Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
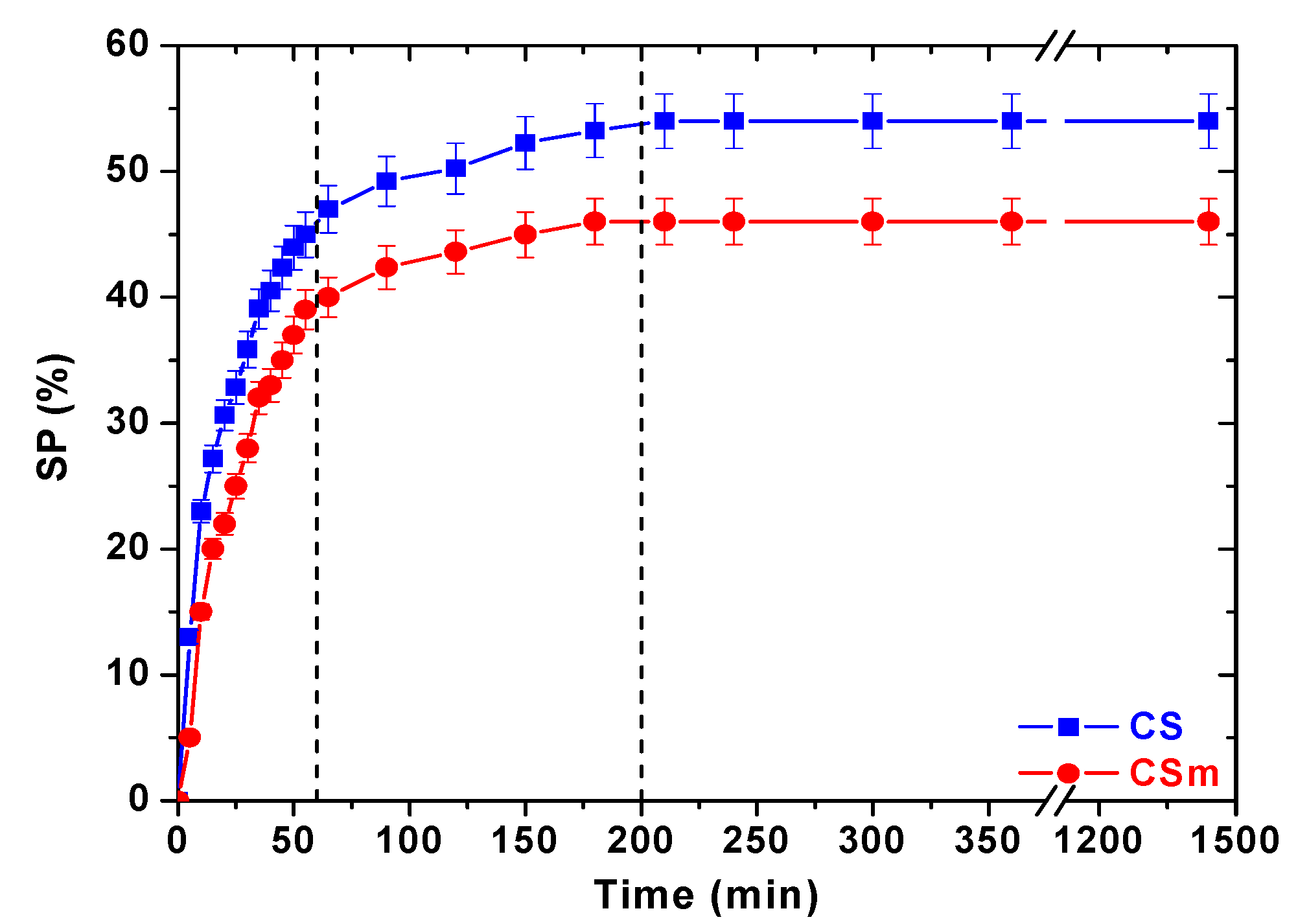

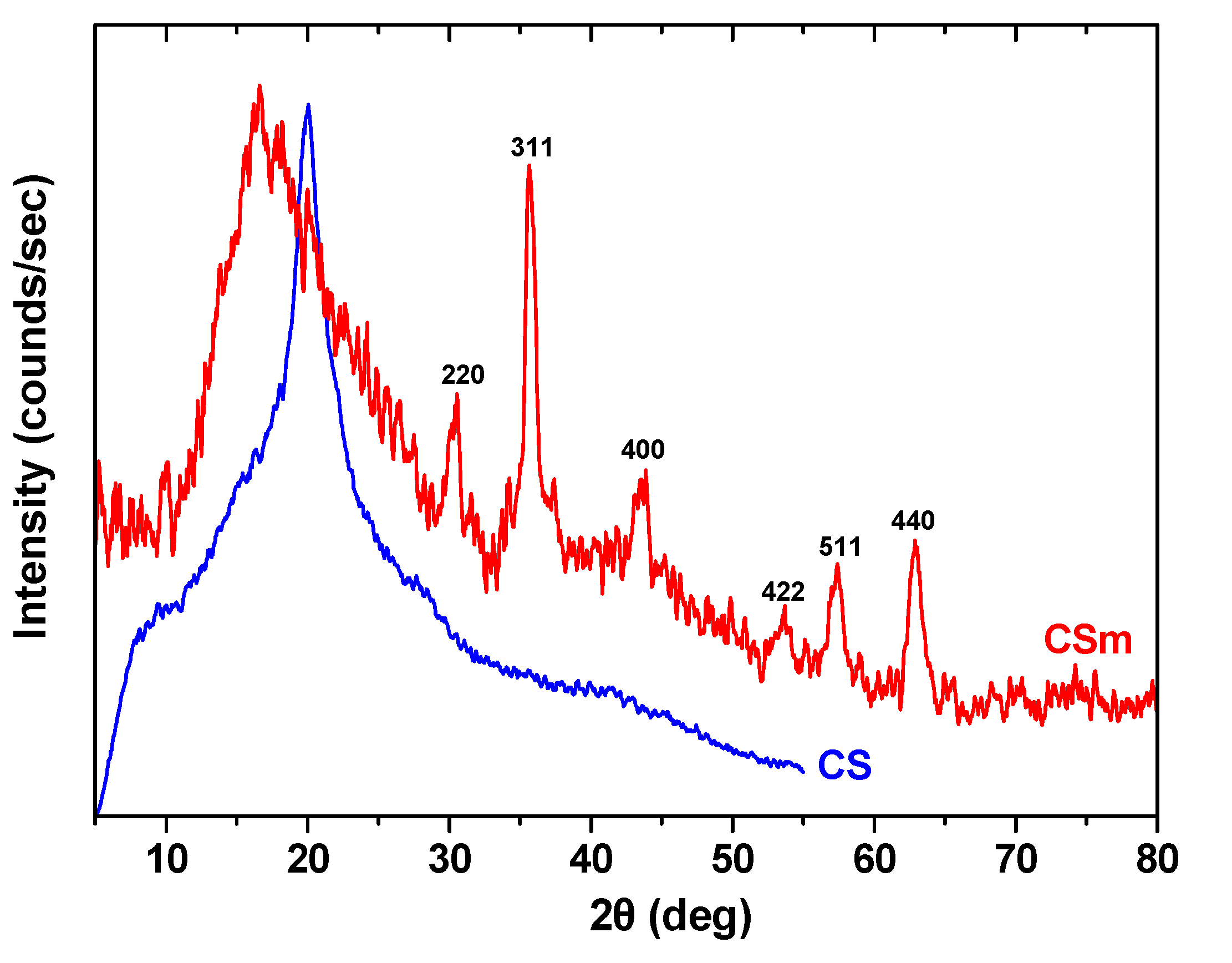
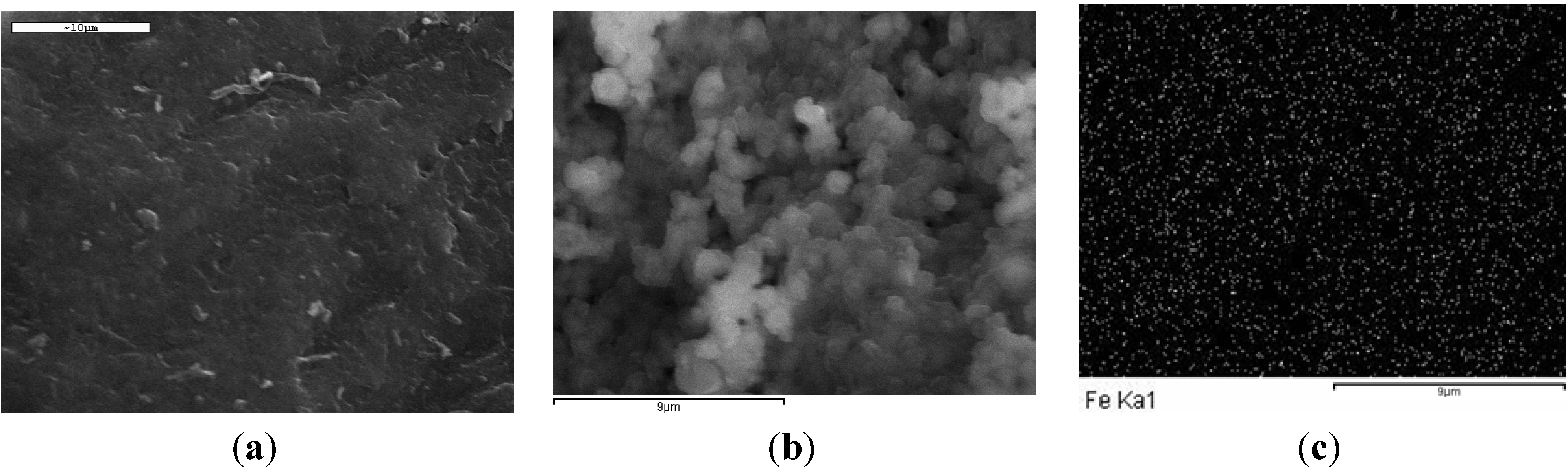

| Property Type | Adsorbent | |
|---|---|---|
| CS | CSm | |
| powder | powder | |
| SP (%) | 54 | 46 |
| Water content (g) | 0.0092 | 0.0108 |
| Particle size (μm) | 75–125 | 75–125 |
| Surface area (m2/g) | <3 | <3 |
| Porosity | non–porous | non–porous |
| Magnetization (emu/g) | – | 5.79 |
| Iron (%) | – | 25.36 |
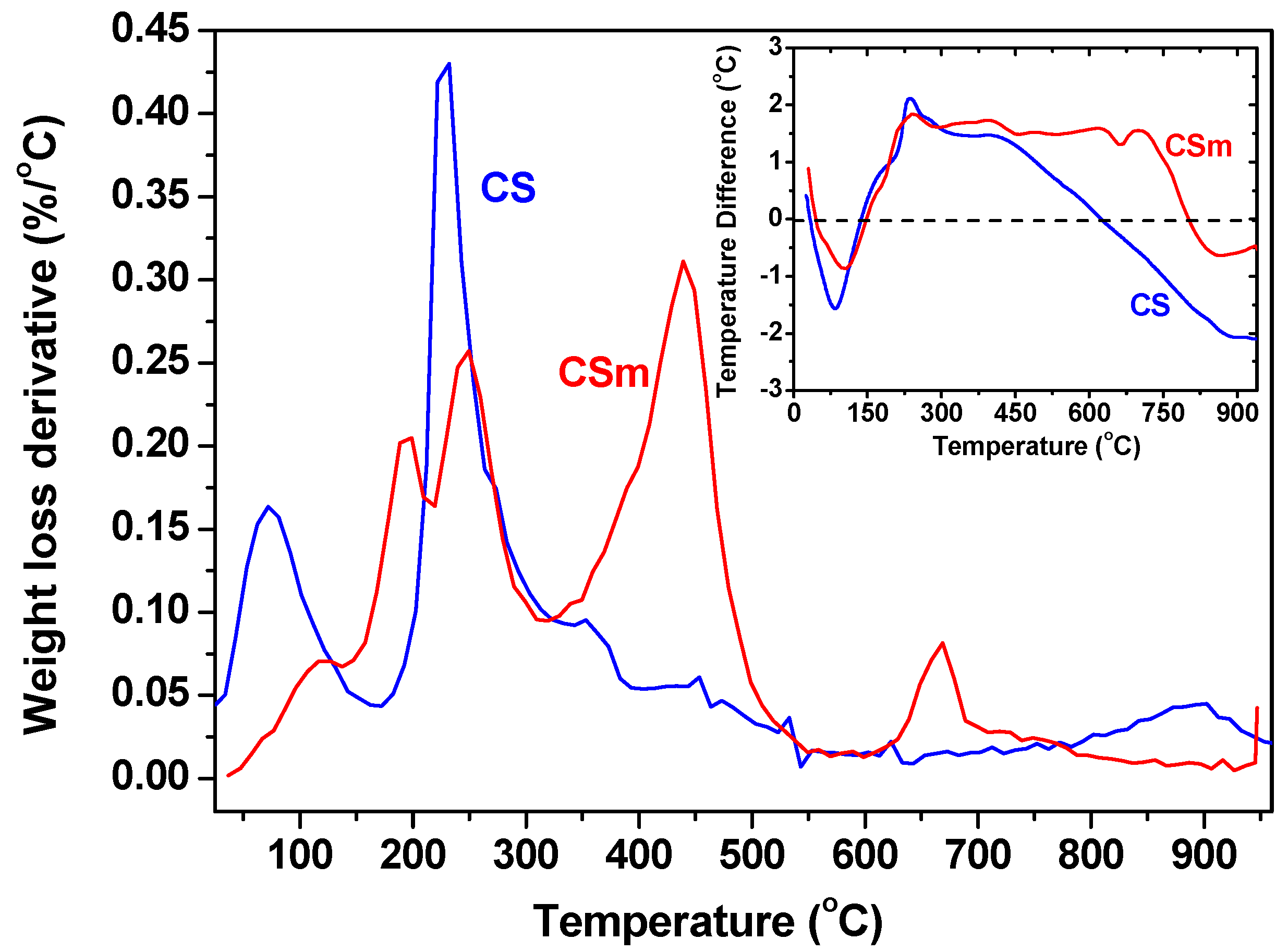
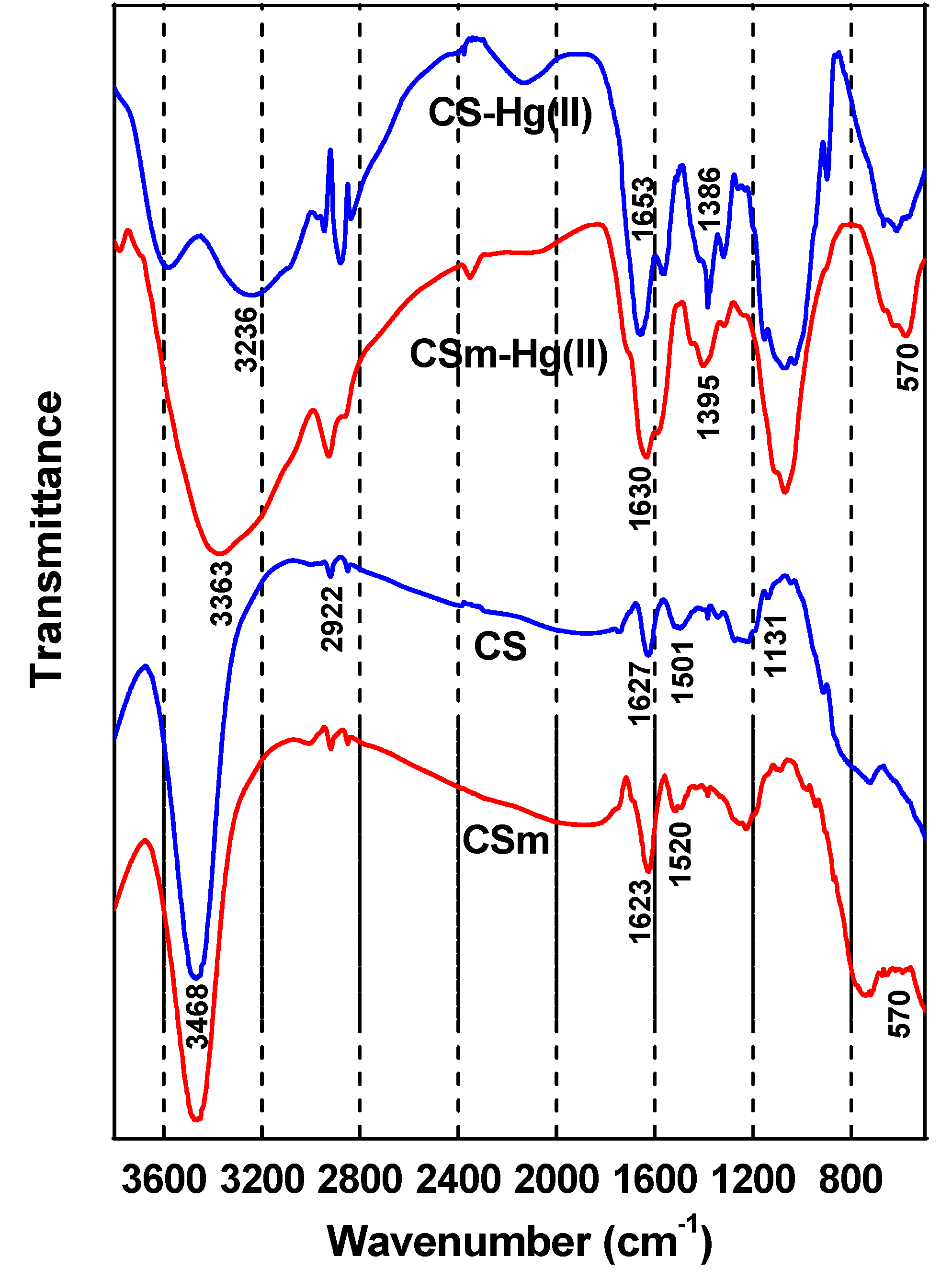
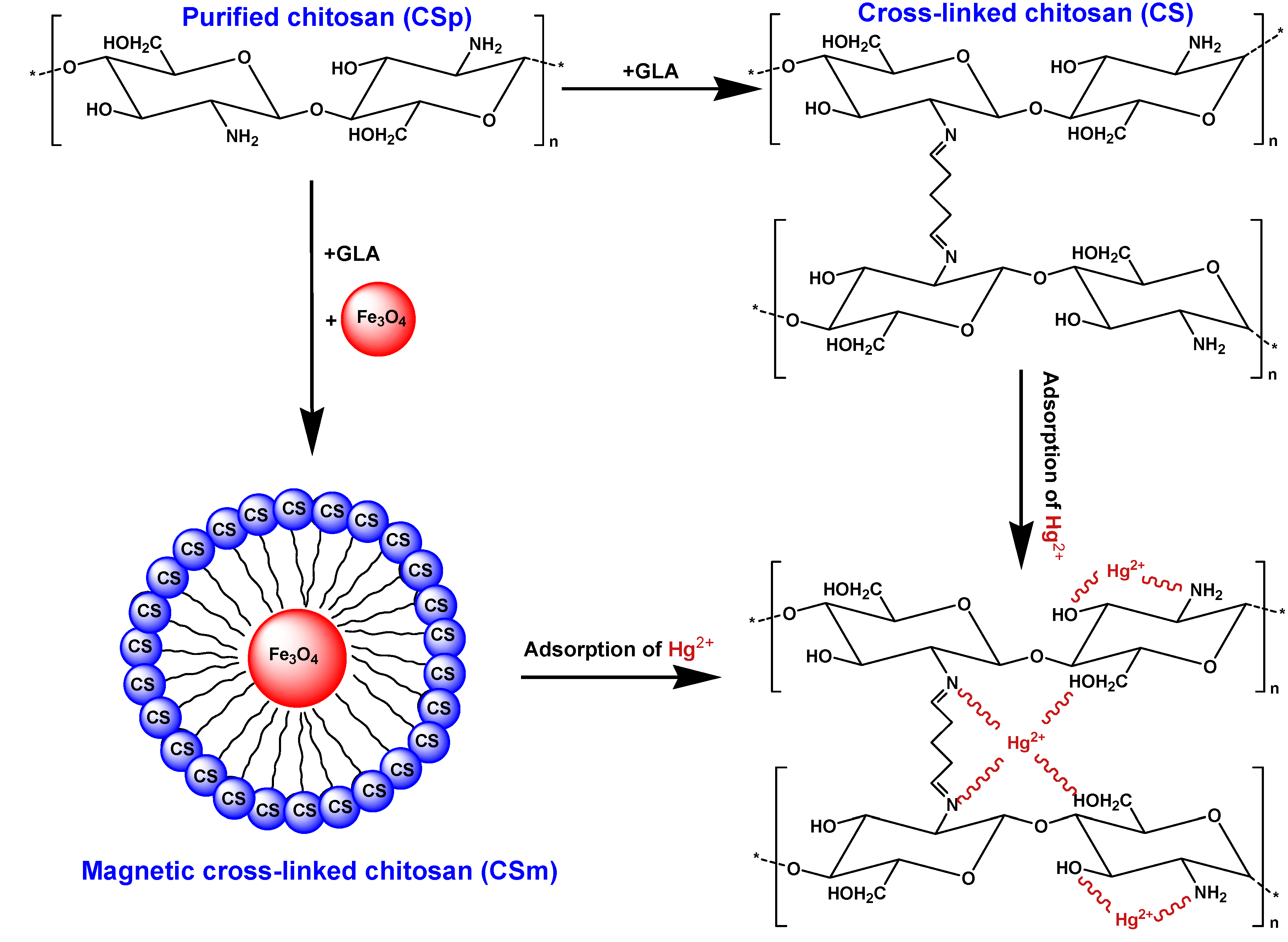
2.2. Adsorption
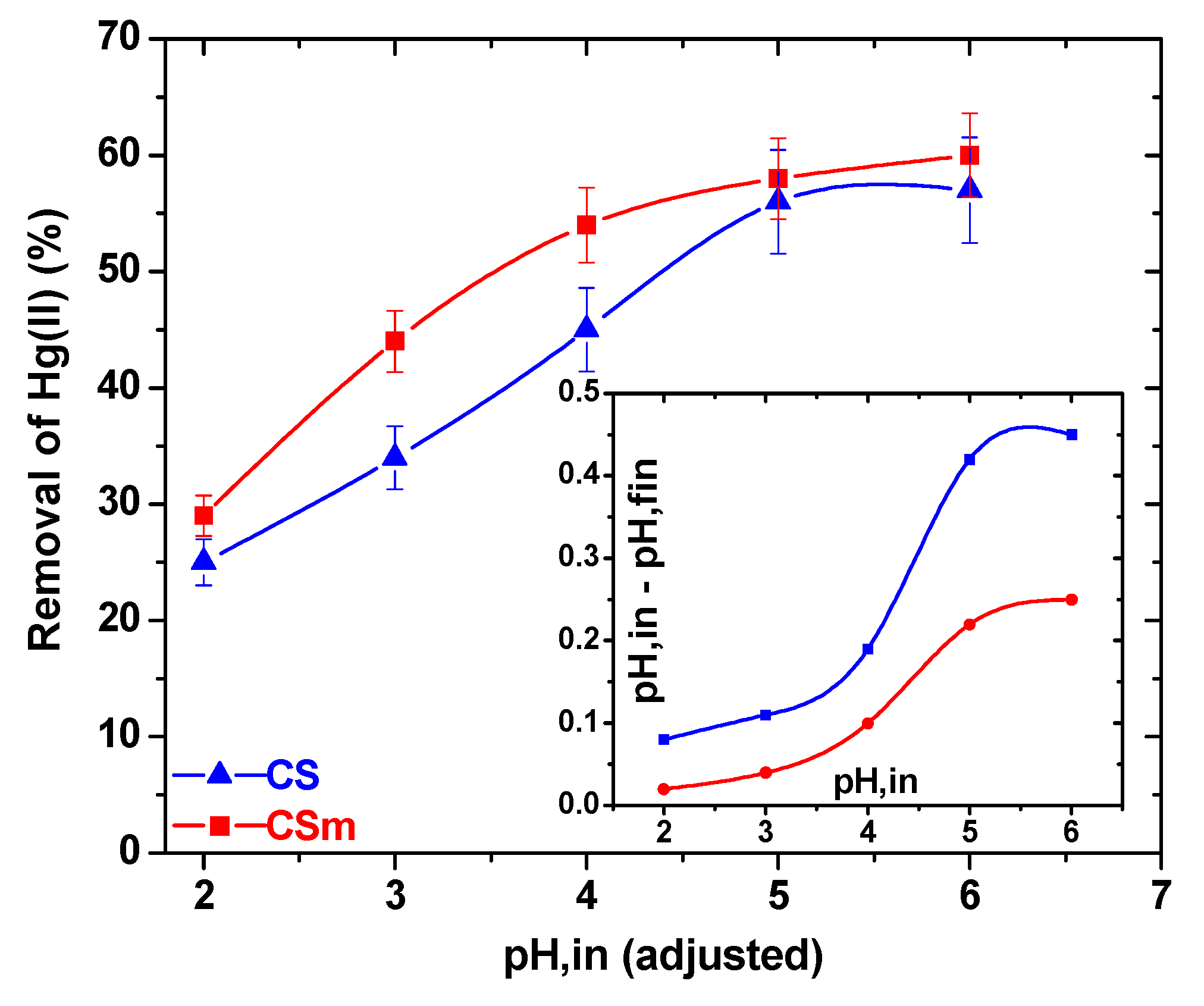
2.2.1. Effect of pH
2.2.2. Effect of Contact Time
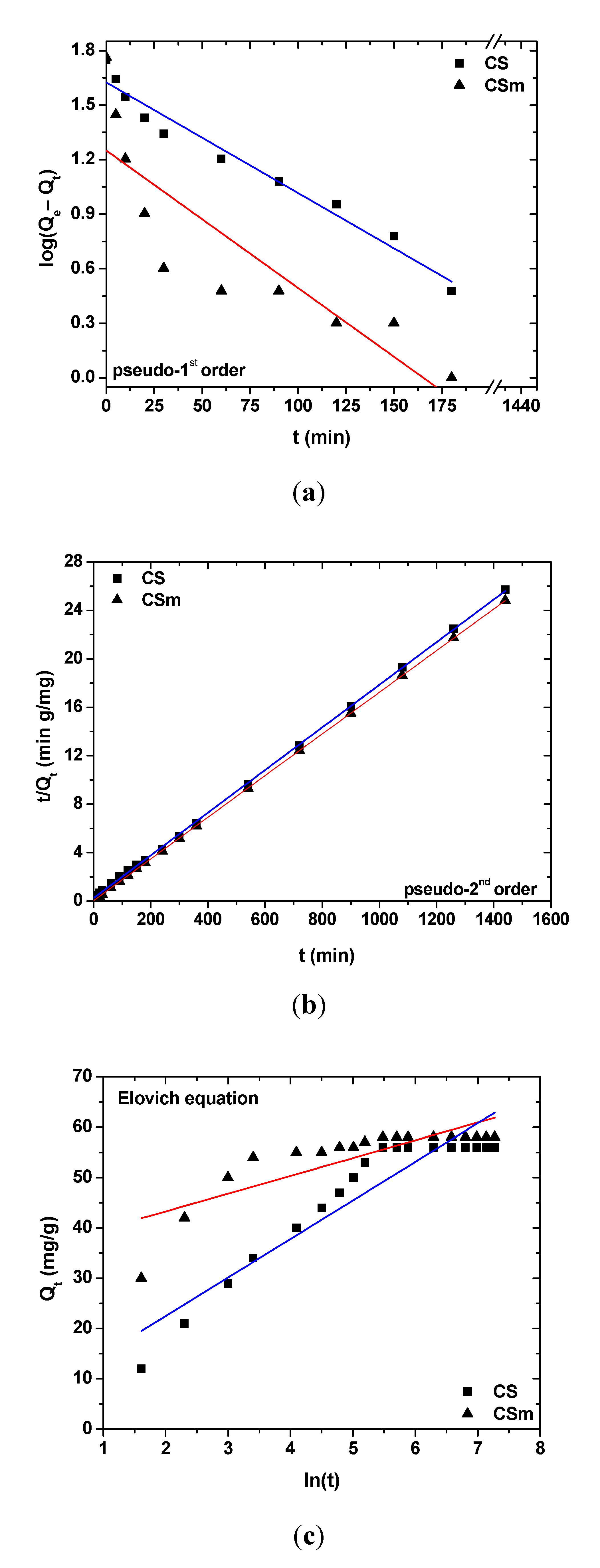
| Pseudo–first order | Pseudo–second order | Elovich equation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe,exp | k1 | Qe,cal | R2 | k2 | Qe,cal | R2 | a | β | R2 | |
| Adsorbent | (mg/g) | (min–1) | (mg/g) | (g mg–1 min–1) | (mg/g) | (mg g–1 min–1) | (g/mg) | |||
| CS | 56 | 0.0138 | 42 | 0.964 | 11.68 × 10–4 | 56 | 0.999 | 39.33 | 0.0276 | 0.658 |
| CSm | 58 | 0.0174 | 18 | 0.729 | 57.65 × 10–4 | 58 | 0.999 | 20.87 | 0.1395 | 0.884 |
2.2.3. Isotherms-Thermodynamics
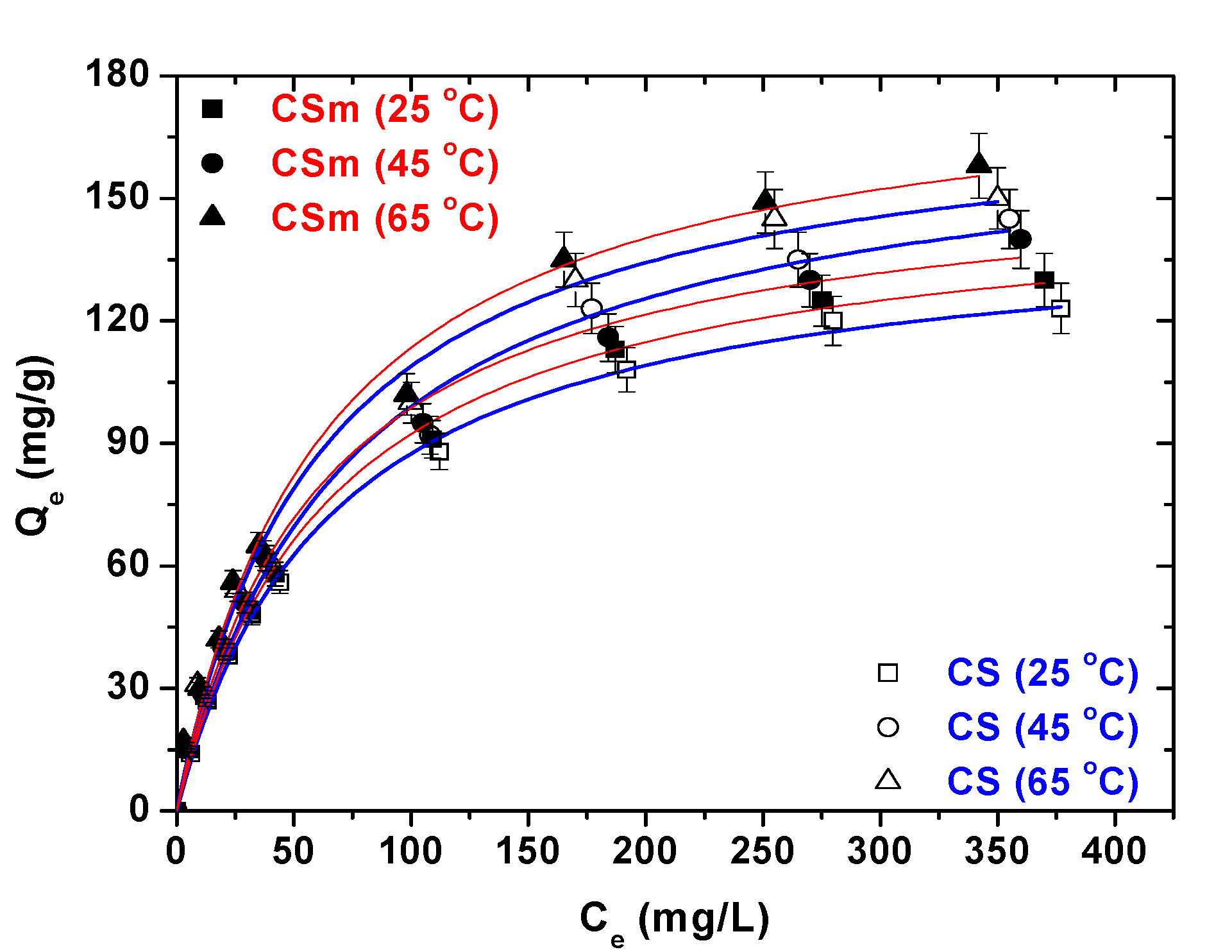
| Adsorbent | Langmuir equation | Freundlich equation | |||||
|---|---|---|---|---|---|---|---|
| T (°C) | Qmax (mg/g) | KL (L/mg) | R2 | KF (mg(n−1)/n L1/n g−1) | n | R2 | |
| CS | 25 | 145 | 0.015 | 0.998 | 10.95 | 2.37 | 0.977 |
| 45 | 171 | 0.014 | 0.992 | 10.93 | 2.22 | 0.976 | |
| 65 | 175 | 0.017 | 0.989 | 13.86 | 2.89 | 0.976 | |
| CSm | 25 | 152 | 0.016 | 0.996 | 11.57 | 2.37 | 0.972 |
| 45 | 158 | 0.017 | 0.994 | 12.54 | 2.39 | 0.988 | |
| 65 | 184 | 0.017 | 0.991 | 13.72 | 2.32 | 0.976 | |
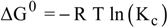


| Adsorbent | C0 (mg/L) | T (K) | Qe (mg/g) | Kc | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (kJ/mol K) |
|---|---|---|---|---|---|---|---|
| CS | 20 | 298 | 14.02 | 2.33 | −2.10 | +18.38 | +0.068 |
| 318 | 15.06 | 3.00 | −2.90 | ||||
| 338 | 16.980 | 5.67 | −4.87 | ||||
| 100 | 298 | 56.02 | 1.27 | −0.60 | +6.10 | +0.023 | |
| 318 | 60.08 | 1.50 | −1.07 | ||||
| 338 | 62.99 | 1.70 | −1.50 | ||||
| 500 | 298 | 123.10 | 0.33 | −0.11 | +5.78 | +0.010 | |
| 318 | 145.04 | 0.41 | −1.44 | ||||
| 338 | 150.03 | 0.43 | −2.44 | ||||
| CSm | 20 | 298 | 15.11 | 3.00 | −0.50 | +13.27 | +0.054 |
| 318 | 16.02 | 4.00 | −0.80 | ||||
| 338 | 17.08 | 5.67 | −3.09 | ||||
| 100 | 298 | 58.12 | 1.38 | −0.20 | +6.21 | +0.024 | |
| 318 | 61.99 | 1.63 | −0.85 | ||||
| 338 | 65.03 | 1.86 | −2.52 | ||||
| 500 | 298 | 130.07 | 0.35 | −0.17 | +5.70 | +0.010 | |
| 318 | 139.89 | 0.39 | −2.03 | ||||
| 338 | 158.01 | 0.46 | −2.80 |
2.3. Desorption-Reuse
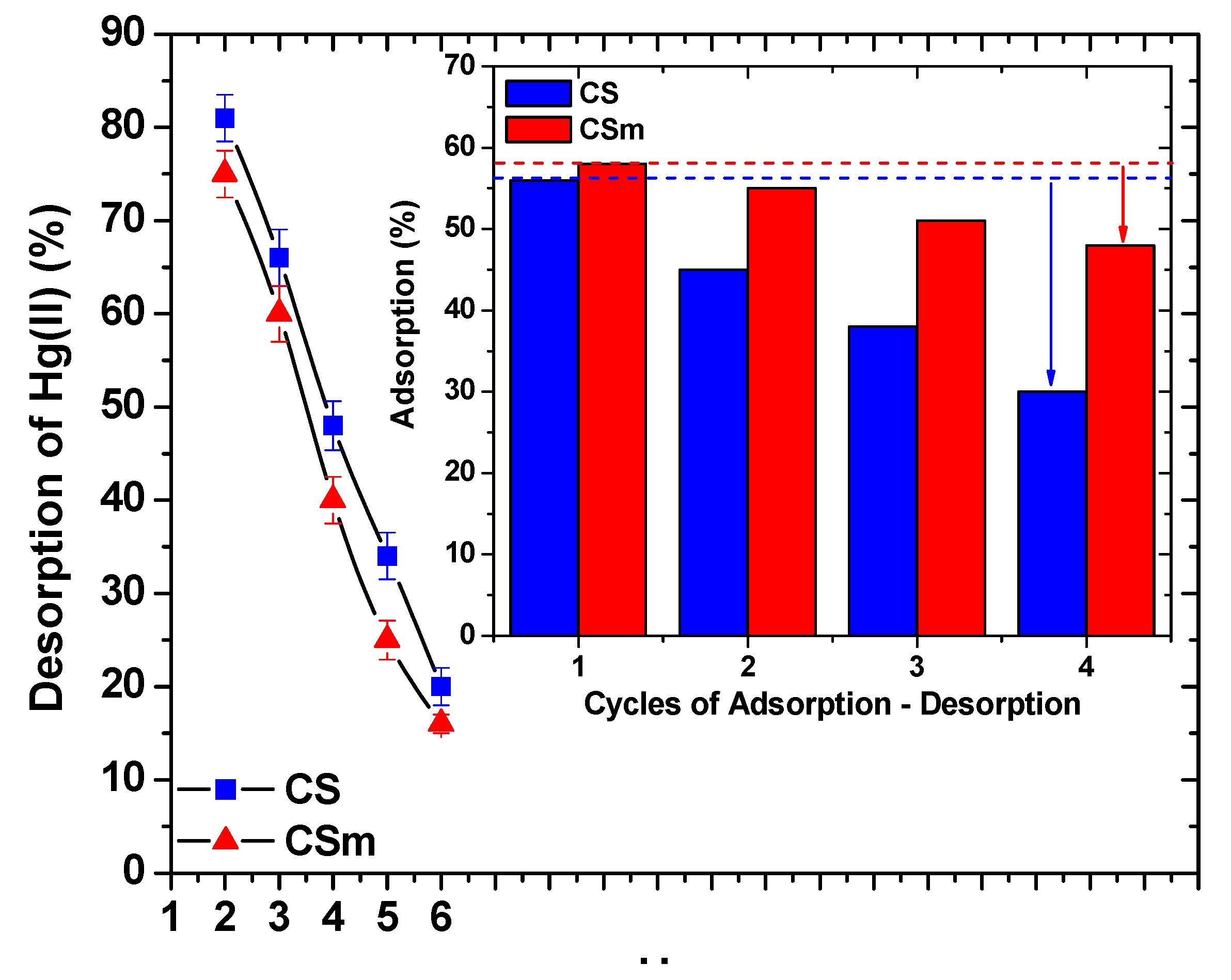
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Adsorbents
3.2.1. Synthesis of Cross–linked Chitosan (CS)
3.2.2. Synthesis of Magnetic Cross–linked Chitosan (CSm)
3.3. Experimental Procedure
3.3.1. Adsorption-Desorption experiments

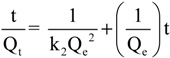

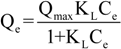

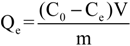
3.3.2. Instrumentation-Characterization
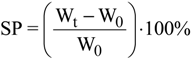
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, H. Biodegradability and Biocompatibility Study of Poly(Chitosan-g-lactic Acid) Scaffolds. Molecules 2012, 17, 3243–3258. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Radwan, A.A.; Alanazi, F.K.; Alsarra, I.A. Microwave irradiation-assisted synthesis of a novel crown ether crosslinked chitosan as a chelating agent for heavy metal ions (M+n). Molecules 2010, 15, 6257–6268. [Google Scholar] [CrossRef]
- Chassary, P.; Vincent, T.; Guibal, E. Metal anion sorption on chitosan and derivative materials: A strategy for polymer modification and optimum use. React. Funct. Polym. 2004, 60, 137–149. [Google Scholar] [CrossRef]
- Batista, A.C.L.; Villanueva, E.R.; Amorim, R.V.S.; Tavares, M.T.; Campos-Takaki, G.M. Chromium (VI) Ion Adsorption Features of Chitosan Film and Its Chitosan/Zeolite Conjugate 13X Film. Molecules 2011, 16, 3569–3579. [Google Scholar] [CrossRef]
- Kim, G.; Kim, N.; Kim, D.; Kwon, J.; Min, B.-H. An electrostatically crosslinked chitosan hydrogel as a drug carrier. Molecules 2012, 17, 13704–13711. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohyd. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Dai, J.; Ren, F.L.; Tao, C.Y. Adsorption behavior of Fe(II) and Fe(III) ions on thiourea cross-linked chitosan with Fe(III) as template. Molecules 2012, 17, 4388–4399. [Google Scholar] [CrossRef]
- Yeganeh, M.; Afyuni, M.; Khoshgoftarmanesh, A.H.; Khodakarami, L.; Amini, M.; Soffyanian, A.R.; Schulin, R. Mapping of human health risks arising from soil nickel and mercury contamination. J. Hazard. Mater. 2013, 244–245, 225–239. [Google Scholar]
- Rocha, C.G.; Zaia, D.A.M.; Alfaya, R.V.d.S.; Alfaya, A.A.d.S. Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J. Hazard. Mater. 2009, 166, 383–388. [Google Scholar] [CrossRef]
- Jeon, C.; Höll, W.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Jeon, C.; Park, K.H. Adsorption and desorption characteristics of mercury(II) ions using aminated chitosan bead. Water Res. 2005, 39, 3938–3944. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloid. Surface. A 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Vieira, R.S.; Beppu, M.M. Interaction of natural and crosslinked chitosan membranes with Hg(II) ions. Colloid. Surface. A 2006, 279, 196–207. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K. Relating interactions of dye molecules with chitosan to adsorption kinetic data. Langmuir 2010, 26, 9617–9626. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Bikiaris, D.N. N-(2-Carboxybenzyl) grafted chitosan as adsorptive agent for simultaneous removal of positively and negatively charged toxic metal ions. J. Hazard. Mater. 2013, 244–245, 29–38. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Vassiliou, A.A.; Lazaridis, N.K. Treatment of real effluents from dyeing reactor: Experimental and modeling approach by adsorption onto chitosan. Chem. Eng. J. 2011, 168, 577–585. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Kyzas, G.Z.; Vassiliou, A.A.; Bikiaris, D.N. Chitosan derivatives as biosorbents for basic dyes. Langmuir 2007, 23, 7634–7643. [Google Scholar] [CrossRef]
- Lv, H.-X.; Zhang, Z.-H.; Wang, X.-P.; Cheng, Q.-Q.; Wang, W.; Huang, X.-H.; Zhou, J.-P.; Zhang, Q.; Hou, L.-L.; Huo, W. A biomimetic chitosan derivates: Preparation, characterization and transdermal enhancement studies of N-arginine chitosan. Molecules 2011, 16, 6778–6790. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, Y.-G.; Peng, Q.-Q.; Hu, X.-J.; Liao, T.; Wang, H.; Lu, M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Yan, H.; Yang, L.; Yang, Z.; Yang, H.; Li, A.; Cheng, R. Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 229–230, 371–380. [Google Scholar] [CrossRef]
- Elwakeel, K.Z. Removal of Cr(VI) from alkaline aqueous solutions using chemically modified magnetic chitosan resins. Desalination 2010, 250, 105–112. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Fang, Z.; Zhang, X.; Zhang, B. Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Selective separation of mercury(II) using magnetic chitosan resin modified with Schiff's base derived from thiourea and glutaraldehyde. J. Hazard. Mater. 2008, 151, 372–379. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Liu, J.; Huang, Q. Adsorption of Hg(II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination 2010, 258, 41–47. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef]
- Seung, H.H. Thermal Reduction of Graphene Oxide. In Physics and Applications of Graphene-Experiments; Mikhailov, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 73–90. Availbale online: http://www.intechopen.com (accesed on 19 April 2011).
- Li, Y.; Chu, J.; Qi, J.; Li, X. An easy and novel approach for the decoration of graphene oxide by Fe3O4 nanoparticles. Appl. Surf. Sci. 2011, 257, 6059–6062. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Liu, L.; Li, B.; Yang, P.; Zhang, L.; Feng, Y. Adsorption of dye from wastewater using chitosan-CTAB modified bentonites. J. Colloid Interface Sci. 2012, 382, 61–66. [Google Scholar] [CrossRef]
- Krishna Rao, K.S.V.; Chung, I.; Ha, C.-S. Synthesis and characterization of poly(acrylamidoglycolic acid) grafted onto chitosan and its polyelectrolyte complexes ith hydroxyapatite. React. Funct. Polym. 2008, 68, 943–953. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liu, Y.-C.; Chang, C.-M.J.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohyd. Polym. 2012, 87, 2538–2545. [Google Scholar] [CrossRef]
- Li, G.-Y.; Jiang, Y.-R.; Huang, K.-L.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J. Alloy. Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Ma, F.; Qu, R.; Sun, C.; Wang, C.; Ji, C.; Zhang, Y.; Yin, P. Adsorption behaviors of Hg(II) on chitosan functionalized by amino-terminated hyperbranched polyamidoamine polymers. J. Hazard. Mater. 2009, 172, 792–801. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, P.; Qu, R.; Chen, H.; Wang, C.; Ren, S. Adsorption kinetics, thermodynamics and isotherm of Hg(II) from aqueous solutions using buckwheat hulls from Jiaodong of China. Food Chem. 2013, 136, 1508–1514. [Google Scholar] [CrossRef]
- Li, Q.; Sun, L.; Zhang, Y.; Qian, Y.; Zhai, J. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II) and Cr(VI) by polyaniline/humic acid composite. Desalination 2011, 266, 188–194. [Google Scholar] [CrossRef]
- Koong, L.F.; Lam, K.F.; Barford, J.; McKay, G. A comparative study on selective adsorption of metal ions using aminated adsorbents. J. Colloid Interface Sci. 2013, 395, 230–240. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Nie, H.L.; Branford-White, C.; He, Z.Y.; Zhu, L.M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J. Colloid Interface Sci. 2009, 330, 29–37. [Google Scholar] [CrossRef]
- Vieira, R.S.; Beppu, M.M. Dynamic and static adsorption and desorption of Hg(II) ions on chitosan membranes and spheres. Water Res. 2006, 40, 1726–1734. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Lv, Z.; Lu, F.; Qiu, H. Preparation of magnetic modified chitosan and adsorption of Zn2+ from aqueous solutions. Colloid. Surface. B. 2011, 88, 574–581. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Yuwei, C.; Jianlong, W. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Method. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K. Copper and chromium(VI) removal by chitosan derivatives-Equilibrium and kinetic studies. Chem. Eng. J. 2009, 152, 440–448. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresource Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C. Introduction to Chemical Engineering Thermodynamics, 4th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Sample Availability: Samples of the adosrbents are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kyzas, G.Z.; Deliyanni, E.A. Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents. Molecules 2013, 18, 6193-6214. https://doi.org/10.3390/molecules18066193
Kyzas GZ, Deliyanni EA. Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents. Molecules. 2013; 18(6):6193-6214. https://doi.org/10.3390/molecules18066193
Chicago/Turabian StyleKyzas, George Z., and Eleni A. Deliyanni. 2013. "Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents" Molecules 18, no. 6: 6193-6214. https://doi.org/10.3390/molecules18066193
APA StyleKyzas, G. Z., & Deliyanni, E. A. (2013). Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents. Molecules, 18(6), 6193-6214. https://doi.org/10.3390/molecules18066193






