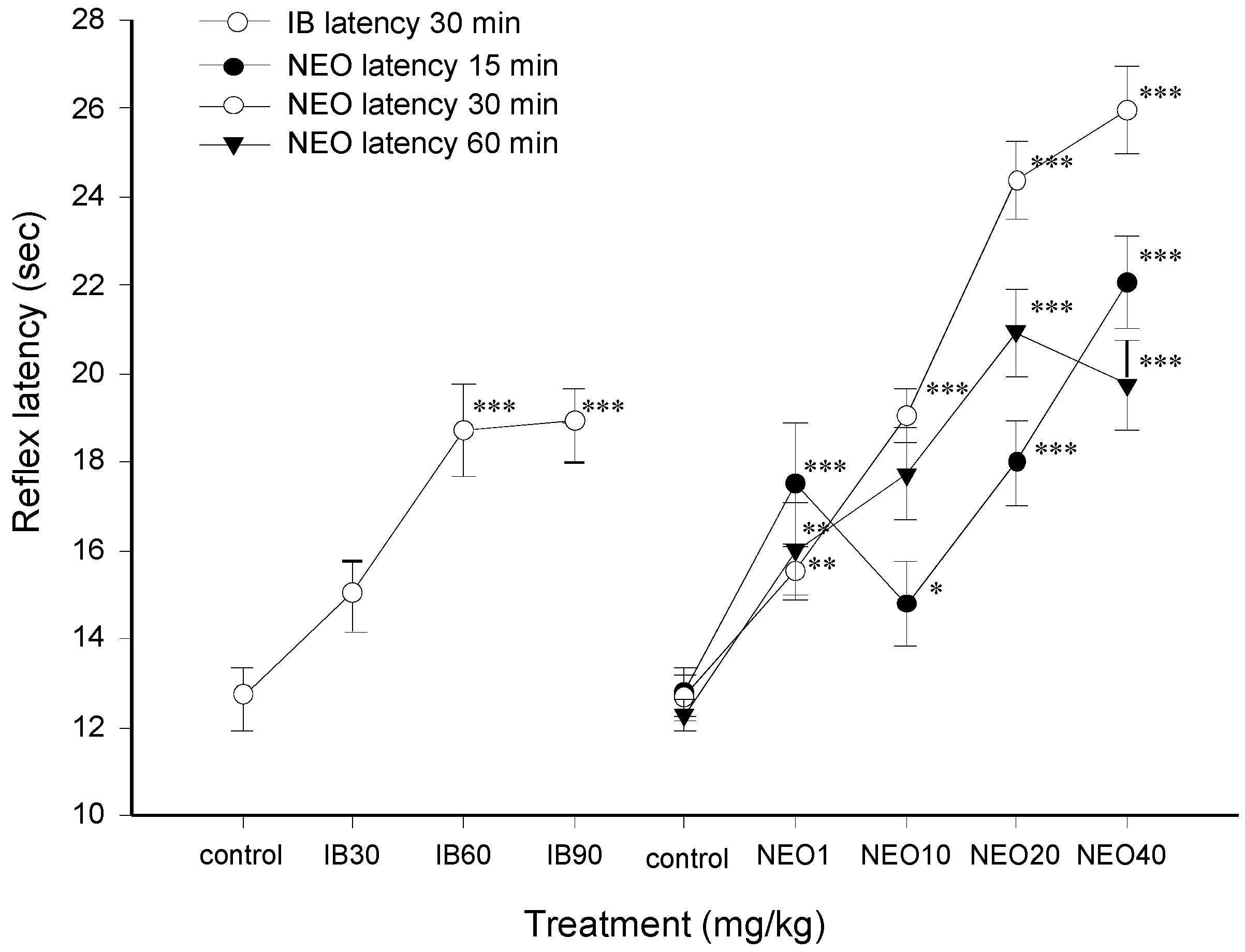
2.2.1. Antinociceptive Effect of NEO
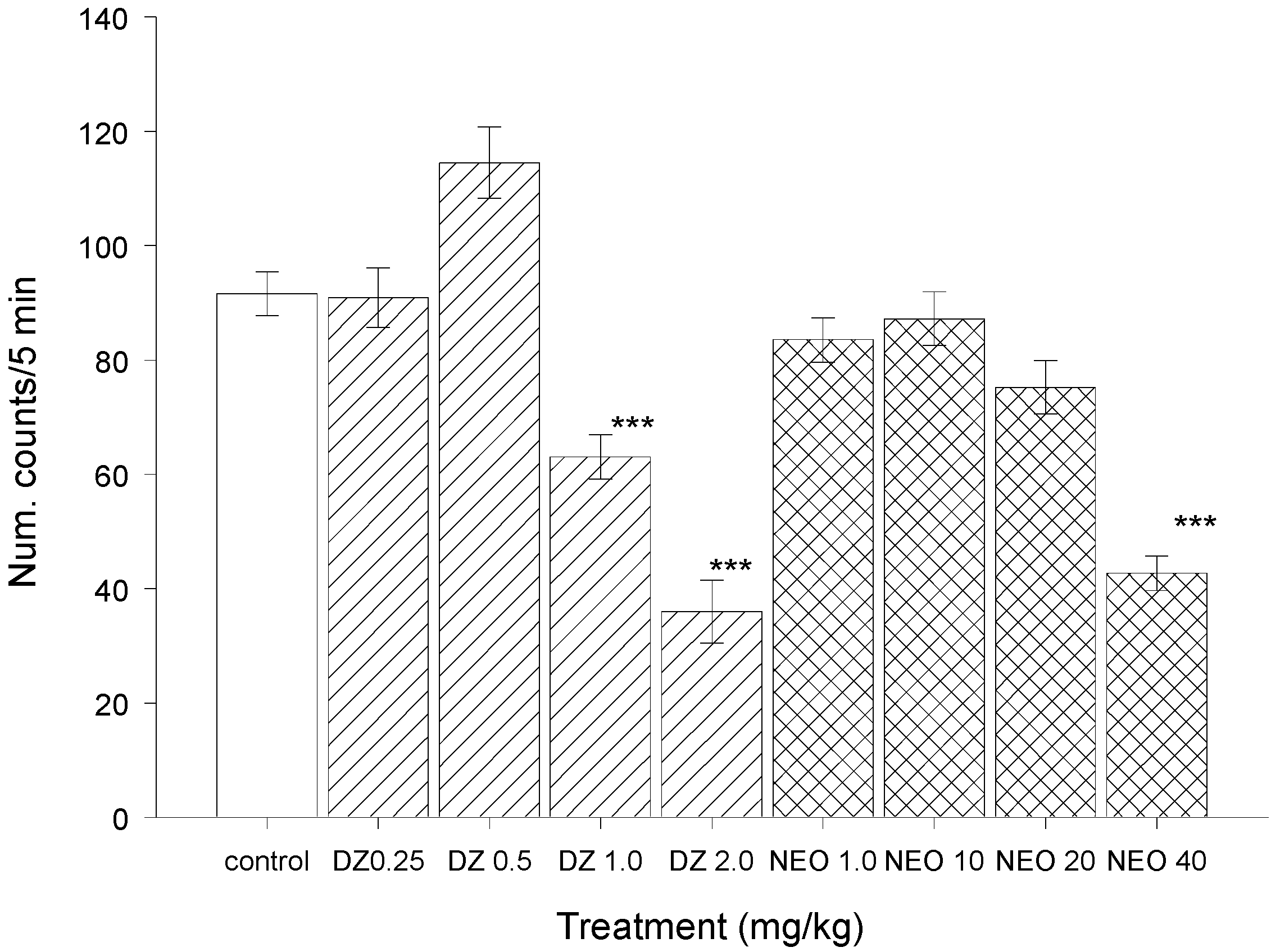
2.2.2. Test of Sodium Pentobarbital-induced Sleeping Time
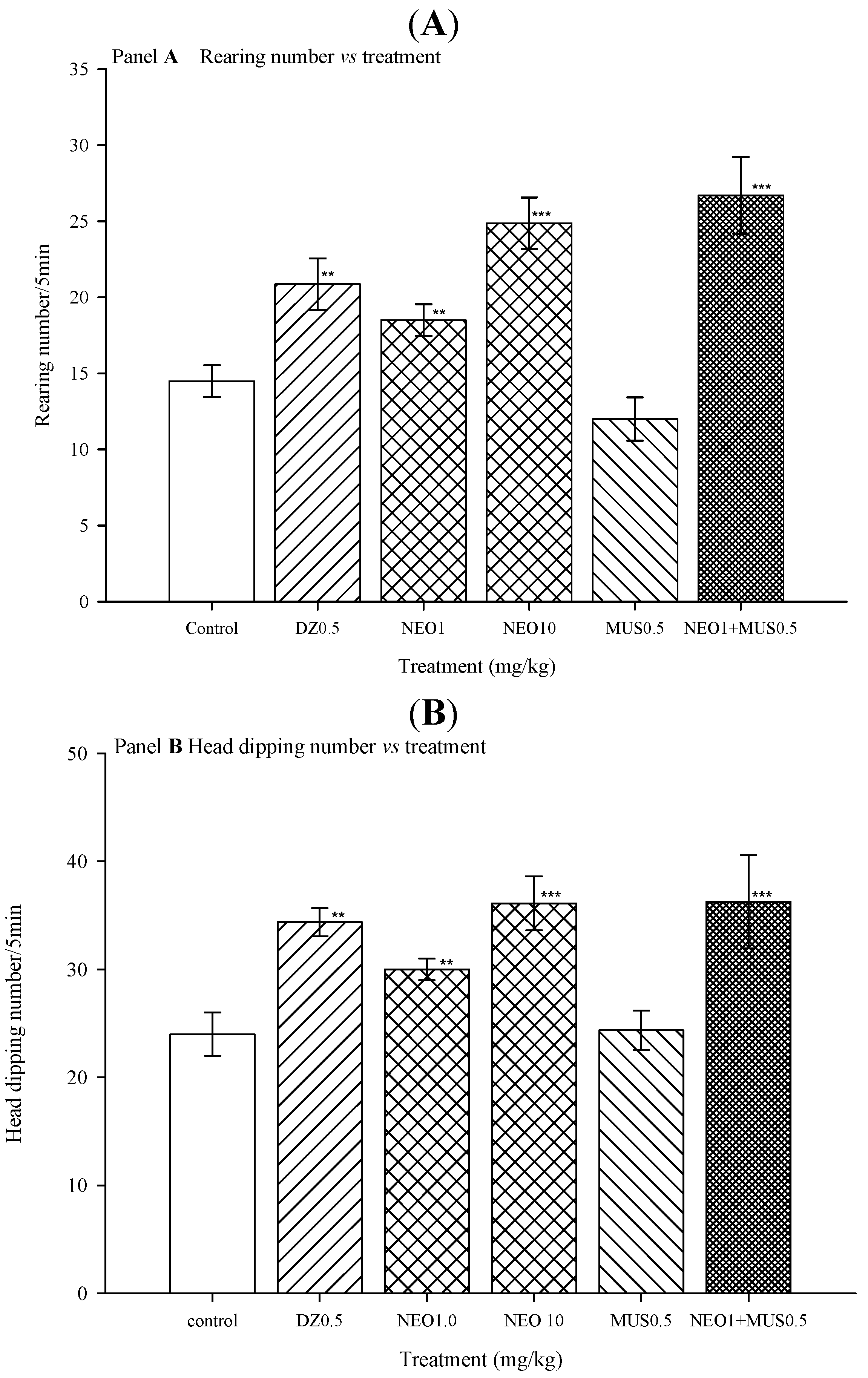
2.2.3. Hole-Board Test
| Treatment (mg/kg) | RN | HDN |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Control | 10.37 ± 0.80 | 26.12 ± 1.55 |
| 1.0 | 17.5 ± 1.74 ** | 32.33 ± 2.16 * |
| 10.0 | 20.70 ± 0.98 *** | 38.20 ± 3.33 ** |
| 20.0 | 5.0 ± 1.08 ++ | 24.77 ± 1.98 |
| 40.0 | 1 ± 0.50 +++ | 6.8 ± 3.88 +++ |
| DZ 0.5 | 20.25 ± 1.66 *** | 42.27 ± 1.55 ** |
| DZ 1.0 | 13.44 ± 2.28 | 34.55 ± 3.12 |
| DZ 2.0 | 9.66 ± 2.27 | 30.55 ± 3.61 |
| DZ 4.0 | 3.08 ± 0.81 +++ | 0.8 ± 0.44 +++ |
| H = 61.73, fd = 8(p ≤ 0.001 ) | H = 57.75, fd = 8(p ≤ 0.001) |
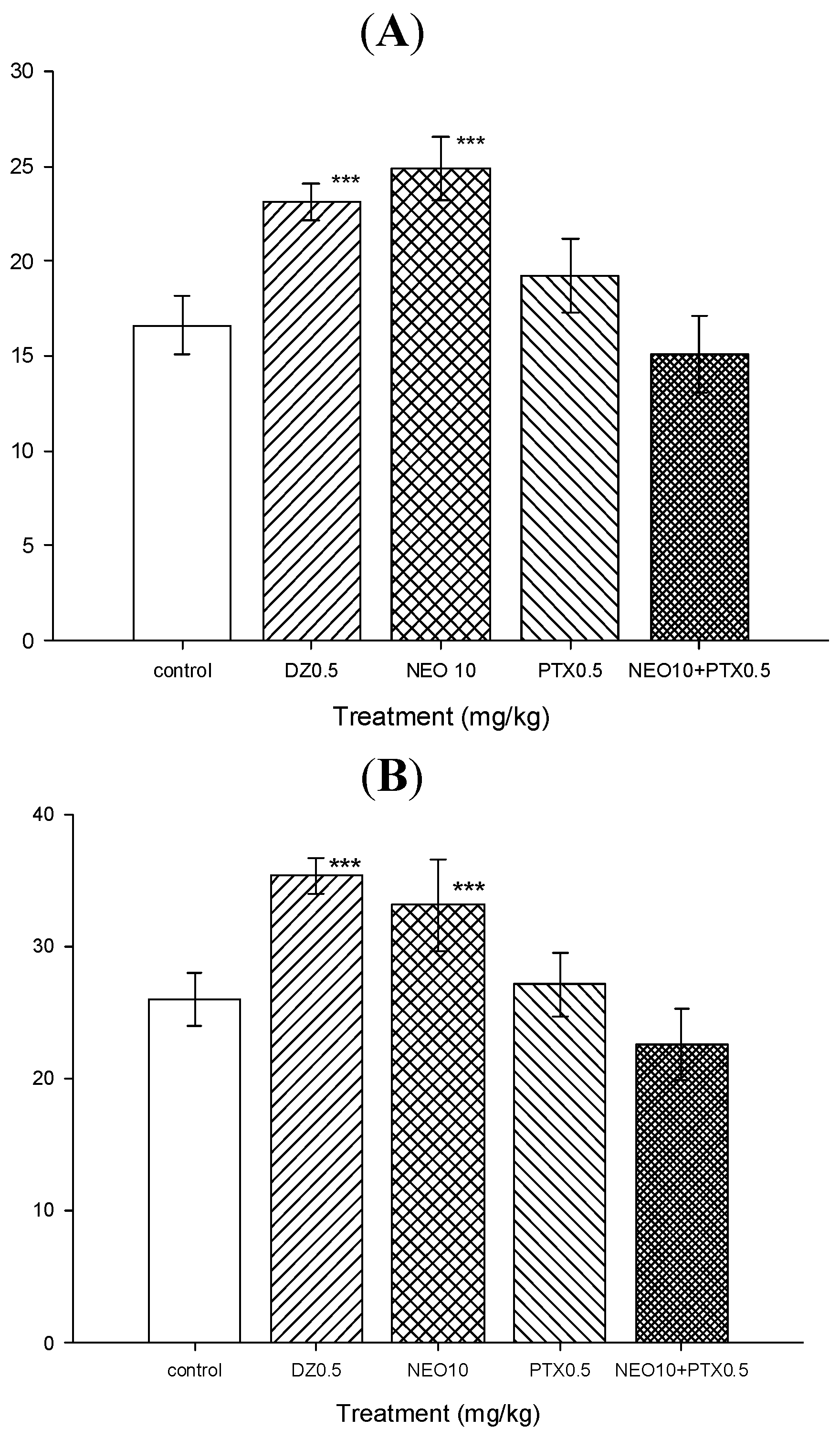
2.2.4. Picrotoxin Blockade and Muscimol Synergism Experiments
3. Experimental
3.1. General
3.2. Plant Material
3.3. Isolation and Identification of 2S-Neoponcirin
3.4. Pharmacological Evaluations
3.4.1. Animals
3.4.2. Drug Preparation and Dosage
3.4.3. Hot Plate Test
3.4.4. Open Field Test
3.4.5. Test of Sodium Pentobarbital-Induced Sleeping Time
3.4.6. Hole-Board Test
3.4.7. Picrotoxin Blockade Experiments
3.4.8. Muscimol Synergism Experiments
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef]
- Paul, P.; Ritra, J.; Ritva, S.; Mackku, H.; Lyly, T.; Eero, P.; Arpo, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 26, 167–181. [Google Scholar]
- Knekt, P.; Järvinen, R.; Seppänen, R.; Heliövaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer's disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Miyazaki, K.; Sakai, S.; Yawo, H.; Nakata, N.; Moriguchi, S.; Fukunaga, K.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinasa A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 2008, 578, 194–200. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Si-Young, S.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef]
- Medina, J.H.; Peña, C.; Levi de Stein, M.; Wolfman, C.; Paladini, A.C. Benzodiazepine-like molecules, as well as other ligands for the brain benzodiazepine receptors, are relatively common constituents of plants. Biochem. Biophys. Res. Com. 1989, 165, 547–553. [Google Scholar] [CrossRef]
- Medina, J.H.; Paladini, A.C.; Wolfman, C.; Levi de Stein, M.; Calvo, D.; Diaz, L.E.; Peña, C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990, 40, 2227–2231. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Martínez-Vázquez, M.; Gallegos-Solís, A.; Heinze, G.; Moreno, J. Depressant effects of Clinopodium mexicanum benth. Govaerts (lamiaceae) on the central nervous system. J. Ethnopharmacol. 2010, 130, 1–8. [Google Scholar] [CrossRef]
- Martínez, M.C.; Fernández, S.P.; Loscalzo, L.L.; Wasowski, C.; Paladini, A.C.; Marder, M.; Medina, J.H.; Viola, H. Hesperidin, a flavonoid glycoside with sedative effect, decreases brain Perk1/2 levels in mice. Pharmacol. Biochem. Behav. 2009, 92, 291–296. [Google Scholar] [CrossRef]
- Fernández, S.; Wasowski, C.; Paladini, A.C.; Marder, M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004, 77, 399–409. [Google Scholar] [CrossRef]
- Fernández, S.P.; Wasowski, C.; Loscalzo, L.M.; Granger, R.E.; Johnston, G.A.; Paladini, A.C.; Marder, M. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 2006, 13, 168–176. [Google Scholar]
- Slade, D.; Ferreira, D.; Marais, J.P.J. Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 2005, 66, 2177–2215. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef]
- Wesolowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaiabolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Petty, F. GABA and mood disorders: A brief review and hypothesis. J. Affect. Disord. 1995, 34, 275–281. [Google Scholar] [CrossRef]
- Leeb-Lundberg, F.; Snowman, A.; Olsen, W.R. Barbiturate receptor sites are coupled to benzodiazepine receptors. Neurobiology 1980, 77, 7468–7472. [Google Scholar]
- Takeda, H.; Tsuji, M.; Matsumiya, T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998, 350, 21–29. [Google Scholar] [CrossRef]
- Nolan, N.A.; Parkes, M.W. The effects of benzodiazepines on the behaviour of mice on a hole-board. Psychopharmacology 1973, 29, 277–288. [Google Scholar] [CrossRef]
- Ashton, H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 1994, 48, 25–40. [Google Scholar] [CrossRef]
- Smith, T.A. Type A gamma-butyric acid (GABAA) receptor subunits and benzodiazepine binding: Significance to clinical syndromes and their treatment. Br. J. Biomed. Sci. 2001, 58, 111–121. [Google Scholar]
- Treit, D. Animal models for the study of anti-anxiety agents: A review. Neurosci. Biobehav. Rev. 1985, 9, 203–222. [Google Scholar] [CrossRef]
- Hanrahan, R.J.; Chebib, M.; Johnston, A.R.G. Flavonoid modulation of GABAA receptors. Br. J. Pharmacol. 2001, 163, 234–245. [Google Scholar] [CrossRef]
- Meotti, F.C.; Posser, T.; Missau, F.C.; Pizzolatti, M.G.; Leal, R.B.; Santos, A.R. Involvement of p38MAPK on the antinociceptive action of myricitrin in mice. Biochem. Pharmacolcol. 2007, 74, 924–931. [Google Scholar] [CrossRef]
- López-Rubalcava, C.; Piña-Medina, B.; Estrada-Reyes, R.; Heinze, G.; Martínez-Vázquez, M. Anxiolytic-like actions of the hexane extract from leaves of Annona cherimolia in two anxiety paradigms: Possible involvement of the GABA/benzodiazepine receptor complex. Life Sci. 2006, 78, 730–737. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. Possible involvement of GABAergic mechanism in protective effect of melatonin against sleep deprivation-induced behaviour modification and oxidative damage in mice. Fundam. Clin. Pharmacol. 2009, 23, 439–448. [Google Scholar] [CrossRef]
- Tsuda, M.; Suzuki, T.; Misawa, M.; Nagase, H. Involvement of the opioid system in the anxiolytic effect of diazepam in mice. Eur. J. Pharmacol. 1996, 307, 7–14. [Google Scholar] [CrossRef]
- Gear, R.W.; Miaskowski, C.; Heller, P.H.; Paul, S.M.; Gordon, N.C.; Levine, J.D. Benzodiazepine mediated antagonism of opioid analgesia. Pain 1997, 71, 25–29. [Google Scholar] [CrossRef]
- Viswanathan, S.; Sambantham, P.T.; Reddy, K.; Kameswaran, L. Gossypin-induced analgesia in mice. Eur. J. Pharmacol. 1984, 98, 289–291. [Google Scholar] [CrossRef]
- Loscalzo, L.M.; Wasowski, C.; Paladini, A.C.; Marder, M. Opioid receptors are involved in the sedative and antinociceptive effects of hesperidin as well as in its potentiation with benzodiazepines. Eur. J. Pharmacol. 2008, 580, 306–313. [Google Scholar] [CrossRef]
- National Institutes of Health. Institutional administrator’s manual for laboratory animal care and use. National NIH publication No. 88-2959; U.S. Department of Health and Human services: Bethesda, Maryland 20892, USA, revised in 1985.
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Martínez-Laurrabaquio, A.; López-Rubalcava, C.; Heinze, G. Neuropharmacological study of Dracocephalum moldovica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 2012, 141, 908–917. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the Neoponcirin is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cassani, J.; Araujo, A.G.E.; Martínez-Vázquez, M.; Manjarrez, N.; Moreno, J.; Estrada-Reyes, R. Anxiolytic-Like and Antinociceptive Effects of 2(S)-Neoponcirin in Mice. Molecules 2013, 18, 7584-7599. https://doi.org/10.3390/molecules18077584
Cassani J, Araujo AGE, Martínez-Vázquez M, Manjarrez N, Moreno J, Estrada-Reyes R. Anxiolytic-Like and Antinociceptive Effects of 2(S)-Neoponcirin in Mice. Molecules. 2013; 18(7):7584-7599. https://doi.org/10.3390/molecules18077584
Chicago/Turabian StyleCassani, Julia, Anna G. Escalona Araujo, Mariano Martínez-Vázquez, Norberto Manjarrez, Julia Moreno, and Rosa Estrada-Reyes. 2013. "Anxiolytic-Like and Antinociceptive Effects of 2(S)-Neoponcirin in Mice" Molecules 18, no. 7: 7584-7599. https://doi.org/10.3390/molecules18077584
APA StyleCassani, J., Araujo, A. G. E., Martínez-Vázquez, M., Manjarrez, N., Moreno, J., & Estrada-Reyes, R. (2013). Anxiolytic-Like and Antinociceptive Effects of 2(S)-Neoponcirin in Mice. Molecules, 18(7), 7584-7599. https://doi.org/10.3390/molecules18077584