Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties
Abstract
:1. Introduction
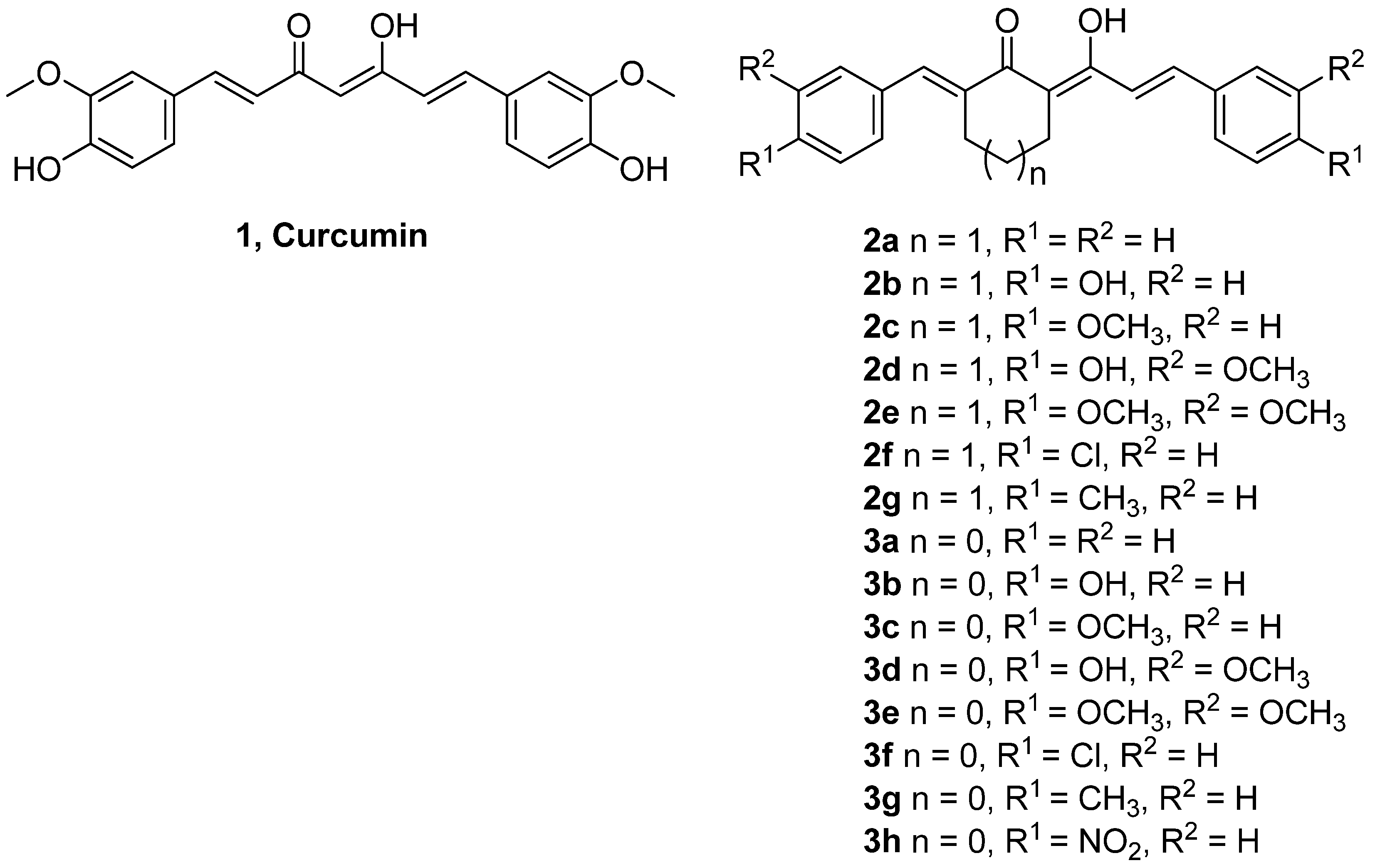
2. Results and Discussion
| Compound | Antioxidant Capacity * | ||
|---|---|---|---|
| DPPH (IC50) (mM) | FRAP (mmole TE/L) | ORAC (mmole TE/L) | |
| Curcumin | 0.86 e | 5.01 ± 0.1 c | 1.89 ± 0.2 a |
| 2a | 2.86 h | 1.13 ± 0.4 g | 0.12 ± 0.01 g |
| 2b | 2.58 g | 3.02 ± 0.02 d | 0.43 ± 0.1 f |
| 2c | 4.07 i | 1.32 ± 0.001 f | 0.11 ± 0.01 g |
| 2d | 0.72 d | 5.35 ± 0.1 b | 0.50 ± 0.02 ef |
| 2e | 2.57 g | 0.47 ± 0.1 k | 0.60 ± 0.1e |
| 2f | 0.71 d | 1.28 ± 0.04 fg | 0.49 ± 0.1 ef |
| 2g | 1.36 f | 1.12 ± 0.02 g | 0.84 ± 0.01 d |
| 3a | 0.07 a | 1.41 ± 0.1 f | 1.12 ± 0.1 c |
| 3b | 0.06 a | 2.29 ± 0.1 e | 0.49 ± 0.1 ef |
| 3c | 0.34 c | 1.09 ± 0.1 gh | 1.17 ± 0.1 b |
| 3d | 0.07 a | 6.70 ± 0.32 a | 0.75 ± 0.05 d |
| 3e | 0.03 a | 0.67 ± 0.02 j | 0.69 ± 0.1 e |
| 3f | 0.09 a | 1.09 ± 0.1 gh | 1.30 ± 0.02 b |
| 3g | 0.51 b | 0.95 ± 0.02 hi | 1.25 ± 0.04 bc |
| 3h | 0.36 c | 1.05 ± 0.01 ghi | 0.84 ± 0.1 d |
| Compound | % Enzyme Inhibition a | ||
|---|---|---|---|
| ACE | Tyrosinase | HIV-I protease | |
| Curcumin | 76.86 bc | 12.61 k | 48.3 c |
| 2a | 19.69 g | 46.1 i | 48.2 c |
| 2b | 88.73 ab | 100 a | 55.5 b |
| 2c | 4.01 g | 40.63 j | 43.7 cd |
| 2d | 84.59 ab | 56.84 h | 37.3 ef |
| 2e | 36.56 f | 100 a | 61.6 a |
| 2f | ND Ω | 60.15 ef | 9.9 i |
| 2g | 15.04 g | 59.44 fg | 20.1 h |
| 3a | 51.96 ef | 70.85 c | 27.0 g |
| 3b | 90.55 ab | 100 a | 41.1 de |
| 3c | 67.42 cd | 58.54 g | 35.9 f |
| 3d | 93.89 a | 79.99 b | 41.8 de |
| 3e | 56.95 de | 100 a | 44.5 cd |
| 3f | 75.12 bc | 61.31 e | 23.3 gh |
| 3g | 46.19 ef | 67.18 d | 22.7 gh |
| 3h | 88.01 ab | 59.22 fg | 44.8 cd |
| Series | Independent | Dependent | Type | Correlation coefficient R | P value |
|---|---|---|---|---|---|
| Series 2 | Hammett σ | ACE | Semi-log | −0.827 | 0.022 |
| Protease | Semi-log | −0.703 | 0.078 | ||
| Hantzsch π | FRAP | Linear | −0.723 | 0.067 | |
| ACE | Linear | −0.886 | 0.008 | ||
| Protease | Semi-log | −0.750 | 0.052 | ||
| cLogP | FRAP | Linear | −0.667 | 0.101 | |
| ACE | Linear | −0.869 | 0.011 | ||
| Protease | Semi-log | −0.792 | 0.034 | ||
| Dipole | ACE | Semi-log | 0.687 | 0.088 | |
| FRAP | ACE | Linear | 0.795 | 0.033 | |
| Series 3 | Hansch π | FRAP | Semi-log | −0.661 | 0.074 |
| ACE | Linear | −0.668 | 0.070 | ||
| Protease | Semi-log | −0.829 | 0.011 | ||
| cLogP | FRAP | Linear | −0.614 | 0.105 | |
| ORAC | Linear | 0.882 | 0.004 | ||
| Protease | Semi-log | −0.874 | 0.005 | ||
| Dipole | DPPH | Semi-log | −0.622 | 0.099 | |
| ORAC | Linear | −0.620 | 0.101 | ||
| DPPH | Tyrosinase | Semi-log | −0.627 | 0.096 | |
| FRAP | ACE | Linear | 0.591 | 0.123 |
2.1. DPPH· Scavenging Assay
2.2. Ferric Reducing Ability of Plasma (FRAP) Assay
2.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4. Angiotensin Converting Enzyme (ACE) Inhibition Assay
2.5. Tyrosinase Inhibition Assay
2.6. HIV-I Protease Inhibition
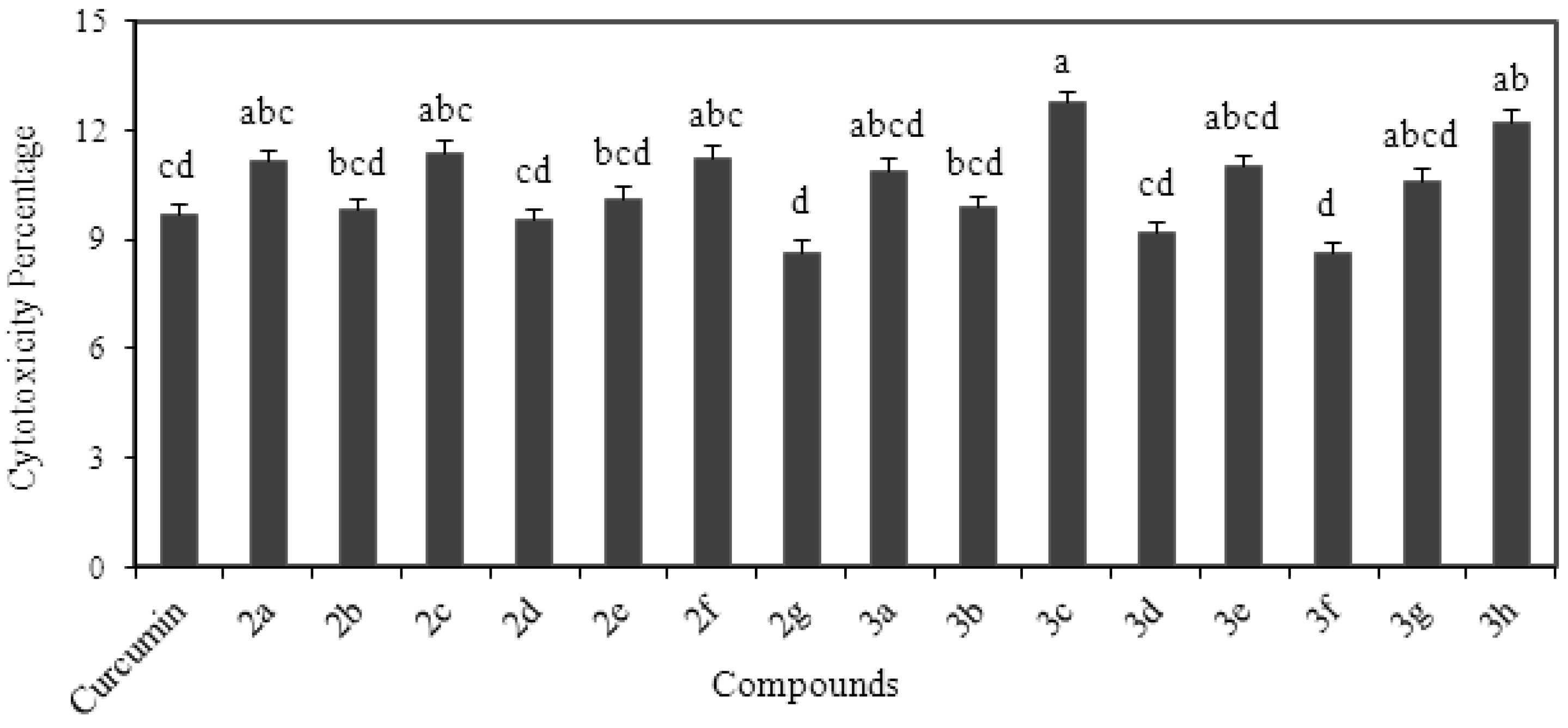
2.7. Cytotoxicity Analysis
3. Experimental
3.1. Materials
3.2. Sample Preparation
3.3. Cell Culture
3.4. DPPH· Scavenging Assay
3.5. Ferric Reducing Ability of Plasma (FRAP) Assay
3.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.7. Angiotensin Converting Enzyme (ACE) Inhibition Assay
3.8. Tyrosinase Inhibition Assay
3.9. HIV I Protease Inhibition Assay
3.10. Cytotoxicity Analysis

3.11. Quantitative Structure Activity Relationship Studies Using Statistical Analysis
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Youssef, D.; Nichols, C.E.; Cameron, T.S.; DeClercq, E.; Balzarini, J.; Jha, A. Design, synthesis and cytostatic activity of novel cyclic curcumin analogs. Bioorg. Med. Chem. Lett. 2007, 17, 5624–5629. [Google Scholar] [CrossRef]
- Zhao, X.C.; Zhang, L.; Yu, H.X.; Sun, Z.; Lin, X.F.; Tan, C.; Lu, R.R. Curcumin protects mouse neuroblastoma Neuro-2A cells against hydrogen-peroxide-induced oxidative stress. Food Chem. 2011, 129, 387–394. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. Role of free radicals in toxicology and disease. J. Basic Clin. Physiol. Pharmacol. 2011, 6, 205–228. [Google Scholar]
- Shang, Y.J.; Jin, X.L.; Shang, X.L.; Tang, J.J.; Liu, G.Y.; Dai, F.; Qian, Y.P.; Fan, G.J.; Liu, Q.; Zhou, B. Antioxidant capacity of curcumin directed analogues: Structure-activity relationship and influence of microenvironment. Food Chem. 2010, 119, 1435–1442. [Google Scholar] [CrossRef]
- Lin, L.; Lee, K.H. Structure-activity relationships of curcumin and its analogues with different biological activities. Stud. Nat. Prod. Chem. 2006, 33, 785–812. [Google Scholar]
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Morimoto, T.; Wada, H.; Takaya, T.; Katanasaka, Y.; Kawamura, T.; Yanagi, S.; Marui, A.; Sakata, R.; Shimatsu, A.; Kimura, T.; Kakeya, H.; Fujita, M.; Hasegawa, K. A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ. J. 2011, 75, 2151–2159. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Hasoya, T.; Nakata, A.; Yamasaki, F.; Abas, F.; Shaari, K.; Lajis, N.H.; Morita, H. Curcumin like diarylpentanoid analogues as melanogenesis inhibitors. J. Nat. Med. 2011, 66, 166–176. [Google Scholar] [CrossRef]
- Lee, K.H.; Aziz, F.H.A.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200. [Google Scholar] [CrossRef]
- McCutchan, F.E. Global Epidemiology of HIV. J. Med. Virol. 2006, 78, S7–S12. [Google Scholar] [CrossRef]
- Mazumder, A.; Raghavan, K.; Weinstein, J.; Kohn, K.W.; Pommier, Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar] [CrossRef]
- Otto, M.J.; Reid, C.D.; Garber, S.; Lam, P.Y.; Scarnati, H.; Bacheler, L.T.; Rayner, M.M.; Winslow, D.L. In vitro anti-human immunodeficiency virus (HIV) activity of XM323, a novel HIV protease inhibitor. Antimicrob. Agents Chemother. 1993, 34, 2606–2611. [Google Scholar] [CrossRef]
- Jha, A.; Zhao, J.; Cameron, T.S.; DeClercq, E.; Balzarini, J.; Manavathu, E.K.; Stables, J.P. Design, synthesis and biological evaluation of novel curcumin analogs as anti-neoplastic agents. Lett. Drug Des. Discov. 2006, 3, 304–310. [Google Scholar] [CrossRef]
- Nichols, C.E.; Youssef, D.; Harris, R.G.; Jha, A. Microwave-assisted synthesis of curcumin analogs. Arkivoc 2006, 13, 64–72. [Google Scholar]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agri. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Dutta, S.; Padhye, S.; Priyadarsini, K.I.; Newton, C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg. Med. Chem. Lett. 2005, 15, 2738–2744. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, Y.; Li, Y.P.; Chen, S.H.; Tan, J.H.; Ou, T.M.; Gu, L.Q.; Huang, Z.S. Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2005, 19, 5596–5604. [Google Scholar] [CrossRef]
- Venkatesan, P.; Rao, M.N.A. Structure activity relationships for the inhibition of lipid peroxidation and the scavenging of free radicals by synthetic symmetrical curcumin analogues. J. Pharm. Pharmacol. 2010, 52, 1123–1128. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, C.; Couto, G.D.; Ouzounian, M.; Sun, M.; Wang, A.B.; Huang, Y.; He, C.W.; Shi, Y.; Chen, X.; et al. Curcumin prevents and reverses murine cardiac hypertrophy. J. Clin. Invest. 2008, 118, 879–893. [Google Scholar] [CrossRef]
- Mazumder, A.; Neamati, N.; Sunder, S.; Schulz, J.; Pertz, H.; Eich, E.; Pommier, Y. Curcumin analogues with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J. Med. Chem. 1997, 40, 3057–3063. [Google Scholar] [CrossRef]
- Lue, B.M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Huber, G.M.; Embree, C.; Forsline, P.L. Red-fleshed apple as a source of functional beverages. Can. J. Plant Sci. 2010, 90, 95–100. [Google Scholar] [CrossRef]
- Huber, G.W.; Rupasinghe, H.P.V. Phenolic profiles and antioxidant properties of apple skin extracts. J. Food Sci. 2009, 74, C693–C700. [Google Scholar] [CrossRef]
- Noh, J.M.; Lee, Y.S. Inhibitory activities of hydroxyphenolic acid-amino acid conjugates on tyrosinase. Food Chem. 2011, 125, 953–957. [Google Scholar] [CrossRef]
- Xu, X.H.; Wan, M.; Dong, H.; But, P.P.; Foo, L.Y. Inhibitory Activity of flavonoids and tannins against HIV-1 Protease. Biol. Pharm. Bull. 2000, 23, 1072–1076. [Google Scholar] [CrossRef]
- Patrick, G.L. An Introduction to Medicinal Chemistry; Oxford University Press Inc.: New York, NY, USA, 2005; p. 271. [Google Scholar]
- Sample Availability: Samples of the compounds 2a–g and 3a–h are available from Dr. Amitabh Jha.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bhullar, K.S.; Jha, A.; Youssef, D.; Rupasinghe, H.P.V. Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties. Molecules 2013, 18, 5389-5404. https://doi.org/10.3390/molecules18055389
Bhullar KS, Jha A, Youssef D, Rupasinghe HPV. Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties. Molecules. 2013; 18(5):5389-5404. https://doi.org/10.3390/molecules18055389
Chicago/Turabian StyleBhullar, Khushwant S., Amitabh Jha, Dani Youssef, and H. P. Vasantha Rupasinghe. 2013. "Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties" Molecules 18, no. 5: 5389-5404. https://doi.org/10.3390/molecules18055389
APA StyleBhullar, K. S., Jha, A., Youssef, D., & Rupasinghe, H. P. V. (2013). Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties. Molecules, 18(5), 5389-5404. https://doi.org/10.3390/molecules18055389







