3.1. General
Melting points were determined on a Büchi B-510 apparatus and are uncorrected. The
1H-NMR (500 MHz) and
13C-NMR (125 MHz) spectra were recorded on a Bruker Ultrashield Plus Spectrometer (BrukerBioSpin GmbH, Rheinstetten, Germany) operating at 500 MHz for
1H and 125 MHz for
13C.
1H and
13C-NMR shifts (δ) are reported in parts per million (ppm) with respect to DMSO-
d6 (2.50 ppm for
1H and 39.7 ppm for
13C). Chemical shifts (δ) were reported in ppm and coupling constants (
J) in Hertz [Hz]. Signal multiplicity was assigned as singlet (s), doublet (d), doublet of doublets (dd), triplet (t), quartet (q), multiplet (m) and broad signal (bs). The low-resolution mass spectra were carried out on a Shimadzu GCMS-QP2010 Plus (Shimadzu Inc., Kyoto, Japan). Analytical conditions: Column: VF-5MS, 30 m × 0.25 mm × 0.25 μm (Varian Inc., Santa Clara, CA, USA); Column temperature: 200 °C for one minute and then increasing to 290 °C at a rate of 10 °C/min and holding for 40 minutes; Injector temperature: 270 °C. The high-resolution mass spectra were recorded with Q-TOF Micromass equipment (Waters, Milford, MA, USA). All the reactions involving microwave instrumentation used the Discover SP system (CEM Inc., Matthews, NC, USA) and were performed in open vessel mode. Analytical thin-layer chromatography (TLC) was performed on precoated silica gel plates (0.25 mm layer thickness) in an appropriate solvent and the spots were visualized under UV light (254 nm and 356 nm). The natural piperine (
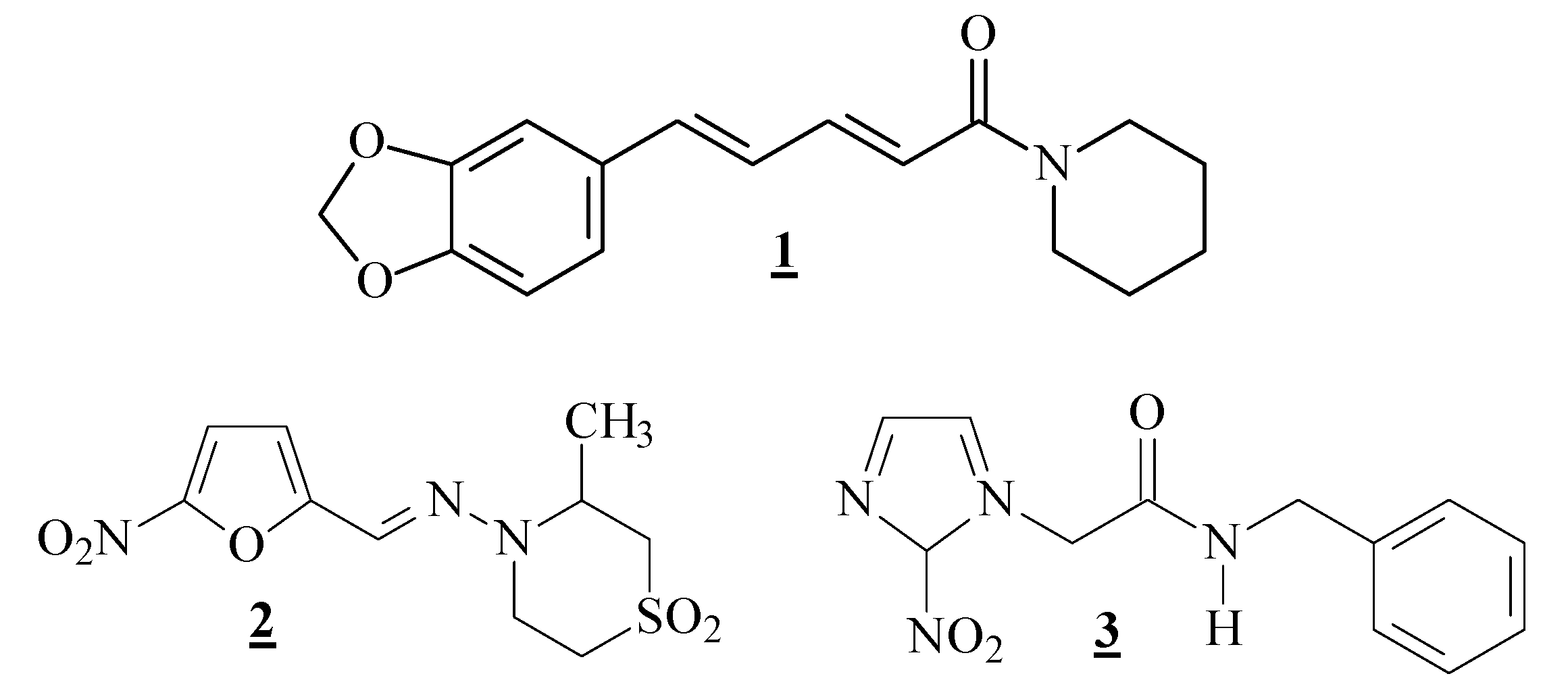
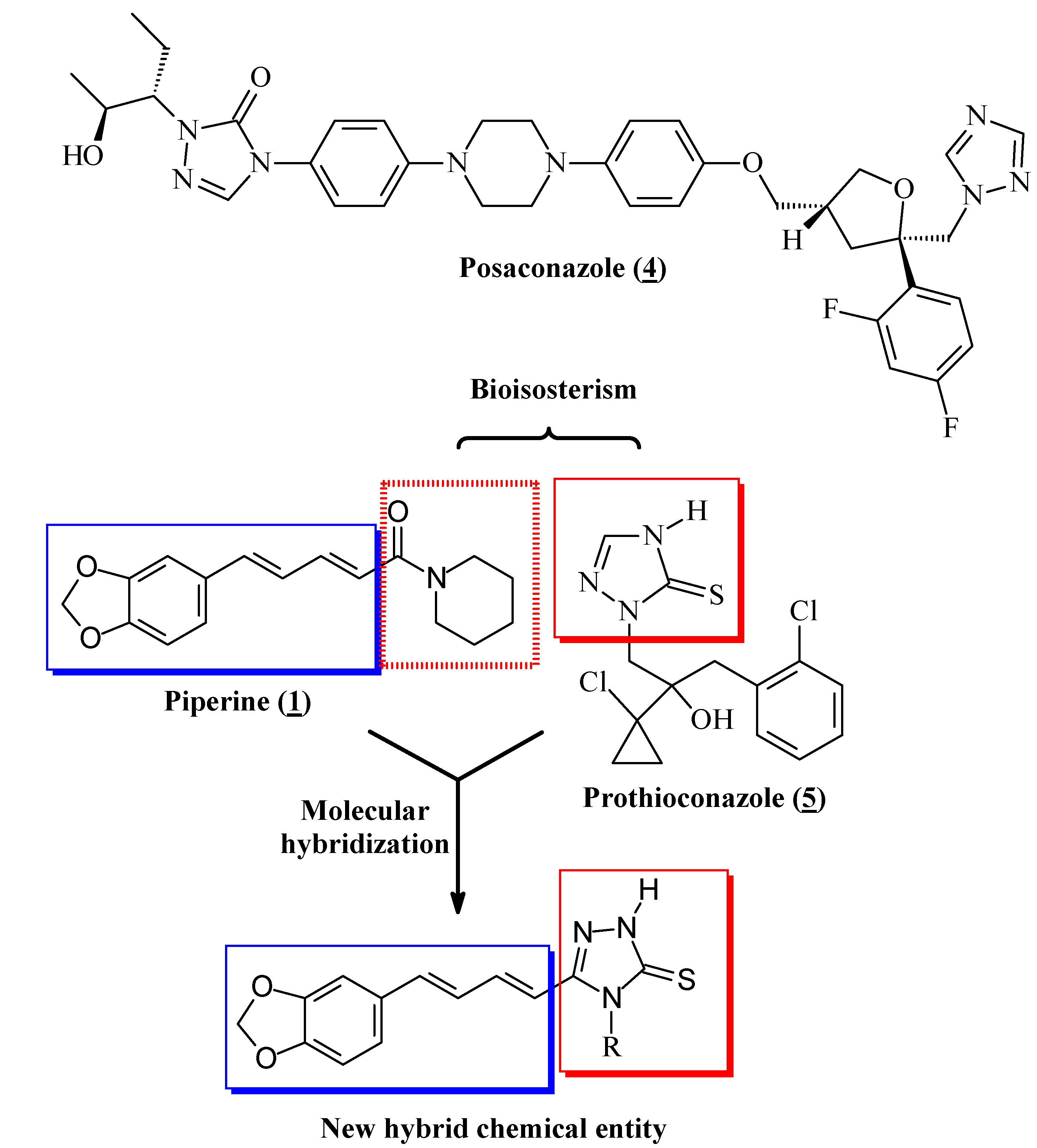
1) was extracted from powdered dry fruits of
P. nigrum purchased from different sources on the market and presented physical and spectroscopic data consistent with that originally described [
6,
36,
37]. The isolation method employed in this study was an adaptation of that previously reported [
6].
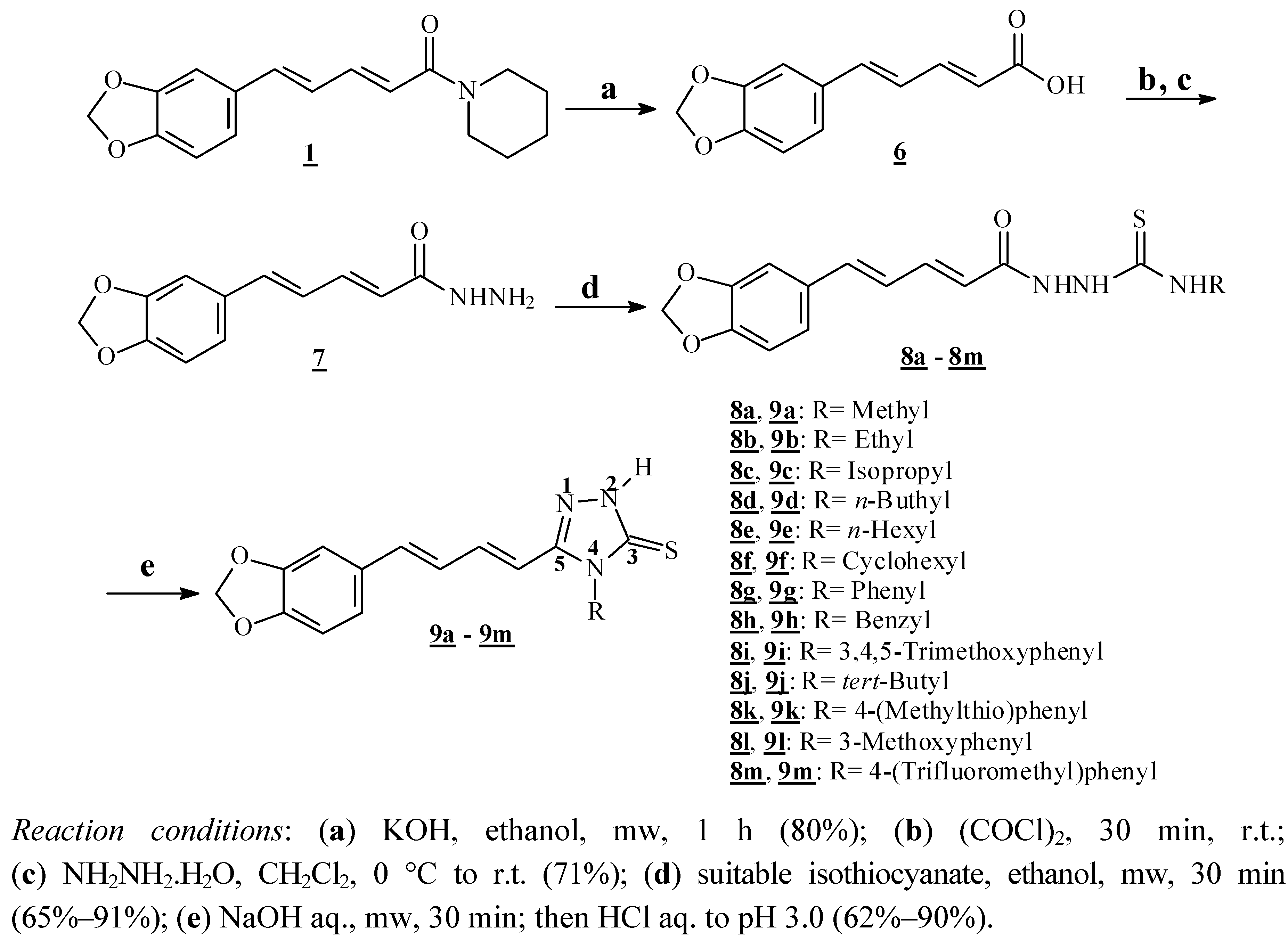
3.1.1. Microwave-assisted Hydrolysis of Natural Piperine: Preparation of 1-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]piperidine (6)
A suspension of piperine (
1, 2.20 g, 7.72 mmol) in an 20% (w/v) ethanolic solution of NaOH (22 mL) was placeded in a 50 mL round bottom flask equipped with a magnetic stirring bar and a reflux apparatus. This suspension was then subjected to microwave irradiation (100 W) for 1 h in open vessel mode. The evolution of the reaction was monitored by TLC. At the end of the reaction the solvent was removed under reduced pressure and hot water was added to solubilize the carboxylate. The warm mixture was filtered and the resulting solution was acidified with concentrated HCl (37% w/v) until pH 3.0, whereupon the free acid precipitated as a yellow solid. After filtration the solid was washed twice with ice water. The acid
6 (1.37 g; 82%) was thus obtained as a yellow solid after recrystallization from ethanol and was characterized by its melting point,
1H- and
13C-NMR. All data were consistent with those previously reported in the literature [
37].
3.1.2. Synthesis of (2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienohydrazide (7)
Acid 6 (500 mg, 2.29 mmol) and oxalyl chloride (1.5 mL, 17.60 mmol) were added to a 10 mL round bottom flask equipped with a magnetic stirring bar, rubber septum and kept under a dry N2 atmosphere. The resulting solution was subjected to stirring at room temperature for about 0.5 h. The evolution of the reaction was accompanied by TLC (indirectly by the reaction of an aliquot with methanol leading to the spontaneous formation of the corresponding methyl ester). After the removal of excess oxalyl chloride the resulting residue was dissolved in dry dichloromethane (5 mL). The resulting solution was added dropwise to an ice-cooled mixture of hydrazine monohydrate (1.0 mL, 20.64 mmol) and dichloromethane (7 mL) placed in a 25 mL round bottom flask equipped with stirring bar, rubber septum and kept under a dry N2 atmosphere. The reaction medium was allowed to reach room temperature (about thirty minutes) and then the solvent was removed under reduced pressure. The product precipitated with addition of ice water to the residue previously obtained and then was filtered, furnishing 375 mg (71% yield) of the hydrazide 7 as a yellow amorphous solid. m.p. = 237–239 °C. 1H-NMR δ (ppm): 9.30 (s, 1H, -NH-), 7.27 (d, 1H, J = 1.40 Hz), 7.19 (dd, 1H, J = 15.01 and 10.40 Hz), 7.00 (dd, 1H, J = 8.00 and 1.40 Hz), 6.86–6.97 (m, 3H), 6.05 (d, 1H, J = 15.10 Hz), 6.06 (s, 2H, -O-CH2-O-), 4.45 (s, 2H, -NH2); 13C-NMR δ (ppm): 165.23, 148.43, 148.19, 139.38, 138.26, 131.27, 125.82, 123.13, 123.05, 108.93, 106.10, 101.75.
3.1.3. General Procedure for the Synthesis of Carbothioamides 8a to 8m
The suitable alkylisothiocyanates (0.517 mmol, 1.2 equiv.) were added to a suspension of hydrazide 7 (100 mg, 0.431 mmol) in ethanol (5 mL). The mixture was placed in a 10 mL round bottom flask equipped with a magnetic stirring bar and a reflux apparatus and then subjected to microwave irradiation (100 W) for 0.5 h in open vessel mode. The evolution of the reaction was monitored by TLC. After elimination of ethanol under reduced pressure and addition of water to the vessel the products were collected and recrystallized from ethanol. All compounds were characterized by 1H- and 13C-NMR. The yields, melting points and spectral data observed for each carbothioamide 8a–8m prepared are as follows:
2-[(2E,4E)-5-(1,3-Bbenzodioxol-5-yl)penta-2,4-dienoyl]-N-methylhydrazinecarbothioamide (8a). Brown amorphous solid, yield 67%, m.p. = 195–196 °C (ethanol). 1H-NMR δ (ppm): 9.89 (s, 1H, NH), 9.27 (s, 1H, NH), 7.98 (s, 1H, NH), 7.32 (s, 1H), 7.29 (dd, 1H, J = 14.98 and 10.56 Hz), 6.93–7.04 (m, 4H), 6.09 (d, 1H, J = 15.00 Hz), 6.07 (s, 2H, -O-CH2-O-), 2.87 (d, 3H, J = 15.00 Hz, -NH-CH3); 13C-NMR δ (ppm): 165.65, 148.46, 141.37, 139.53, 131.20, 125.53, 123.51, 122.40, 108.96, 106.18, 101.81, 31.40.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-ethylhydrazinecarbothioamide (8b). Brown amorphous solid, yield 90%, m.p. = 192–193 °C. 1H-NMR δ (ppm): 9.86 (s, 1H, NH), 9.18 (s, 1H, NH), 8.00 (t, 1H, J = 5.20 Hz, NH), 7.29 (d, 1H, J = 1.40 Hz), 7.25 (dd, 1H, J = 15.18 and 9.91 Hz), 7.00 (dd, 1H, J = 8.28 and 1.51 Hz), 6.91–6.99 (m, 3H), 6.08 (d, 1H, J = 15.30 Hz), 6.05 (s, 2H, -O-CH2-O-), 3.39-3.49 (m, 2H, -NH-CH2-CH3), 1.05 (t, 3H, J = 7.00 Hz, -NH-CH2-CH3); 13C-NMR δ (ppm): 181.65, 165.61, 148.42, 141.35, 139.46, 131.16, 125.48, 123.38, 122.36, 108.94, 106.20, 101.76, 38.96, 14.87.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-isopropylhydrazinecarbothioamide (8c). Pale yellow amorphous solid, yield 66%, m.p. = 193–194 °C. 1H-NMR δ (ppm): 9.82 (s, 1H, NH), 9.16 (s, 1H, NH), 7.67 (d, 1H, J = 7.80 Hz, NH), 7.31 (s, 1H), 7.26 (dd, 1H, J = 14.98 and 10.25 Hz), 6.93–7.03 (m, 4H), 6.10 (d, 1H, J = 15.00 Hz), 6.07 (s, 2H, -O-CH2-O-), 4.34–4.46 (m, 1H, -NH-CH-(CH3)2), 1.12 (d, 6H, J = 6.60 Hz, -NH-CH-(CH3)2); 13C-NMR δ (ppm): 165.49, 148.46, 141.20, 139.47, 131.22, 125.53, 123.48, 122.56, 108.96, 106.17, 101.81, 46.24, 22.39.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-buthylhydrazinecarbothioamide (8d). Yellow amorphous solid, yield 87%, m.p. = 173–175 °C. 1H-NMR δ (ppm): 9.87 (s, 1H, NH), 9.19 (s, 1H, NH), 8.00 (s, 1H, NH), 7.33 (s, 1H), 7.27 (dd, 1H, J = 14.82 and 10.72 Hz), 6.94–6.98 (m, 2H), 7.02–7.30 (m, 2H), 6.10 (d, 1H, J = 15.00 Hz), 6.08 (s, 2H,-O-CH2-O-), 3.48-3.44 (m, 2H, -NH-CH2-CH2-CH2-CH3), 1.49 (m, 2H, -NH-CH2-CH2-CH2-CH3), 1.28 (m, 2H, -NH-CH2-CH2-CH2-CH3), 0.90 (t, 3H, J = 6.90 Hz, NH-CH2-CH2-CH2-CH3); 13C-NMR δ (ppm): 165.57, 148.46, 148.40, 141.27, 139.49, 131.22, 125.53, 123.49, 122.49, 108.96, 106.17, 101.81, 43.82, 31.39, 19.92, 14.31.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-hexylhydrazinecarbothioamide (8e). Yellow amorphous solid, yield 90%, m.p. = 165–167 °C. 1H-NMR δ (ppm): 9.86 (s, 1H, NH), 9.18 (s, 1H, NH), 7.99 (s, 1H, NH), 7.32 (s, 1H), 7.26 (dd, 1H, J =14.98 and 10.56 Hz), 6.93–7.02 (m, 2H), 7.03–7.24 (m, 2H), 6.11 (d, 1H, J = 15.00 Hz), 6.07 (s, 2H, -O-CH2-O-), 3.41–3.42 (m, 2H, -NH-CH2-CH2-CH2-CH2-CH2-CH3), 1.48-1.66 (m, 2H, -NH-CH2-CH2-CH2-CH2-CH2-CH3), 1.27–1.37 (m, 6H, -NH-CH2-CH2-CH2-CH2-CH2-CH3), 0.88 (t, 3H, J = 5.00 Hz, -NH-CH2-CH2-CH2-CH2-CH2-CH3); 13C-NMR δ (ppm): 165.65, 164.17, 148.48, 141.35, 139.56, 131.30, 125.62, 123.57, 122.56, 109.04, 106.25, 101.88, 44.21, 31.62, 29.26, 26.48, 22.65, 14.50.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-cyclohexylhydrazinecarbothioamide (8f). Yellow amorphous solid, yield 91%, m.p. = 192–194 °C. 1H-NMR δ (ppm): 9.84 (s, 1H, NH), 9.18 (s, 1H, NH), 7.65 (d, 1H, NH, J = 2.52 Hz), 7.32 (s, 1H), 7.25 (dd, 1H, J = 14.98 and 10.56 Hz), 7.03 (d, 1H, J = 8.20 Hz), 6.93-7.04 (m, 3H), 6.10 (d, 1H, J = 15.13 Hz), 6.07 (s, 2H, -O-CH2-O-), 4.07–4.19 (bs, 1H, -NH-CH-(CH2-CH2)2-CH2), 1.06–1.89 (m,10H, -NH-CH-(CH2-CH2)2-CH2); 13C-NMR δ (ppm): 162.92, 148.39, 141.20, 139.46, 131.22, 125.54, 123.47, 122.55, 108.96, 106.17, 101.81, 53.37, 32.35, 25.67, 25.40.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-phenylhydrazinecarbothioamide (8g). Yellow amorphous solid, yield 87%, m.p. = 149–150 °C. 1H-NMR δ (ppm): 10.11 (s, 1H, NH), 9.74 (s, 1H, NH), 9.68 (s, 1H, NH), 7.44–7.48 (bs, 2H), 7.34–7.36 (m, 2H), 7.33 (s, 1H), 7.27–7.33 (m, 1H), 7.16–7.18 (m, 1H), 6.84-7.04 (m, 4H), 6.15 (d, 1H, J = 13.87 Hz), 6.07 (s, 2H, -O-CH2-O-); 13C-NMR δ (ppm): 148.46, 141.32, 139.70, 139.54, 131.22, 130.43, 128.47, 126.47, 125.56, 123.51, 122.53, 108.97, 106.18, 101.81.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-benzylhydrazinecarbothioamide (8h). Yellow amorphous solid, yield 84%, m.p. = 196–197 °C. 1H-NMR δ (ppm): 9.98 (s, 1H, NH), 9.41 (s, 1H, NH), 8.59 (bs, 1H, NH), 7.32 (s, 1H), 7.29–7.32 (m, 5H), 7.24–7.27 (m, 1H), 6.94–7.04 (m, 4H), 6.11 (d, 1H, J = 15.00 Hz), 6.07 (s, 2H, -O-CH2-O-), 4.74 (d, 2H, J = 5.30 Hz); 13C-NMR δ (ppm): 165.71, 148.41, 141.37, 139.94, 139.56, 131.21, 128.51, 127.60, 127.08, 125.53, 123.51, 122.45, 108.97, 106.18, 101.82, 41.17.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-(3,4,5-trimethoxyphenyl) hydrazinecarbo-thioamide (8i). Pale yellow amorphous solid, yield 77%, m.p. = 189–191 °C. 1H-NMR δ (ppm): 10.08 (s, 1H, NH), 9.67 (s, 1H, NH), 9.60 (s, 1H, NH), 7.32 (s, 1H), 7.27–7.32 (m, 1H), 7.03 (dd, 1H, J = 7.88 and 1.26 Hz), 6.86–7.06 (m, 3H), 6.91 (s, 2H), 6.16 (d, 1H, J = 11.03 Hz), 6.07 (s, 2H, -O-CH2-O-), 3.76 (s, 6H), 3.66 (s, 3H); 13C-NMR δ (ppm): 153.79, 148.46, 148.42, 141.42, 139.59, 135.40, 135.07, 131.21, 125.55, 123.51, 122.43, 108.97, 106.19, 104.42, 101.81, 60.56, 56.31.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-tert-butylhydrazinecarbothioamide (8j). Yellow amorphous solid, yield 80%, m.p. = 135–136 °C. 1H-NMR δ (ppm): 10.18 (s, 1H, NH), 9.95 (s, 1H, NH), 9.15 (s, 1H, NH), 7.30 (s, 1H), 7.24–7.30 (m, 1H), 7.02 (dd, 1H, J = 8.16 and 1.38 Hz), 6.92–6.99 (m, 3H), 6.13 (d, 1H, J = 14.81 Hz), 6.07 (s, 2H, -O-CH2-O-), 1.46 (s, 9H, -C-(CH3)3); 13C-NMR δ (ppm): 178.73, 169.55, 148.44, 141.54, 139.58, 131.17, 125.50, 123.48, 121.91, 108.94, 106.14, 101.81, 53.19, 29.09.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-[4-(methylthio)phenyl] hydrazinecarbothio-amide (8k). Yellow amorphous solid, yield 84%, m.p.= 175–177 °C. 1H-NMR δ (ppm): 10.24 (s, 1H, NH), 9.80 (s, 2H, NH), 7.55-7.64 (m, 1H), 7.55 (bs, 1H), 7.36-7.45 (m, 4H),7.06–7.18 (m, 4H), 6.27 (d, 1H, J = 14.10 Hz), 6.20 (s, 2H, -O-CH2-O-), 2.65 (s, 3H, -S-CH3); 13C-NMR δ (ppm): 148.48, 141.36, 139.57, 136.96, 131.24, 126.30, 125.57, 123.52, 122.53, 121.67, 108.99, 106.22, 101.83, 15.63.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-(3-methoxyphenyl) hydrazinecarbothio-amide (8l). Dark green amorphous solid, yield 65%, m.p.= 174–175 °C. 1H-NMR δ (ppm): 10.09 (s, 1H, NH), 9.70 (s, 2H, NH), 7.32 (s, 1H), 7.22–7.38 (m, 3H), 6.93–7.08 (m, 5H), 6.74 (d, 1H, J = 8.03 Hz), 6.13 (d, 1H, J = 15.30 Hz), 6.07 (s, 2H, -O-CH2-O-), 3.75 (s, 3H); 13C-NMR δ (ppm): 181.15, 172.77, 164.55, 148.45, 141.33, 140.79, 139.54, 131.19, 131.13, 125.53, 123.50, 122.45, 118.68, 114.96, 111.62, 108.94, 106.15, 101.79, 55.54.
2-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)penta-2,4-dienoyl]-N-[4-(trifluoromethyl)phenyl] hydrazinecarbo-thioamide (8m). Pale yellow amorphous solid, yield 83%, m.p. = 177–178 °C. 1H-NMR δ (ppm): 10.18 (s, 1H, NH), 10.15 (s, 1H, NH), 9.93 (s, 1H, NH), 7.69–7.80 (m, 4H), 7.33 (s, 1H), 7.27–7.34 (m, 1H), 6.93–7.08 (m, 4H), 6.16 (d, 1H, J = 14.80 Hz), 6.07 (s, 2H, -O-CH2-O-); 13C-NMR δ (ppm): 181.27, 164.55, 148.45, 143.54, 141.54, 139.69, 131.16, 130.10, 125.49, 124.81 (q, CF3, JC-F = 270 Hz), 123.53, 122.28, 121.60, 117.13, 108.94, 106.16, 101.80.
3.1.4. General Procedure to the Synthesis of Triazoles 9a to 9m
In a 10 mL bottom rounded flask equipped with a magnetic stirring bar and a reflux apparatus were placed the corresponding hydrazine carbothioamide 8a–8m (1.37 mmol) and an aqueous solution of NaOH (2 mL, 1 equiv.). This mixture was then subjected to microwave irradiation (100 W) for 0.5 h in open vessel mode. The evolution of the reaction was monitored by TLC. Then the mixture was acidified with a 10% aqueous solution of HCl (w/v) until pH 3, filtered and the solids were washed twice with ice water (5 mL). After recrystallization from ethanol all the triazoles were characterized by NMR and low- and high-resolution mass spectra. All compounds were characterized by 1H- and 13C- NMR. The yields, melting points and spectral data observed for each carbothioamide 9a–9m prepared are as follows:
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-methyl-2,4-dihydro-3H-1,2,-triazole-3-thione (9a). Brown amorphous solid, yield 71%, m.p. = 220–222 °C. 1H-NMR δ (ppm): 13.78 (s, 1H, NH), 7.28 (dd, 1H, J = 15.40 and 11.00 Hz), 7.23 (s, 1H), 6.90–7.07 (m, 4H), 6.54 (d, 1H, J = 15.40 Hz), 6.07 (s, 2H, -O-CH2-O-), 3.39 (s, 3H, -N-CH3); 13C-NMR δ (ppm): 167.11, 150.15, 148.45, 148.24, 137.55, 137.02, 131.31, 126.50, 123.00, 113.95, 109.03, 105.91, 101.77, 30.40; MS (m/z): 287; HRMS (ES)-m/z: [M+H]+, found 288.0807. C15H15N3O2S requires 288.0806.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-ethyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9b). Brown amorphous solid, yield 62%, m.p. = 208–209 °C. 1H-NMR δ (ppm): 13.76 (s, 1H, NH), 7.28 (dd, 1H, J = 15.30 and 10.70 Hz), 7.20 (s, 1H), 6.88–7.07 (m, 4H), 6.57 (d, 1H, J = 15.30 Hz), 6.05 (s, 2H, -O-CH2-O-), 4.08 (q, 2H, J = 7.03 Hz, -N-CH2-CH3), 1.19 (t, 3H, J = 7.03 Hz, -NH-CH2-CH3); 13C-NMR δ (ppm): 166.46, 149.55, 148.44, 148.23, 137.56, 137.21, 131.32, 126.50, 122.95, 113.50, 109.02, 105.90, 101.75, 38.33, 14.22; MS (m/z): 301; HRMS (ES)-m/z: [M+H]+, found 302.0963. C15H15N3O2S requires 302.0963.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-isopropyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9c). Pale yellow amorphous solid, yield 90%, m.p. = 210–211 °C. 1H-NMR δ (ppm): 13.78 (s, 1H, NH), 7.15-7.27 (m, 2H), 7.20 (s, 1H), 6.87–7.00 (m, 3H), 6.75 (d, 1H, J = 15.00 Hz), 6.07 (s, 2H, -O-CH2-O-), 5.09-5.14 (m, 1H, -NH-CH-(CH3)2), 1.47 (d, 6H, J = 5.00 Hz, -NH-CH-(CH3)2); 13C-NMR δ (ppm): 166.46, 149.57, 148.46, 148.22, 137.23, 131.44, 126.77, 122.96, 114.55, 109.06, 105.84, 101.81, 46.61, 20.78; MS (m/z): 315; HRMS (ES)-m/z: [M+H]+, found 316.1120. C16H17N3O2S requires 316.1119.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-butyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9d). Yellow amorphous solid, yield 71%, m.p. = 173–174 °C. 1H-NMR δ (ppm): 13.78 (s, 1H, NH), 7.29 (dd, 1H, J = 15.10 and 11.00 Hz), 7.22 (s, 1H), 6.89–7.09 (m, 4H), 6.58 (d, 1H, J = 15.10 Hz), 6.07 (s, 2H, -O-CH2-O-), 4.04 (t, 2H, J = 6.78 Hz, -NH-CH2-CH2-CH2-CH3), 1.60–1.63 (m, 2H, -NH-CH2-CH2-CH2-CH3), 1.31-1.36 (m, -NH-CH2-CH2-CH2-CH3), 0.93 (t, 3H, J = 7.20 Hz, -NH-CH2-CH2-CH2-CH3); 13C-NMR δ (ppm): 166.74, 149.79, 148.48, 148.27, 137.58, 137.17, 131.55, 125.53, 126.55, 123.06, 113.56, 109.06, 105.89, 101.81, 42.92, 30.87, 19.78, 14.12; MS (m/z): 329; HRMS (ES)-m/z: [M+H]+, found 330.1276. C17H19N3O2S requires 330.1276.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9e). Dark green amorphous solid, yield 79%, m.p. = 124–125 °C. 1H-NMR δ (ppm): 13.77 (s, 1H, NH), 7.27–7.32 (m, 1H), 7.22 (s, 1H), 6.89–7.06 (m, 4H), 6.57 (d, 1H, J = 15.45 Hz), 6.07 (s, 2H, -O-CH2-O-), 4.03 (t, 2H, J = 7.57 Hz, -NH-CH2-CH2-CH2-CH2-CH2-CH3), 1.62–1.64 (m, 2H, -NH-CH2-CH2-CH2-CH2-CH2-CH3), 1.27–1.30 (m, 6H, -NH-CH2-CH2-CH2-CH2-CH2-CH3), 0.84–0.90 (m, 3H, -NH-CH2-CH2-CH2-CH2-CH2-CH3); 13C-NMR δ (ppm): 166.73, 149.79, 148.48, 137.58, 137.19, 131.35, 126.54, 123.06, 113.56, 109.07, 105.89, 101.81, 43.09, 31.26, 28.66, 26.06, 22.47, 14.36; MS (m/z): 357; HRMS (ES)-m/z: [M+H]+, found 358.1589. C19H23N3O2S requires 358.1589.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-cyclohexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9f). Yellow amorphous solid, yield 91%, m.p. = 192–194 °C. 1H-NMR δ (ppm): 13.76 (s, 1H, NH), 7.19 (d, 1H, J = 1.89 Hz), 7.19–7.24 (m, 1H), 7.11–7.17 (m, 1H), 6.99 (dd, 1H, J = 10.00 and 5.00 Hz), 6.93 (d, 1H, J = 8.20 Hz), 6.85 (d, 1H, J = 15.13 Hz), 6.75 (d, 1H, J = 14.50 Hz), 6.05 (s, 2H, -O-CH2-O-), 4.55–4.75 (bs, 1H, -NH-CH-(CH2-CH2)2-CH2), 1.33–2.08 (m, 10H, -NH-CH-(CH2-CH2)2-CH2); 13C-NMR δ (ppm): 166.63, 149.63, 148.47, 148.21, 141.20, 137.22, 131.45, 126.80, 122.97, 114.63, 109.06, 105.82, 101.81, 55.45, 32.35, 26.13, 24.87; MS (m/z): 355; HRMS (ES)-m/z: [M+H]+, found 356.1433. C19H21N3O2S requires 356.1432.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9g). Yellow amorphous solid, yield 89%, m.p. = 244–245 °C. 1H-NMR δ (ppm): 14.00 (s, 1H, NH), 7.58–7.65 (m, 3H), 7.44 (dd, 2H, J = 8.04 and 1.42 Hz), 7.20 (d, 1H, J = 1.58 Hz), 7.15 (dd, 1H, J = 15.00 and 10.00 Hz), 6.94–7.00 (m, 2H), 6.89 (d, 1H, J = 7.88 Hz), 6.78 (d, 1H, J = 15.45 Hz), 6.04 (s, 2H, -O-CH2-O-) 5.94 (d, 1H, J = 15.45 Hz);13C-NMR δ (ppm): 168.03, 149.83, 148.25, 137.79, 137.37, 134.02, 131.31, 130.10, 129.06, 126.54, 123.29, 113.40, 108.93, 105.91, 101.75; MS (m/z): 349; HRMS (ES)-m/z: [M+H]+, found 350.0963. C19H15N3O2S requires 350.0963.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-benzyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9h). Yellow amorphous solid, yield 76%, m.p. = 187–188 °C. 1H-NMR δ (ppm): 13.97 (s, 1H, NH), 7.37–7.40 (m, 2H), 7.28–7.32 (m, 3H), 7.21 (d, 1H, J = 1.26 Hz), 7.21–7.26 (m, 1H), 6.93 (d, 1H, J = 15.45 Hz), 6.93–6.99 (m, 2H), 6.85 (d, 1H, J = 15.45 Hz), 6.42 (d, 1H, J = 15.45 Hz), 6.06 (s, 2H, -O-CH2-O-), 5.35 (s, 2H); 13C-NMRδ (ppm): 167.49, 150.06, 148.30, 137.94, 137.38, 136.42, 131.24, 129.24, 128.21, 127.37, 126.35, 123.20, 113.40, 109.01, 105.95, 101.80, 45.92; MS (m/z): 363; HRMS (ES)-m/z: [M+H]+, found 364.1120. C20H17N3O2S requires 364.1119.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-(3,4,5-trimethoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (9i). Pale yellow amorphous solid, yield 81%, m.p. = 242–243 °C. 1H-NMR δ (ppm): 13.94 (s, 1H, NH), 7.21 (d, 1H, J = 1.58 Hz), 7.17–7.22 (m, 1H), 7.03 (dd, 1H, J = 15.00 and 10.00 Hz), 6.96 (dd, 1H, J = 10.00 and 5.00 Hz), 6.90 (d, 1H, J = 7.88 Hz), 6.81 (d, 1H, J = 15.00 Hz), 6.80 (s, 2H), 6.03 (d, 1H, J = 15.00 Hz), 6.04 (s, 2H, -O-CH2-O-), 3.80 (s, 6H), 3.78 (s, 3H); 13C-NMR δ (ppm): 168.01, 153.73, 150.06, 148.43, 148.22, 138.41, 137.58, 137.16, 131.39, 129.53, 126.74, 123.23, 113.65, 108.94, 106.85, 105.89, 101.75, 60.59, 56.77; MS (m/z): 439; HRMS (ES)-m/z: [M+H]+, found 440.1280. C22H21N3O5S requires 440.1280.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-tert-butyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (9j). Yellow amorphous solid, yield 50%, m.p. = 183–184 °C. 1H-NMR δ (ppm): 7.63 (s, 1H, NH), 7.22 (s, 1H), 6.84 (d, 1H, J = 15.06 Hz), 6.89–7.04 (m, 4H), 6.48 (d, 1H, J = 15.31 Hz), 6.06 (s, 2H, -O-CH2-O-), 1.36 (s, 9H, -C-(CH3)3); 13C-NMR δ (ppm): 161.97, 157.91, 148.42, 148.12, 136.76, 135.29, 131.41, 126.51, 122.87, 113.17, 109.00, 105.87, 101.76, 51.50, 28.79; MS (m/z): 329; HRMS (ES)-m/z: [M+H]+, found 330.1276. C17H20N3O2S requires 330.1276.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-[4-(methylthio)phenyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (9k). Pale yellow amorphous solid, yield 75%, m.p. = 209–211 °C. 1H-NMR δ (ppm): 13.98 (s, 1H, NH), 7.47 (d, 2H, J = 10.00 Hz), 7.37 (d, 2H, J = 10.00 Hz), 7.20 (d, 1H, J = 1.00 Hz), 7.16–7.22 (m, 1H), 7.00 (dd, 1H, J = 15.00 and 10.00 Hz), 6.95 (dd, 1H, J = 10.00 and 5.00 Hz), 6.90 (d, 1H, J = 8.03 Hz), 6.81 (d, 1H, J = 15.31 Hz), 6.04 (s, 2H, -O-CH2-O-), 5.95 (d, 1H, J = 15.56 Hz), 2.52 (s, 3H, -S-CH3); 13C-NMR δ (ppm): 148.48, 141.36, 139.57, 136.96, 131.24, 126.30, 125.57, 123.52, 122.53, 121.67, 108.99, 106.22, 101.83, 15.63; MS (m/z): 396; HRMS (ES)-m/z: [M+H]+, found 396.0840. C20H18N3O2S2 requires 396.0840.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-(3-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (9l). Yellow amorphous solid, yield 80%, m.p. = 248–249 °C. 1H-NMR δ (ppm): 13.97 (s, 1H, NH), 7.53 (t, 1H, J = 8.16 Hz), 7.20 (d, 1H, J = 1.51 Hz), 7.13–7.18 (m, 2H), 7.04–7.05 (m, 1H), 6.95–6.99 (m, 3H), 6.90 (d, 1H, J = 8.03 Hz), 6.80 (d, 1H, J = 15.56 Hz), 5.97 (d, 1H, J = 15.31 Hz), 6.04 (s, 2H, -O-CH2-O), 3.83 (s, 3H); 13C-NMR δ (ppm): 167.62, 160.36, 149.81, 148.40, 148.22, 137.71, 137.27, 135.00, 131.32, 130.87, 126.57, 123.23, 121.07, 115.74, 114.84, 113.42, 108.91, 105.88, 101.73, 56.01; MS(m/z): 379; HRMS (ES)-m/z: [M+H]+, found 380.1069. C20H18N3O3S requires 380.1068.
5-[(1E,3E)-4-(1,3-Benzodioxol-5-yl)buta-1,3-dien-1-yl]-4-[4-(trifluoromethyl)phenyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (9m). Brown amorphous solid, yield 70%, m.p. = 232–233 °C. 1H-NMR δ (ppm): 14.09 (s, 1H, NH), 8.03 (d, 2H, J = 8.53Hz), 7.75 (d, 2H, J = 8.28 Hz), 7.20 (d, 1H, J = 1.51 Hz), 7.13–7.18 (m, 1H), 6.95–7.00 (m, 2H), 6.90 (d, 1H, J = 8.03Hz), 6.83 (m, 1H), 5.96 (d, 1H, J = 15.31 Hz), 6.04 (s, 2H, -O-CH2-O-); 13C-NMR δ (ppm): 167.78, 149.61, 148.41, 148.27,137.87, 137.70, 137.55, 131.29, 130.29, 130.30, 127.04 (q, JC-F = 270.00 Hz)127.19, 126.51, 123.26, 113.14, 108.93, 105.82, 101.75; MS(m/z): 417; HRMS (ES)-m/z: [M+H]+, found 418.0837. C20H15F3N3O2S requires 418.0837.