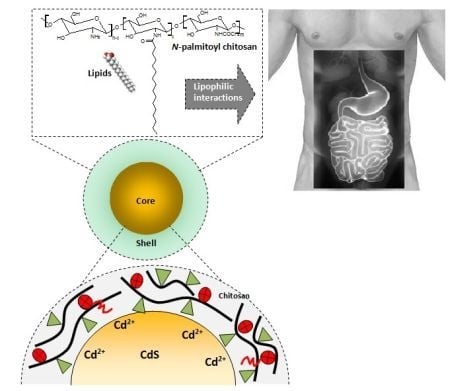
One-Step Biofunctionalization of Quantum Dots with Chitosan and N-palmitoyl Chitosan for Potential Biomedical Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Methods of CdS Precursor Solutions
2.3. Preparation of Reference Chitosan (CHI) 1.0 % (w/v) Solution
2.4. Procedure for Preparation of N-Palmitoyl Chitosan (C-Pal)
2.5. Synthesis of CdS Nanoparticles in CHI and C-Pal Solutions
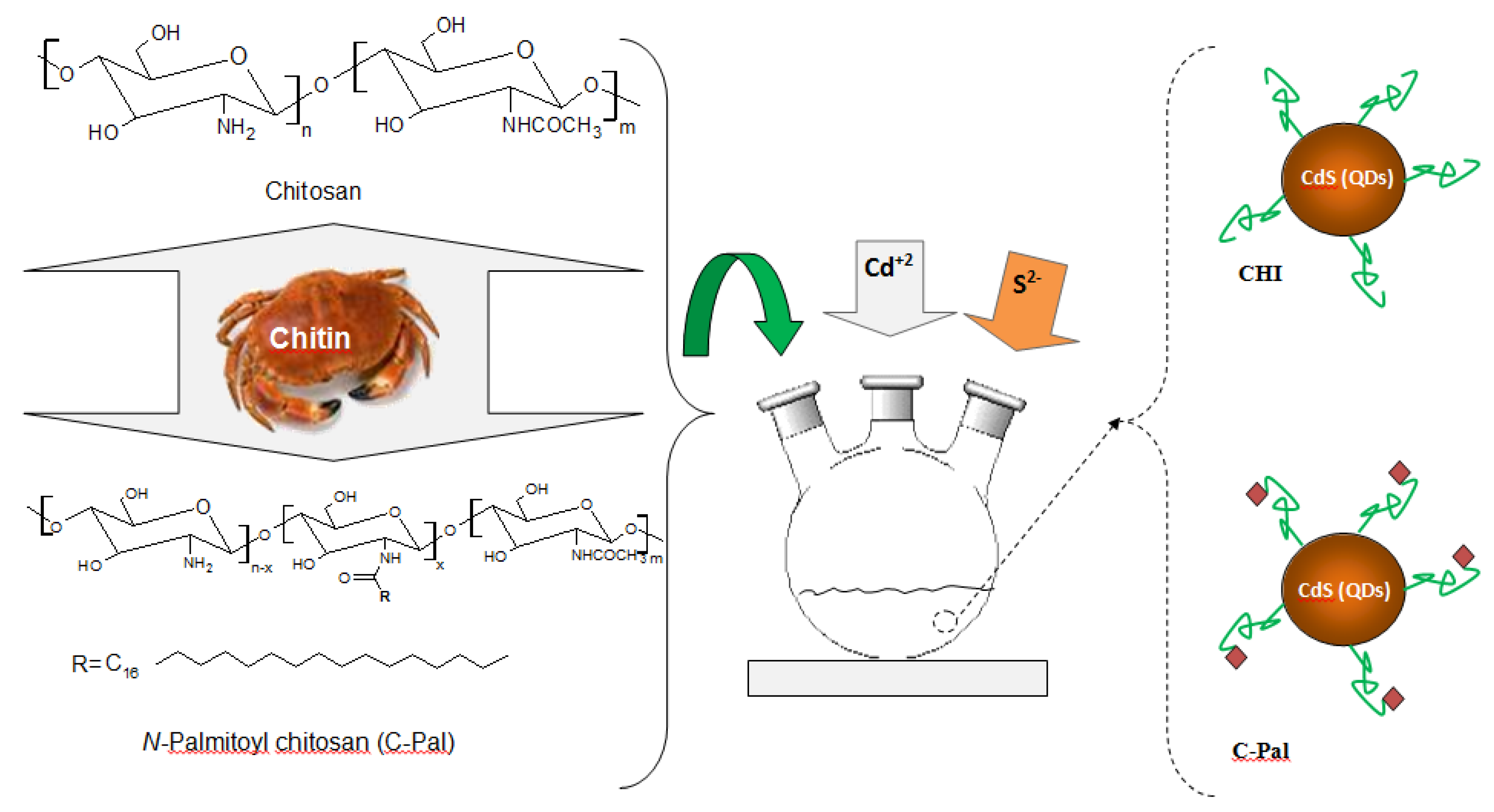
2.6. Characterization of Carbohydrates
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.2. Thermal Analysis (TG/DSC)
2.6.3. Degree of Swelling (DS) in Phosphate Buffer (PBS)
2.6.4. Surface Contact Angle (SCA)
2.6.5. Qualitative and Scanning Electron Microscopy (SEM) analysis
2.7. Characterization of CdS Quantum Dots
2.7.1. UV-Visible Spectroscopy (UV-Vis)
2.7.2. Photoluminescence spectroscopy (PL)
2.7.3. Transmission Electron Microscopy (TEM)
2.7.4. Dynamic Light Scattering (DLS) analysis
3. Results and Discussion
3.1. Characterization of Carbohydrates
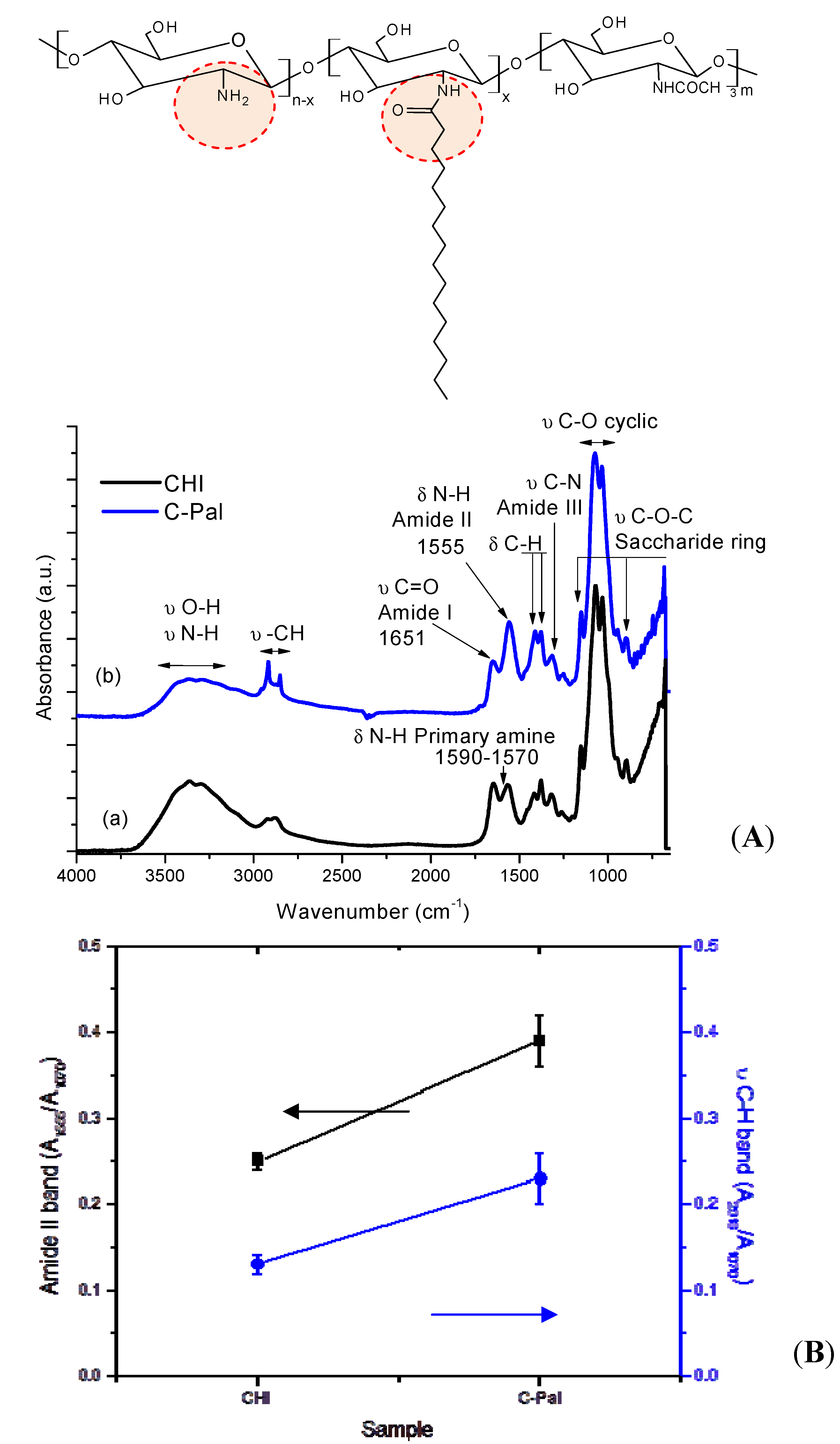
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
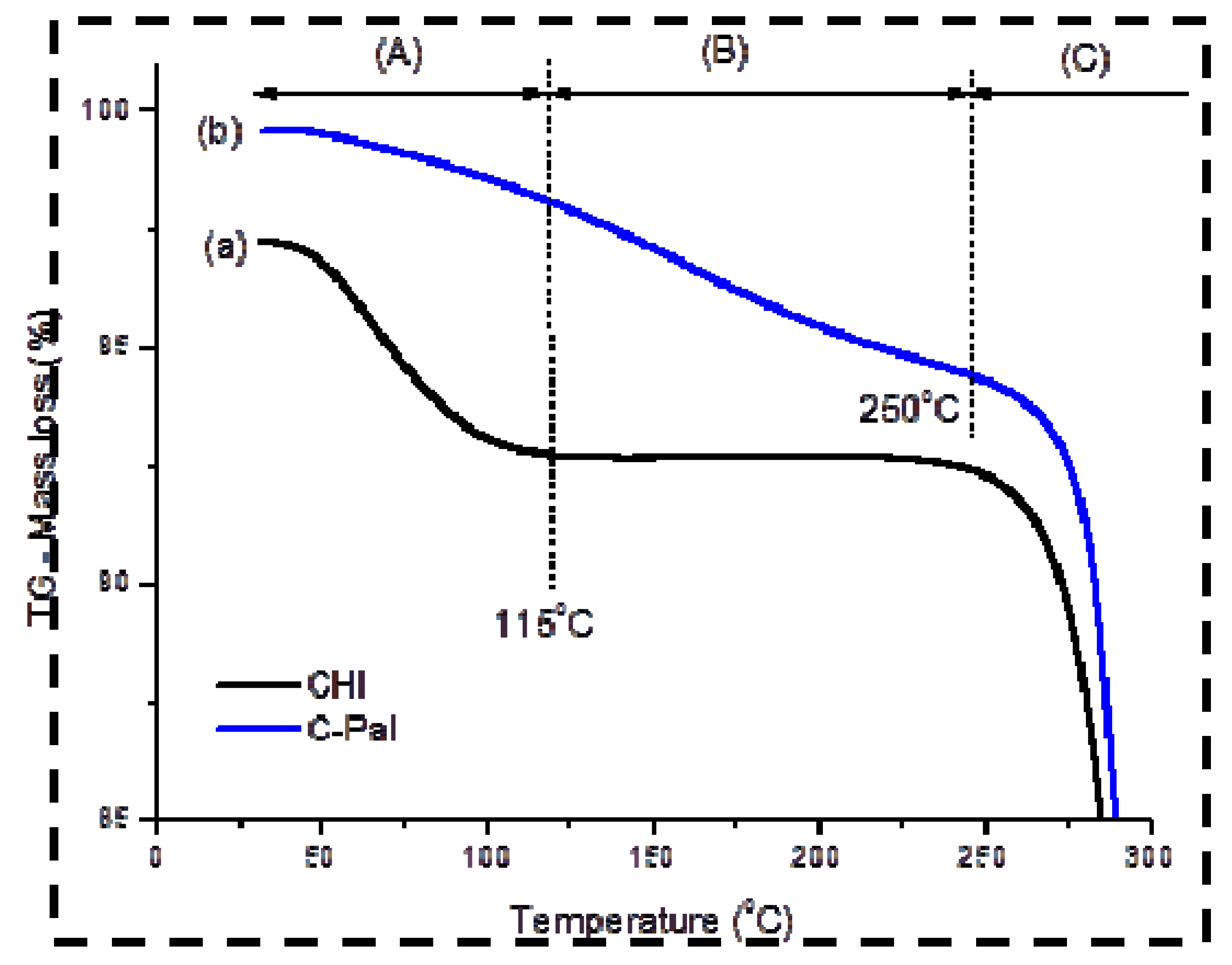
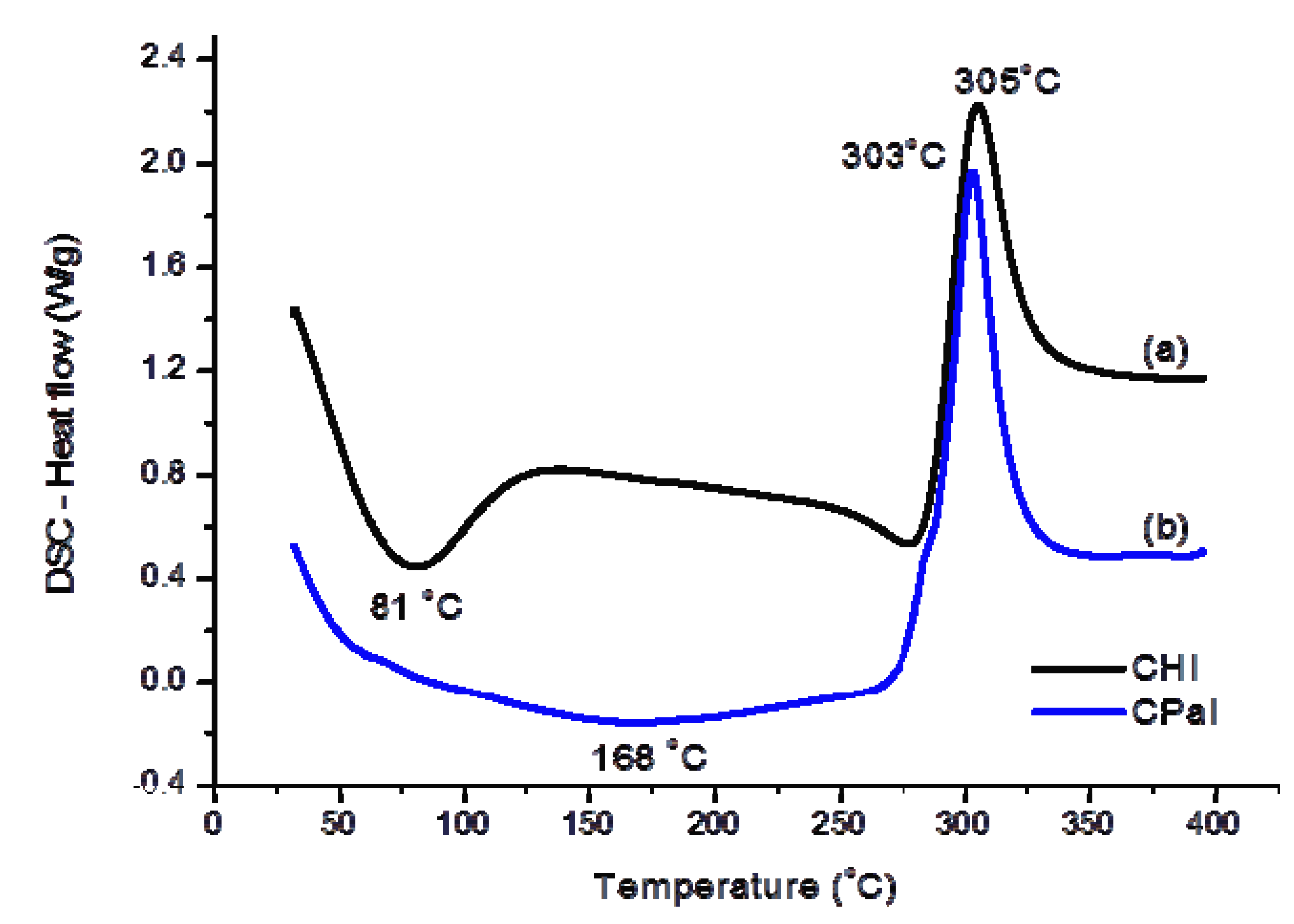
3.1.2. Thermal Analysis (TG/DSC)

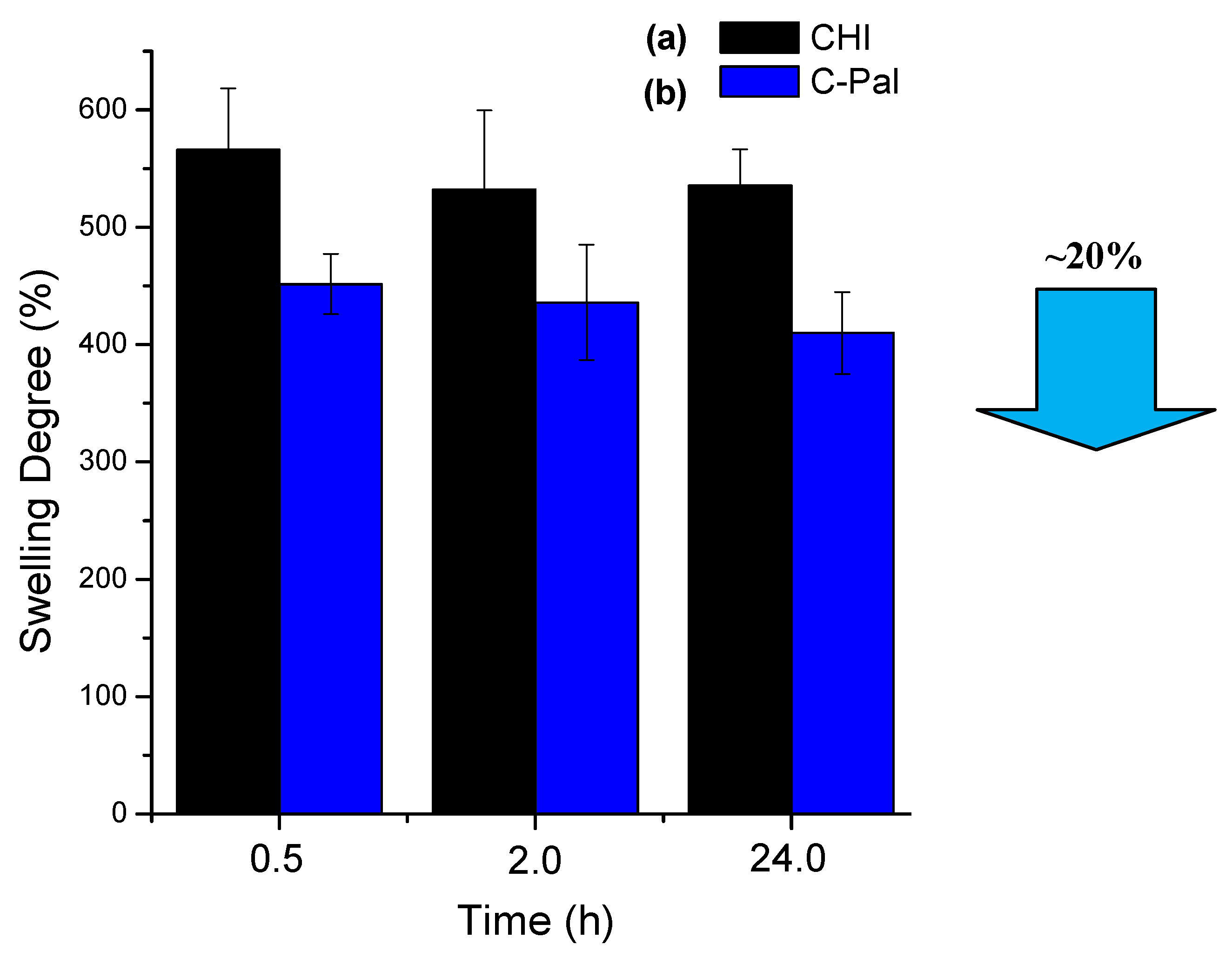
3.1.3. Degree of Swelling (DS) in Phosphate Buffer (PBS)
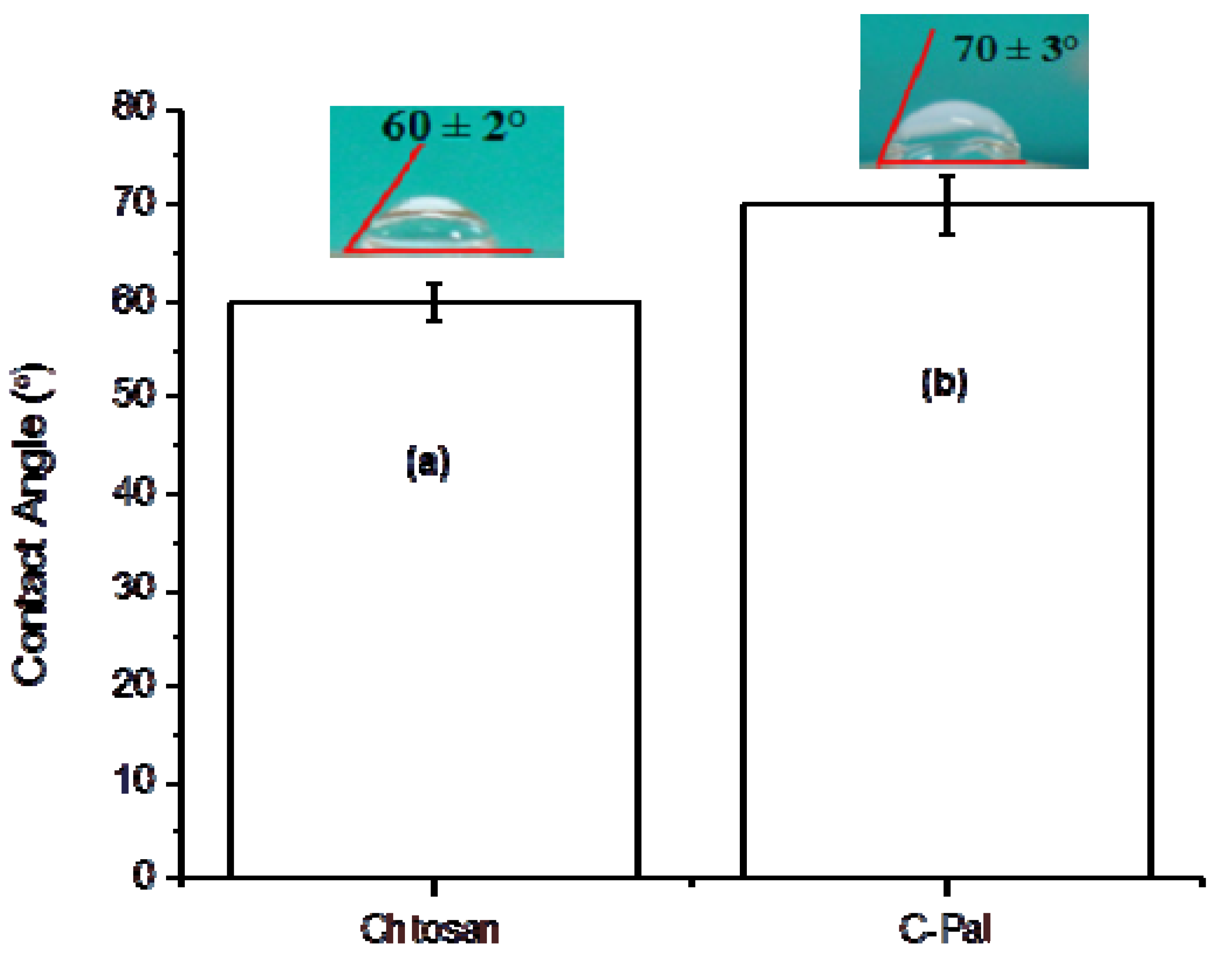
3.1.4. Surface Contact Angle (SCA)
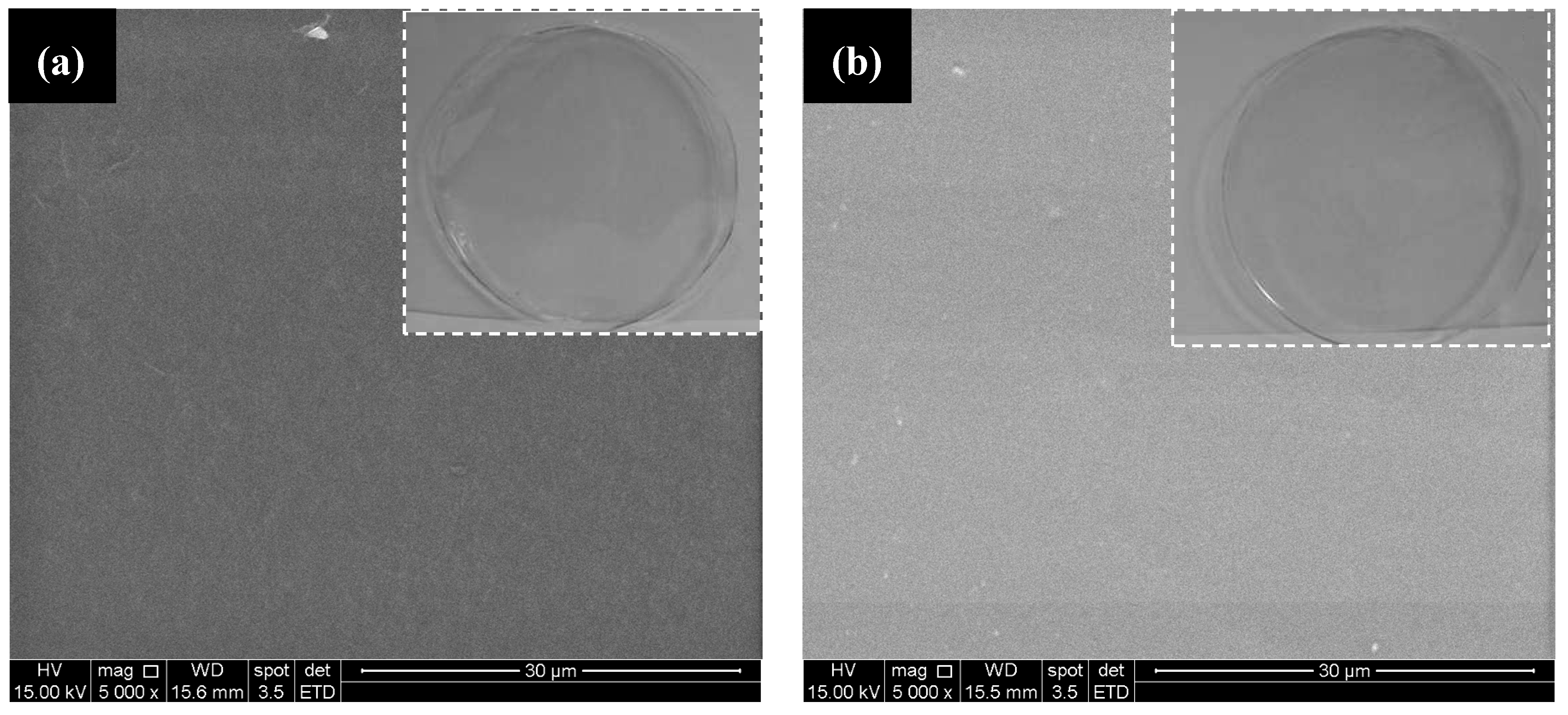
3.1.5. Qualitative and Scanning Electron Microscopy (SEM) Analysis
3.2. Characterization of CdS Quantum Dots
3.2.1. UV-Visible Spectroscopy (UV-Vis)
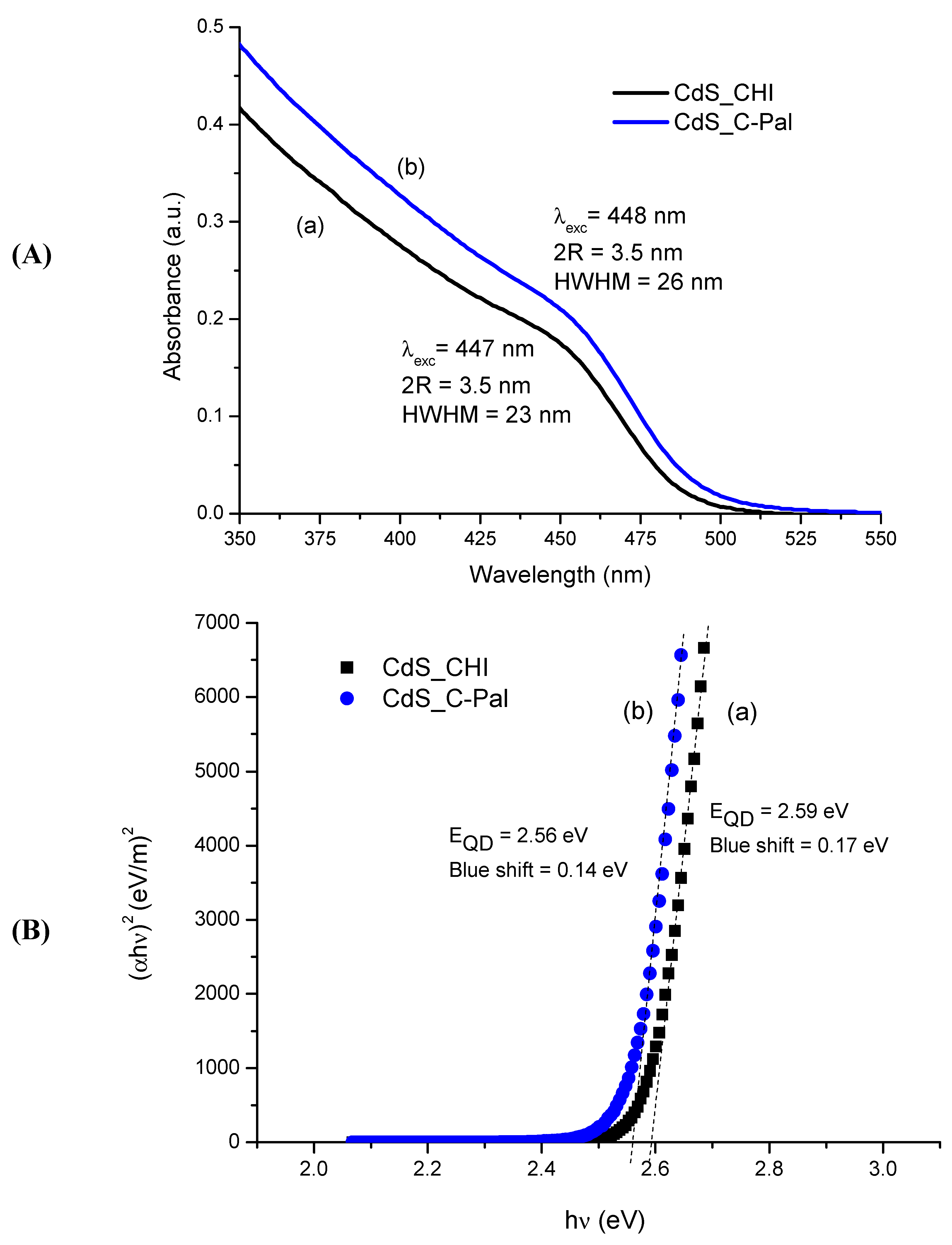
| System | Parameters | Values after 5 days |
|---|---|---|
| CdS-CHI | Band Gap (eV) | 2.59 ± 0.02 |
| Blue Shift (eV) | 0.17 ± 0.02 | |
| λexc (nm) | 447 ± 2 | |
| 2R (nm) | 3.5 ± 0.1 | |
| HWHM (nm) | 23 ± 1 | |
| CdS-CPal | Band Gap (eV) | 2.56 ± 0.02 |
| Blue Shift (eV) | 0.14 ± 0.02 | |
| λexc (nm) | 448 ± 2 | |
| 2R (nm) | 3.5 ± 0.1 | |
| HWHM (nm) | 26 ± 1 |

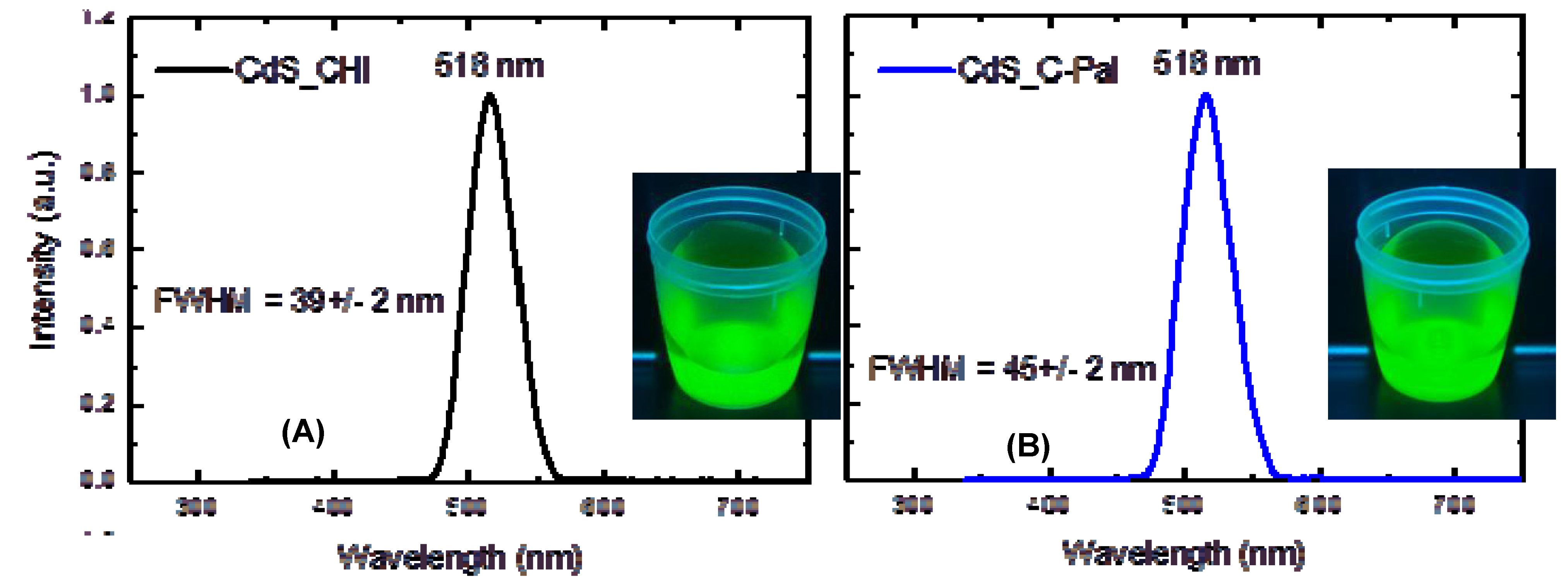
3.2.2. Photoluminescence Spectroscopy (PL)
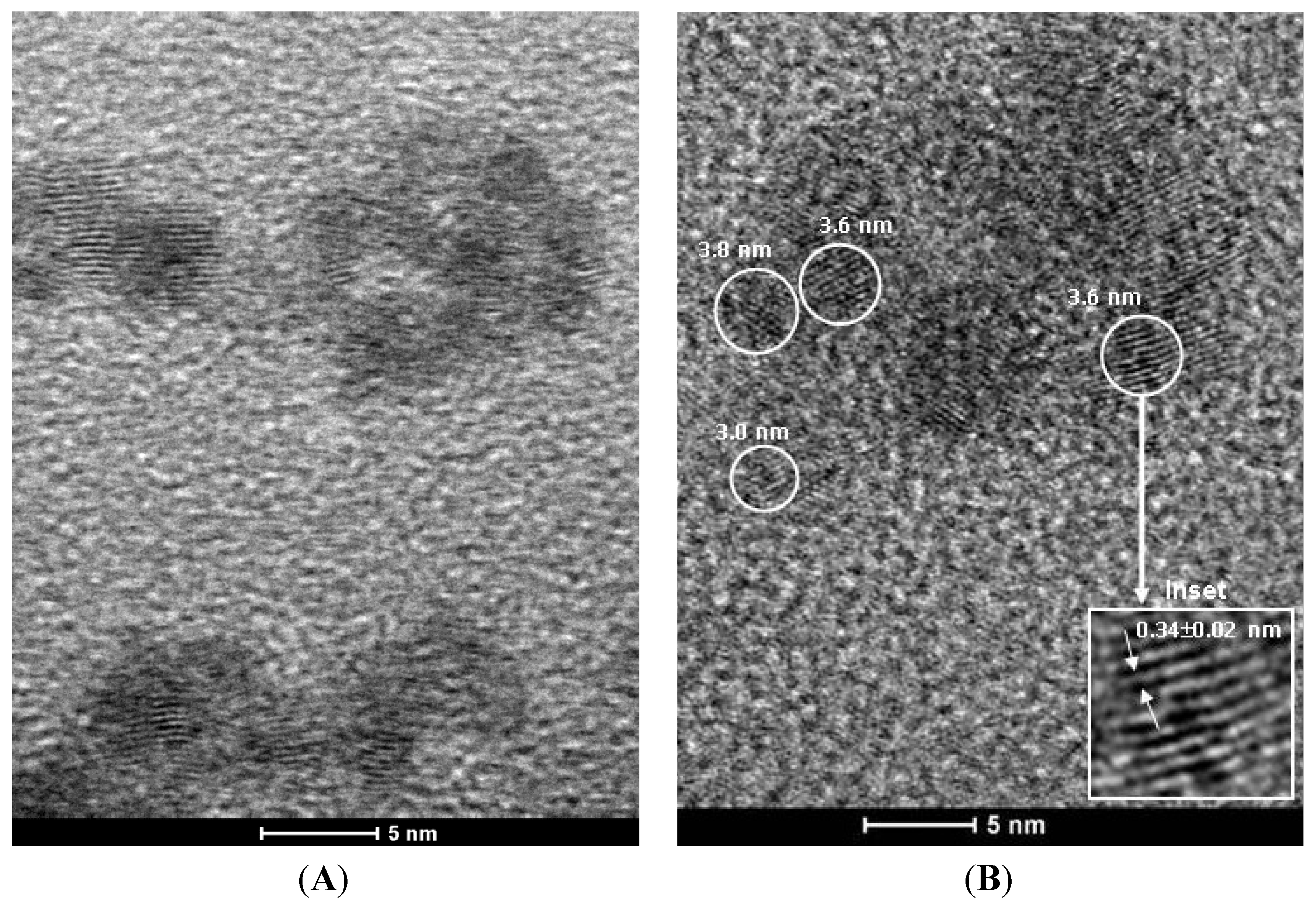
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. Dynamic Light Scattering (DLS) Analysis
3.3. Biofunctionalized QDs for Potential Bioapplications
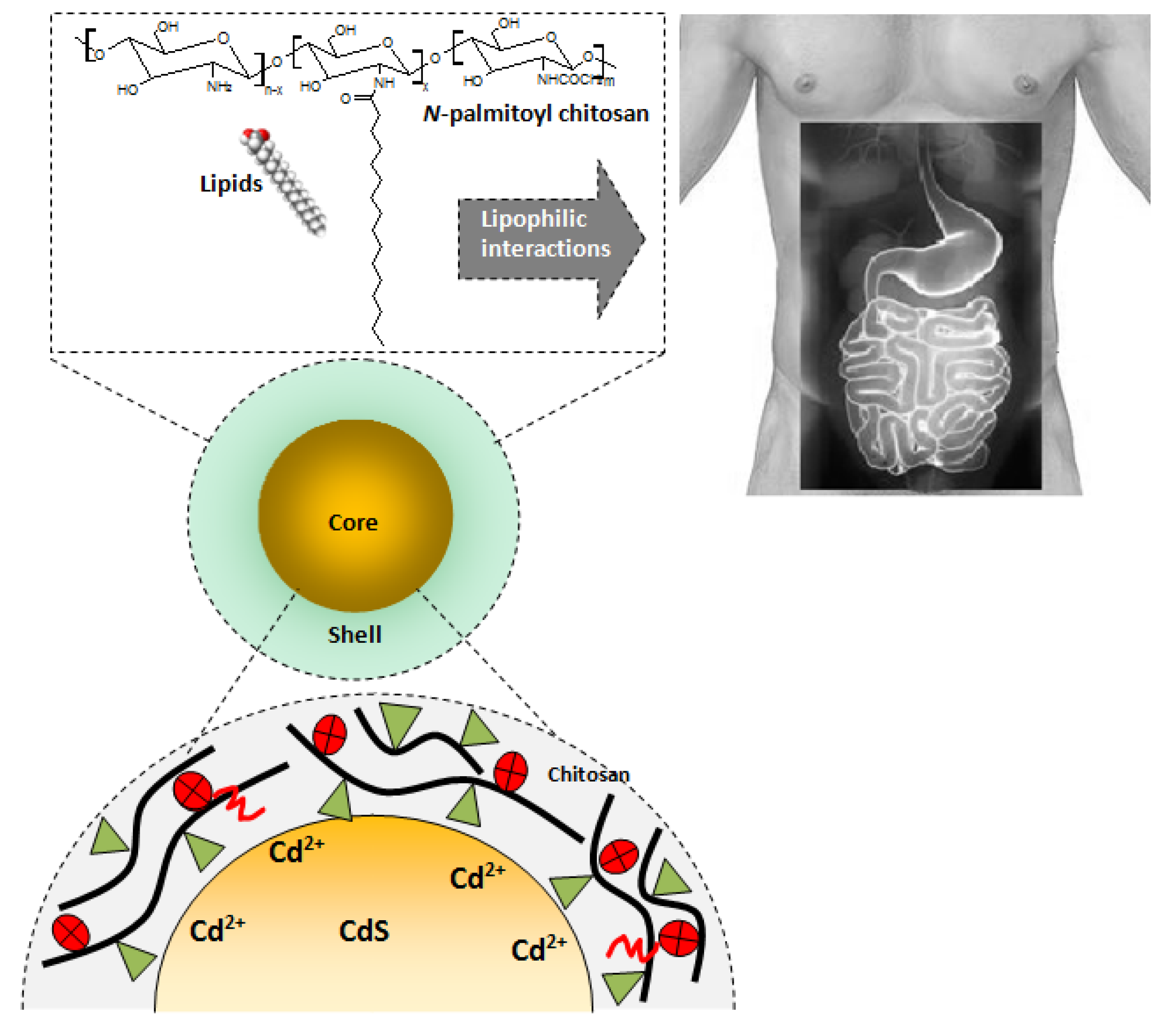
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wydro, P.; Krajewska, B.; Hac-Wydro, K. Chitosan as a lipid binder: A langmuir monolayer study of chitosan-lipid interactions. Biomacromolecules 2007, 8, 2611–2617. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, X.K.; Zhong, M.; Li, Z.J. Study of carbohydrate–protein interactions using glyco-QDs with different fluorescence emission wavelengths. Carbohydr. Res. 2012, 361, 189–194. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahi, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Chen, S.C.; Su, C.J.; Hsiao, C.W.; Chen, Y.M.; Chen, H.L.; Sung, H.W. PH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan. Biomaterials 2009, 30, 4877–4888. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progr. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Costa, E.S.; Barbosa-Stancioli, E.F.; Vasconcelos, W.; Mansur, H.; Mansur, A.A.P. Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohyd. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohyd. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Bispo, V.M.; Mansur, A.A.P.; Barbosa-Stancioli, E.F.; Mansur, H.S. Biocompatibility of nanostructured chitosan/poly(vinyl alcohol) blends chemically crosslinked with genipin for biomedical applications. J. Biomed. Nanotechnol. 2010, 6, 166–175. [Google Scholar] [CrossRef]
- Mansur, H.S.; Costa, E.S., Jr.; Mansur, A.A.P.; Barbosa-Stancioli, E.F. Cytocompatibility evaluation in cell-culture systems of chemically crosslinked chitosan/PVA hydrogels. Mater. Sci. Eng. C Biomim. Mater. Sens. Syst. 2009, 29, 1574–1583. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P. CdSe Quantum Dots stabilized by carboxylic-functionalized PVA: Synthesis and UV-Vis spectroscopy characterization. Mater. Chem. Phys. 2011, 125, 709–717. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P.; González, J.C. Synthesis and characterization of CdS quantum Dots with carboxylic-functionalized poly (vinyl alcohol) for bioconjugation. Polymer 2011, 52, 1045–1054. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P.; González, J.C. Biomolecule-quantum dot systems for bioconjugation applications. Colloids Surf. B 2011, 84, 360–368. [Google Scholar] [CrossRef]
- Mansur, A.; Mansur, H.; González, J. Enzyme-polymers conjugated to quantum-dots for sensing applications. Sensors 2011, 11, 9951–9972. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P. Fluorescent Nanohybrids: Quantum-dots Coupled to Polymer-Recombinant Protein Conjugates for the Recognition of Biological Hazards. J. Mater. Chem. 2012, 22, 9006–9018. [Google Scholar] [CrossRef]
- Božanić, D.K.; Djoković, D.; Bibić, N.; Nair, P.S.; Georges, M.K.; Radhakrishnan, T. Biopolymer-protected CdSe nanoparticles. Carbohydr. Res. 2009, 344, 2383–2387. [Google Scholar] [CrossRef]
- Chaudhuri, R.J.; Paria, S. Core/Shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Mansur, H.S. Quantum dots and nanocomposites. Wiley Int. Rev. Nanomed. Nanobiotechnol. 2010, 2, 113–129. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P.; Curti, E.; de Almeida, M.V. Functionalized-Chitosan/Quantum Dots Nano-hybrids for Nanomedicine Applications: Towards Biolabeling and Biosorbing Phosphate Metabolites. J. Mater. Chem. B 2013, 1, 1696–1711. [Google Scholar] [CrossRef]
- Esquenet, C.; Terech, P.; Boué, F.; Buhler, E. Structural and rheological properties of hydrophobically modified polysaccharide associative networks. Langmuir 2004, 20, 3583–3592. [Google Scholar] [CrossRef]
- Li, Z.; Du, Y.; Zhang, Z.; Pang, D. Preparation and characterization of CdS quantum dots chitosan biocomposite. React. Funct. Polym. 2003, 55, 35–43. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.; Yao, J.; Fu, Y.; Guan, Y. Chitosan hydrogel films as a template for mild biosynthesis of CdS quantum dots with highly efficient photocatalytic activity. Appl. Surf. Sci. 2012, 258, 3513–3518. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Termsarasab, U.; Cho, H.J.; Kim, D.H.; Chong, S.; Chung, S.J.; Shim, C.K.; Moon, H.T.; Kim, D.D. Chitosan oligosaccharide-arachidic acid-based nanoparticles for anti-cancer drug delivery. Int. J. Pharm. 2013, 441, 373–380. [Google Scholar] [CrossRef]
- Xu, J.; McCarthy, S.P.; Gross, R.A.; Kaplan, D.L. Chitosan film acylation and effects on biodegradability. Macromolecules 1996, 29, 3436–3440. [Google Scholar] [CrossRef]
- Le Tien, C.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-acylated chitosan: Hydrophobic matrices for controlled drug release. J. Control. Release 2003, 93, 1–13. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Monal, W.A.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polymer Edn 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A.F. Determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1980, 2, 115–116. [Google Scholar] [CrossRef]
- Santos, J.E.; Soares, J.P.; Dockal, E.R.; Campana-Filho, S.P.; Cavalheiro, E.T.G. Caracterização de quitosanas comerciais de diferentes origens. Polímeros 2003, 13, 242–249. [Google Scholar]
- Jiang, M.; Wanga, K.; Kennedy, J.F.; Nie, J.; Yu, Q; Ma, G. Preparation and characterization of water-soluble chitosan derivative by Michael addition reaction. Int. J. Biol. Macromol. 2010, 47, 696–699. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Elgannoudi, E.S.; Mainal, A.; Yahaya, A.H. Characterization of chitosan in acetic acid: Rheological and thermal studies. Turk. J. Chem. 2010, 34, 47–56. [Google Scholar]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohyd. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Yasuda, T.; Okuno, T. Contact angle of water on polymer surfaces. Langmuir 1994, 10, 2435–2439. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Weller, H.; Schmidt, H.M.; Koch, U.; Fojtik, A.; Baral, S.; Henglein, A.; Kunath, W.; Weiss, K.; Dieman, E. The Size distribution of semiconductor quantum dots (QDs). Chem. Phys. Lett. 1986, 124, 557–560. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Dai, Q.; Li, D.; Jiang, S.; Chen, H.; Wang, Y.; Kan, S.; Liu, B.; Cui, Q.; Zou, G. Synthesis of monodisperse CdSe nanocrystals directly to air: Monomer reactivity tuned by the selenium ligand. J. Cryst. Growth 2006, 292, 14–18. [Google Scholar] [CrossRef]
- Yu, W.W.; Falkner, J.C.; Shih, B.S.; Colvin, V.L. Preparation and characterization of monodisperse PbSe semiconductor nanocrystals in a noncoordinating solvent. Chem. Mater. 2004, 16, 3318–3322. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Yu, W.W.; Wang, Y.A.; Peng, X. Formation and stability of size-, shape-, and structure-controlled CdTe nanocrystals: Ligand effects on monomers and nanocrystals. Chem. Mater. 2003, 15, 4300–4308. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P.; Curti, E.; de Almeida, M.V. Bioconjugation of quantum-dots with chitosan and N,N,N-trimethyl chitosan. Carbohydr. Polym. 2012, 90, 189–196. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ghsoh, S.S.; Chattopadhyay, A. Quantum dot impregnated-chitosan film for heavy metal ion sensing and removal. Langmuir 2012, 28, 15687–15696. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Ho, Y.C.; Chen, Y.M.; Peng, S.F.; Ke, C.J.; Chen, K.J.; Mi, F.L.; Sung, H.W. The characteristics, cellular uptake and intracellular trafficking of nanoparticles made of hydrophobically-modified chitosan. J. Control. Release 2010, 146, 152–159. [Google Scholar] [CrossRef]
- Chiandotti, R.S.; Rodrigues, P.C.; Akcelrud, L. Grafting of chitosan with acyl derivatives. J. Braz. Chem. Soc. 2010, 21, 1910–1916. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-biointerface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Lakowicz, J.; Gryczynski, I.; Gregorz, P.; Murphy, C. Emission spectral properties of cadmium sulfide nanoparticles with multiphonon excitation. J. Phys. Chem. B 2002, 106, 5365–5370. [Google Scholar] [CrossRef]
- Smyntyna, V.; Skobeeva, V.; Malushin, N. The nature of emission centers in CdS nanocrystals. Rad. Meas. 2007, 42, 693–696. [Google Scholar] [CrossRef]
- Ramsden, J.J.; Gratzel, M. Photoluminescence of small cadmium sulphide particles. J. Chem. Soc. Faraday Trans. 1984, 80, 919–933. [Google Scholar] [CrossRef]
- Pons, T.; Uyeda, H.T.; Medintz, I.L.; Mattoussi, H. Hydrodynamic dimensions, electrophoretic mobility, and stability of hydrophilic quantum dots. J. Phys. Chem. B 2006, 220, 20308–20316. [Google Scholar]
- Mochalova, A.E.; Smirnova, L.A.; Zaitsev, S.D.; Semchikov, Y.D.; Zaitseva, I.I.; Pavlov, G.M. Hydrodynamic and molecular characteristics of graft copolymers of chitosan with acrylamide. Polym. Sci. Ser. B 2007, 49, 232–235. [Google Scholar] [CrossRef]
- Dowling, M.B.; Kumar, R.; Keibler, M.A.; Hess, J.R.; Bochicchio, G.V.; Raghavan, S.R. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials 2011, 32, 3351–3357. [Google Scholar] [CrossRef]
- Maezaki, Y.; Tsuji, K.; Nakagawa, Y.; Kawai, Y.; Akimoto, M.; Tsugita, T. Hypochloesterolemic effect of chitosan in adult males. Biosci. Biotech. Biochem. 1993, 57, 1439–1444. [Google Scholar] [CrossRef]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release 2012, 163, 34–35. [Google Scholar] [CrossRef]
- Jiang, G.B.; Liao, D.Q.K.; Wang, H. Novel polymer micelles prepared from chitosan grafted, hydrophobic palmitoyl groups for drug delivery. Mol. Pharm. 2006, 3, 152–160. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Santos, J.C.C.; Mansur, A.A.P.; Mansur, H.S. One-Step Biofunctionalization of Quantum Dots with Chitosan and N-palmitoyl Chitosan for Potential Biomedical Applications. Molecules 2013, 18, 6550-6572. https://doi.org/10.3390/molecules18066550
Santos JCC, Mansur AAP, Mansur HS. One-Step Biofunctionalization of Quantum Dots with Chitosan and N-palmitoyl Chitosan for Potential Biomedical Applications. Molecules. 2013; 18(6):6550-6572. https://doi.org/10.3390/molecules18066550
Chicago/Turabian StyleSantos, Joyce C. C., Alexandra A. P. Mansur, and Herman S. Mansur. 2013. "One-Step Biofunctionalization of Quantum Dots with Chitosan and N-palmitoyl Chitosan for Potential Biomedical Applications" Molecules 18, no. 6: 6550-6572. https://doi.org/10.3390/molecules18066550
APA StyleSantos, J. C. C., Mansur, A. A. P., & Mansur, H. S. (2013). One-Step Biofunctionalization of Quantum Dots with Chitosan and N-palmitoyl Chitosan for Potential Biomedical Applications. Molecules, 18(6), 6550-6572. https://doi.org/10.3390/molecules18066550