Different Analytical Approaches in Assessing Antibacterial Activity and the Purity of Commercial Lysozyme Preparations for Dairy Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purity of Commercial Lysozyme Preparations
| Sample | Country of Purchase 1 | Type of preparation | Water (g/100 g) | Nitrogen (g/100 g) | Total Protein 2 (g/100 g) | Sugars 3 (g/100 g) | HEW proteinsother than LSZ 4 (+/−) |
|---|---|---|---|---|---|---|---|
| 1 | Netherland (M) | Granular | 5.00 | 17.38 | 91.9 | n.d. | + |
| 2 | Italy (D) | Granular | 6.71 | 16.73 | 88.5 | n.d. | + |
| 3 | Italy (D) | Granular | 6.67 | 16.75 | 88.6 | n.d. | - |
| 4 | Germany (M) | Powder | 7.61 | 16.48 | 87.2 | 0 | +++ |
| 5 | Italy (D) | Granular | 6.37 | 16.81 | 88.9 | n.d. | + |
| 6 | Italy (D) | Powder | 5.07 | 15.67 | 82.9 | 7.6 (g) | +++ |
| 7 | Italy (M) | Granular | 8.66 | 16.86 | 89.2 | n.d. | + |
| 8 | Italy (D) | Granular | 5.10 | 16.77 | 88.7 | n.d. | ++ |
| 9 | Switzerland (M) | Granular | 5.70 | 17.20 | 91.0 | n.d. | - |
| 10 | Italy (D) | Granular | 5.00 | 17.44 | 92.3 | n.d. | ++ |
| 11 | Germany (D) | Powder | 8.06 | 16.84 | 89.1 | n.d. | + |
| 12 | Spain (D) | Granular | 6.60 | 12.53 | 66.3 | 24.0 (g) | - |
| 13 | Spain (D) | Liquid 5 | n.d. | 4.15 | 22.0 | n.d. | + |
| 14 | Spain (D) | Granular | 5.50 | 13.31 | 70.4 | 17.3 (s) | ++ |
| 15 | Belgium (M) | Powder | 4.10 | 17.14 | 90.7 | n.d. | + |
| 16 | Canada (M) | Granular | 5.40 | 17.31 | 91.6 | n.d. | + |
| 17 | Poland (M) | Powder | 5.00 | 16.11 | 85.2 | 0 | ++++ |
| Sample | Type of preparation | Anhydrous basis (mg/g) | Product basis 1 (g/100 g product) | Protein basis (g/100 g protein) | |||
|---|---|---|---|---|---|---|---|
| Potency | Potency | Concentration | Potency | Concentration | |||
| 1 | Granular | 1008 | 96 ± 1.73 | 100 ± 0.71 | 104 | 109 | |
| 2 | Granular | 929 | 87 ± 0.06 | 83 ± 0.70 | 98 | 94 | |
| 3 | Granular | 1040 | 97 ± 1.58 | 97 ± 2.11 | 110 | 109 | |
| 4 | Powder | 872 | 81 ± 0.13 | 75 ± 0.70 | 92 | 86 | |
| 5 | Granular | 968 | 91 ± 0.46 | 86 ± 1.68 | 102 | 96 | |
| 6 | Powder | 753 | 71 ± 0.51 | 66 ± 1.95 | 86 | 80 | |
| 7 | Granular | 949 | 87 ± 0.17 | 80 ± 1.36 | 97 | 90 | |
| 8 | Granular | 985 | 93 ± 0.23 | 95 ± 4.28 | 105 | 107 | |
| 9 | Granular | 1027 | 97 ± 0.02 | 99 ± 2.10 | 106 | 109 | |
| 10 | Granular | 1001 | 95 ± 0.41 | 95 ± 1.42 | 103 | 103 | |
| 11 | Powder | 960 | 88 ± 0.32 | 79 ± 2.13 | 99 | 89 | |
| 12 | Granular | 747 | 70 ± 2.90 | 76 ± 0.69 | 105 | 114 | |
| 13 2 | Liquid | n.d. | 26 ± 0.56 | 24 ± 1.41 3 | 118 | 108 3 | |
| 14 | Granular | 746 | 70 ± 1.13 | 70 ± 0.70 | 100 | 99 | |
| 15 | Powder | 985 | 94 ± 0.07 | 84 ± 0.71 | 104 | 93 | |
| 16 | Granular | 1025 | 97 ± 1.40 | 93 ± 2.11 | 106 | 101 | |
| 17 | Powder | 643 | 61 ± 0.24 | 52 ± 0.72 | 72 | 61 | |
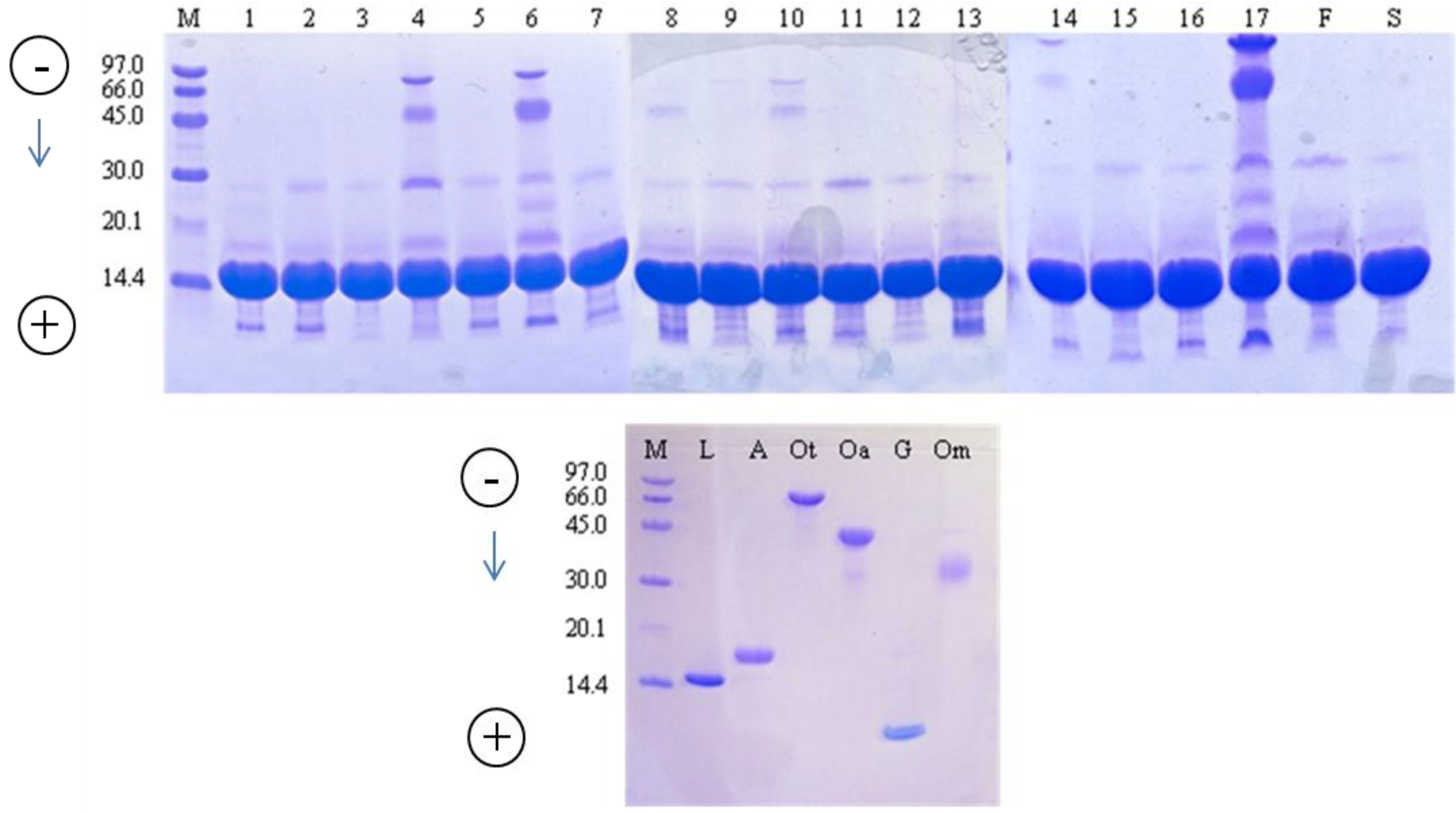
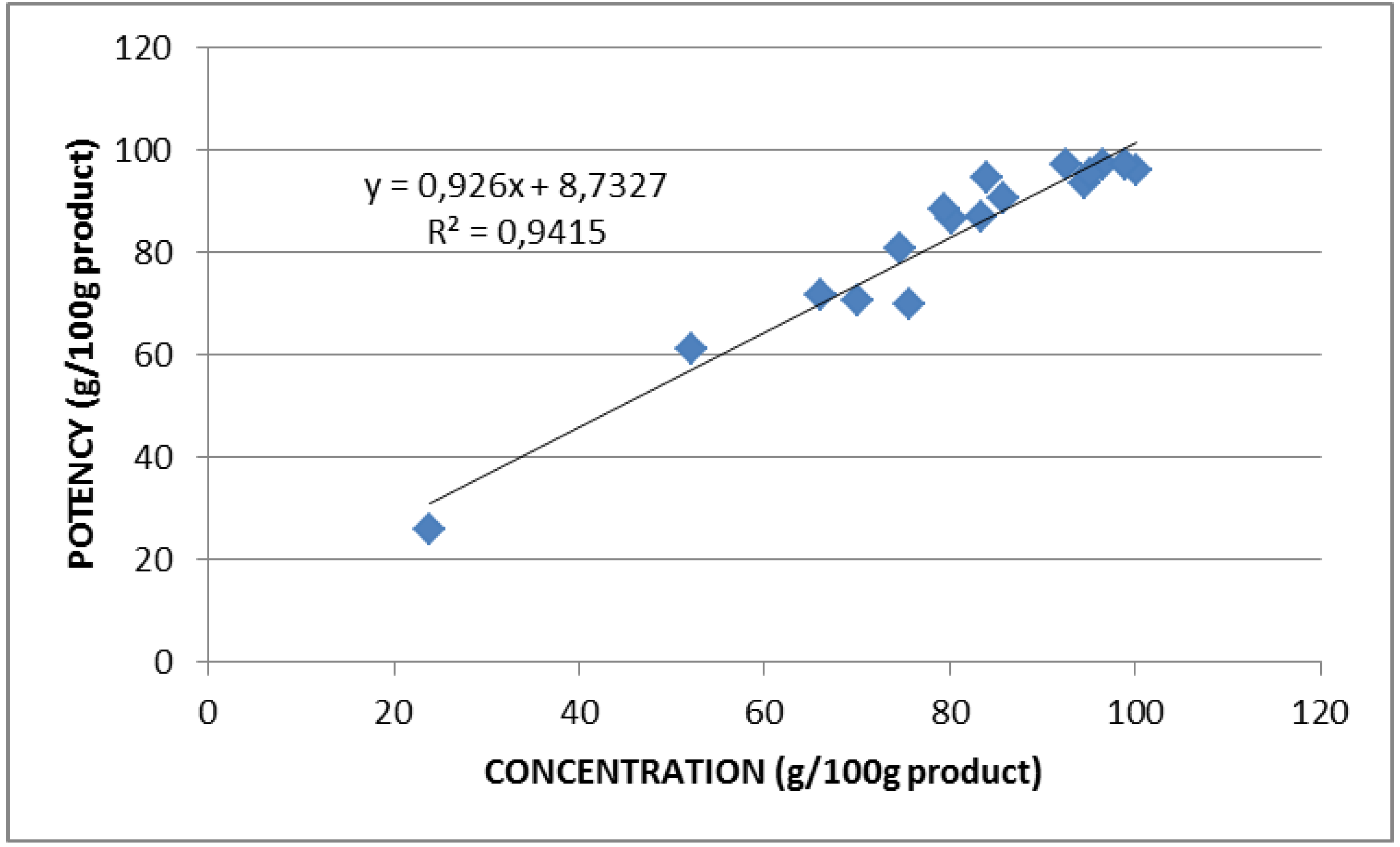
2.2. Lysozyme Concentration and Potency

3. Experimental
3.1. Lysozyme Samples and Reference Materials
3.2. Composition Analysis
3.3. SDS-PAGE Analysis
3.4. Determination of Lysozyme Potency
3.5. HPLC-FLD Analysis
4. Conclusions
Conflicts of Interest
References
- Durance, T.D. Separation, Purification, and Thermal Stability of Lysozyme and Avidin from Chicken Egg White. In Egg Uses and Processing Technologies. New Developments; Sim, J.S., Nakai, S., Eds.; CAB International Press: Wallingford, UK, 1994; pp. 77–93. [Google Scholar]
- Tirelli, A.; de Noni, I. Evaluation of lysozyme stability in young red wine and model systems by a validated HPLC method. Food Chem. 2007, 106, 1564–1570. [Google Scholar] [CrossRef]
- Silvetti, T.; Brasca, M.; Lodi, R.; Vanoni, L.; Chiolerio, F.; de Groot, M.; Bravi, A. Effects of lysozyme on the microbiological stability and organoleptic properties of unpasteurized beer. J. Inst. Brew. 2010, 116, 33–40. [Google Scholar] [CrossRef]
- Stadhouders, J. Prevention of butyric acid fermentation by the use of nitrate. Bull. Int. Dairy Fed. 1990, 251, 40–46. [Google Scholar]
- Van den Berg, G.; Meijer, W.C.; Düsterhöft, E.M.; Smit, G. Gouda and Related Cheeses. In Cheese: Chemistry, Physics and Microbiology, 3rd; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Chapman & Hall: London, UK, 2004; Volume 2, pp. 103–140. [Google Scholar]
- Lodi, R. The use of lysozyme to control butyric acid fermentation. Bull. Int. Dairy Fed. 1990, 251, 51–54. [Google Scholar]
- Bärtschi, F.; Muralt, L.; Rieder, K.; Kämpfer, U; Schaller, J. Bestimmung von Lysozym in Käse mittels LC-MS. Mitt. Lebensm. Hyg. 2006, 97, 478–488. [Google Scholar]
- Cunningham, F.E.; Proctor, V.A.; Goetsch, S.J. Egg-white lysozyme as a food preservative: An overview. World Poultry Sci. J. 1991, 47, 141–163. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No. 1129/2011 of 11 November 2011. Off. J. Eur. Union 2011, L295, 1–177.
- Codex Alimentarius. Codex-STAN 283–1978, rev. 2010; General Standard for Cheese. 2010; pp. 1–7.
- Pellegrino, L.; Tirelli, A. A sensitive HPLC method to detect hen’s egg white lysozyme in milk and dairy products. Int. Dairy J. 2000, 10, 435–442. [Google Scholar] [CrossRef]
- European Food Safety Authority. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to a notification from AMAFE on egg lysozyme used as additive in food pursuant to Article 6 paragraph 11 of Directive 2000/13/EC. EFSA J. 2005, 186, 1–5. [Google Scholar]
- European Parliament and Council Regulation No 1169/2011 of 25 October 2011. Off. J. Eur. Union 2011, L 304, 18–63.
- Mine, Y.; Zhang, J.W. Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J. Agric. Food Chem. 2002, 50, 2679–2683. [Google Scholar] [CrossRef]
- Bernhisel-Broadbent, J.; Dintzis, H.M.; Dintzis, R.Z.; Sampson, H.A. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J. Allergy Clin. Immun. 1994, 93, 1047–1059. [Google Scholar] [CrossRef]
- Iaconelli, A.; Fiorentini, L.; Bruschi, S.; Rossi, F.; Mingrone, G.; Piva, G. Absence of allergic reactions to egg white lysozyme additive in Grana Padano cheese. J. Am. Coll. Nutr. 2008, 27, 326–331. [Google Scholar] [CrossRef]
- Fremont, S.; Kanny, G.; Nicolas, J.P.; Moneret-Vautrin, D.A. Prevalence of lysozyme sensitization in an egg-allergic population. Allergy 1997, 52, 224–228. [Google Scholar] [CrossRef]
- Holen, E.; Elsayed, S. Characterization of four major allergens of hen egg white by IEF/SDS-PAGE combined with electrophoretic transfer and IgE immunoautoradiography. Int. Arch. Allergy Imm. 1990, 91, 136–141. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA) 2006. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/ (accessed on 18 March 2013).
- Gorin, G.; Wang, S.F.; Papapavlou, L. Assay of lysozyme by its lytic action on M.lysodeikticus cells. Anal. Biochem. 1971, 39, 113–127. [Google Scholar] [CrossRef]
- McKenzie, H.A.; White, F.H., Jr. Determination of lysozyme activity at low levels with emphasis on the milk enzyme. Anal. Biochem. 1986, 157, 367–374. [Google Scholar] [CrossRef]
- Weth, F.; Schroeder, T.; Buxtorf, U.P. Determination of lysozyme content in eggs and egg products using SDS-gel electrophoresis. Z. Lebensm. Unters. For. 1988, 187, 541–545. [Google Scholar] [CrossRef]
- Kerkaert, B.; Mestdagh, F.; de Meulenaer, B. Detection of hen’s egg white lysozyme in food: Comparison between a sensitive HPLC and a commercial ELISA method. Food Chem. 2010, 120, 580–584. [Google Scholar] [CrossRef]
- Schneider, N.; Becker, C.M.; Pischetsrieder, M. Analysis of lysozyme in cheese by immunocapture mass spectrometry. J. Chromatogr. B 2010, 878, 201–206. [Google Scholar] [CrossRef]
- Schneider, N.; Werkmeister, K.; Becker, C.M.; Pischetsrieder, M. Prevalence and stability of lysozyme in cheese. Food Chem. 2011, 128, 145–151. [Google Scholar] [CrossRef]
- International Organization for Standardization. Milk and milk products—Determination of hen’s egg white lysozyme by HPLC; ISO/TS 27105:2009|IDF 216:2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Commission Regulation (EU) No 231/2012 of 9 March 2012. Off. J. Eur. Union 2012, L 83, 1–295.
- Isengard, H.; Haschka, E.; Merkh, G. Determination of water content in lactose by Karl Fischer titration. Interlaboratory collaborative study. Bull. Int. Dairy Fed. 2011, 450, 2–14. [Google Scholar]
- Separovich, F.; Lam, Y.H.; Ke, X.; Chan, H.K. A solid-state NMR study of protein hydration and stability. Pharmacol. Res. 1998, 15, 1816–1821. [Google Scholar] [CrossRef]
- Nagendra, H.G.; Sukumar, N.; Vijayan, M. Role of water in plasticity, stability, and action of proteins: The crystal structures of lysozyme at very low levels of hydration. Proteins 1998, 32, 229–240. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2005; pp. 571–607. [Google Scholar]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Maroufi, B.; Ranjbar, B.; Khajeh, K.; Naderi-Manesh, K.; Yaghoubi, H. Structural studies of hen egg-white lysozyme dimer: Comparison with monomer. Biochim. Biophys. Acta 2008, 1784, 1043–1049. [Google Scholar] [CrossRef]
- Desfougères, Y.; Jardin, J.; Lechevalier, V.; Pezennec, S.; Nau, F. Succinimidyl residue formation in hen egg-white lysozyme favors the formation of intermolecular covalent bonds without affecting its tertiary structure. Biomacromolecules 2011, 12, 156–166. [Google Scholar] [CrossRef]
- Onuma, K.; Inaka, K. Lysozyme dimer association: Similarities and differences compared with lysozyme monomer association. J. Cryst. Growth 2008, 310, 1174–1181. [Google Scholar] [CrossRef]
- Hytonen, V.P.; Laitinen, O.H.; Grapputo, A.; Kettunen, A.; Savolainen, J.; Kalkkinen, N.; Marttila, A.T.; Nordlund, H.R.; Nyholm, T.K.M.; Paganelli, G.; et al. Characterization of poultry egg-white avidins and their potential as a tool in pretargeting cancer treatment. Biochem. J. 2003, 372, 219–225. [Google Scholar] [CrossRef]
- Korpela, J. Avidin, a high affinity biotin-binding protein as a tool and subject of biological research. Med. Biol. 1984, 62, 5–26. [Google Scholar]
- Desert, C.; Guérin-Dubiard, C.; Nao, F.; Jan, G.; Val, F.; Maillard, J. Comparison of different electrophoretic separation of hen egg white protein. J. Agric. Food Chem. 2001, 49, 4553–4561. [Google Scholar] [CrossRef]
- Suzuki, M.; Fujii, H.; Fujigaki, H.; Shinoda, S.; Takahashi, K.; Saito, K.; Wada, H.; Kimoto, M.; Kondo, N.; Seishima, M. Lipocalin-type prostaglandin D synthase and egg white cystatin react with IgE antibodies from children with egg allergy. Allergol. Int. 2010, 59, 175–183. [Google Scholar] [CrossRef]
- Mann, K. The chicken egg white proteome. Proteomics 2007, 7, 3558–3568. [Google Scholar] [CrossRef]
- Odani, S.; Koide, T.; Ono, T.; Takahashi, Y.; Suzuki, J. Covalent structure of a low-molecular-mass protein, meleagrin present in turkey (Melagris-gallopavo) ovomucoid preparation. J. Biochem-Tokyo 1989, 105, 660–663. [Google Scholar]
- Simpson, R.J.; Morgan, F.J. Isolation and complete amino-acid-sequence of a basic low-molecular weight protein from black swan egg-white. J. Biochem. 1983, 22, 476–481. [Google Scholar]
- Gong, D.; Wilson, P.W.; Bain, M.M.; Mc Dade, K.; Kalina, J.; Hervé-Grépinet, V.; Nys, Y.; Dunn, I.C. Gallin: An antimicrobial peptide member of a new avian defensin family, the ovodefensins, has been subject to recent gene duplication. BMC Immunol. 2010, 11. [Google Scholar] [CrossRef]
- Miguel, M.; Manso, M.A.; López-Fandiño, R.; Ramos, M. Comparative study of egg white proteins from different species by chromatographic and electrophoretic methods. Eur. Food Res. Technol. 2005, 221, 542–546. [Google Scholar] [CrossRef]
- International Organization for Standardization. Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method; ISO 1871:2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. Milk and Milk Products—Determination of Lactose Content by High-Performance Liquid Chromatography (Reference Method); ISO 22662|IDF 198:2007; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Sample Availability: Samples are available by contacting the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brasca, M.; Morandi, S.; Silvetti, T.; Rosi, V.; Cattaneo, S.; Pellegrino, L. Different Analytical Approaches in Assessing Antibacterial Activity and the Purity of Commercial Lysozyme Preparations for Dairy Application. Molecules 2013, 18, 6008-6020. https://doi.org/10.3390/molecules18056008
Brasca M, Morandi S, Silvetti T, Rosi V, Cattaneo S, Pellegrino L. Different Analytical Approaches in Assessing Antibacterial Activity and the Purity of Commercial Lysozyme Preparations for Dairy Application. Molecules. 2013; 18(5):6008-6020. https://doi.org/10.3390/molecules18056008
Chicago/Turabian StyleBrasca, Milena, Stefano Morandi, Tiziana Silvetti, Veronica Rosi, Stefano Cattaneo, and Luisa Pellegrino. 2013. "Different Analytical Approaches in Assessing Antibacterial Activity and the Purity of Commercial Lysozyme Preparations for Dairy Application" Molecules 18, no. 5: 6008-6020. https://doi.org/10.3390/molecules18056008
APA StyleBrasca, M., Morandi, S., Silvetti, T., Rosi, V., Cattaneo, S., & Pellegrino, L. (2013). Different Analytical Approaches in Assessing Antibacterial Activity and the Purity of Commercial Lysozyme Preparations for Dairy Application. Molecules, 18(5), 6008-6020. https://doi.org/10.3390/molecules18056008






