Synthesis and Antimicrobial Activity of Some New Thieno[2,3-b]thiophene Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
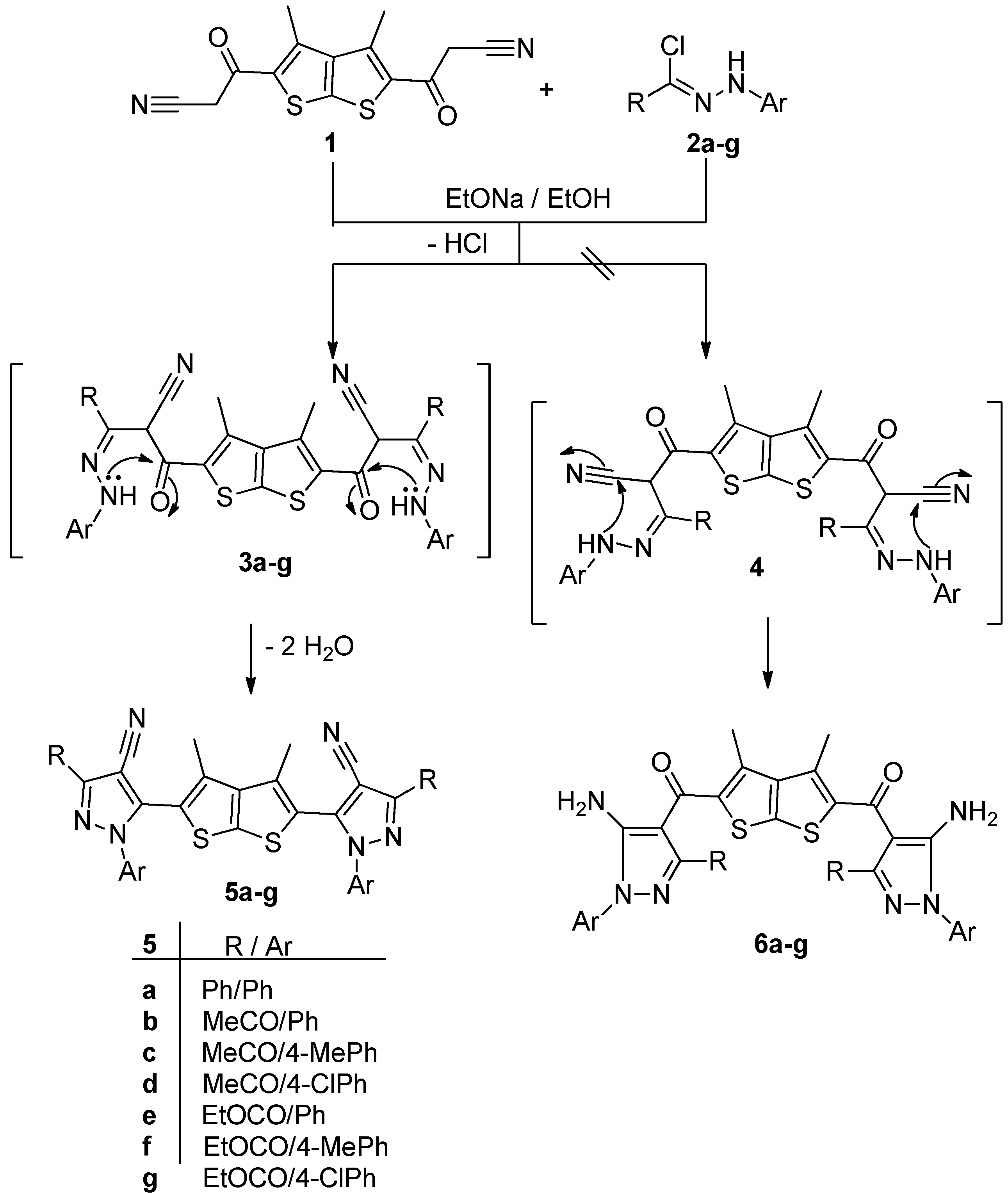

2.2. Antimicrobial Evaluation
| Compound Tested | Micoorganisms | |||
|---|---|---|---|---|
| Aspergillus Fumigatus | Geotrichum Candidum | Candida albicans | Syncephalastrum racemosum | |
| 5c | 17.3 ± 0.4 | 21.3 ± 0.4 | NA | 14.8 ± 0 |
| 5d | 22.4 ± 0.5 | 29.7 ± 0.2 | NA | 19.8 ± 0.8 |
| 11 | 18.9 ± 0.3 | 23.4 ± 0.4 | NA | 16.5 ± 0.2 |
| Amphotericin B | 23.7 ± 0.1 | 28.7 ± 0.2 | 25.4 ± 0.1 | 19.7 ± 0.2 |
| Compound Tested | Gram-Positive Bacteria | Gram-Negative Bacteria | ||
|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E. coli | |
| 5c | 18.6 ± 0.3 | 20.9 ± 0.5 | 17.4 ± 0.2 | 20.3 ± 0.4 |
| 5d | 23.8 ± 0.5 | 27.4 ± 0.6 | 20.7 ± 0.2 | 26.8 ± 0.2 |
| 11 | 20.4 ± 0.4 | 23.1 ± 0.2 | 19.6 ± 0.1 | 22.2 ± 0.5 |
| Penicillin G | 23.8 ± 0.2 | 32.4 ± 0.3 | - | - |
| Streptomycin | - | - | 20.3 ± 0.1 | 24.9 ± 0.3 |


3. Experimental
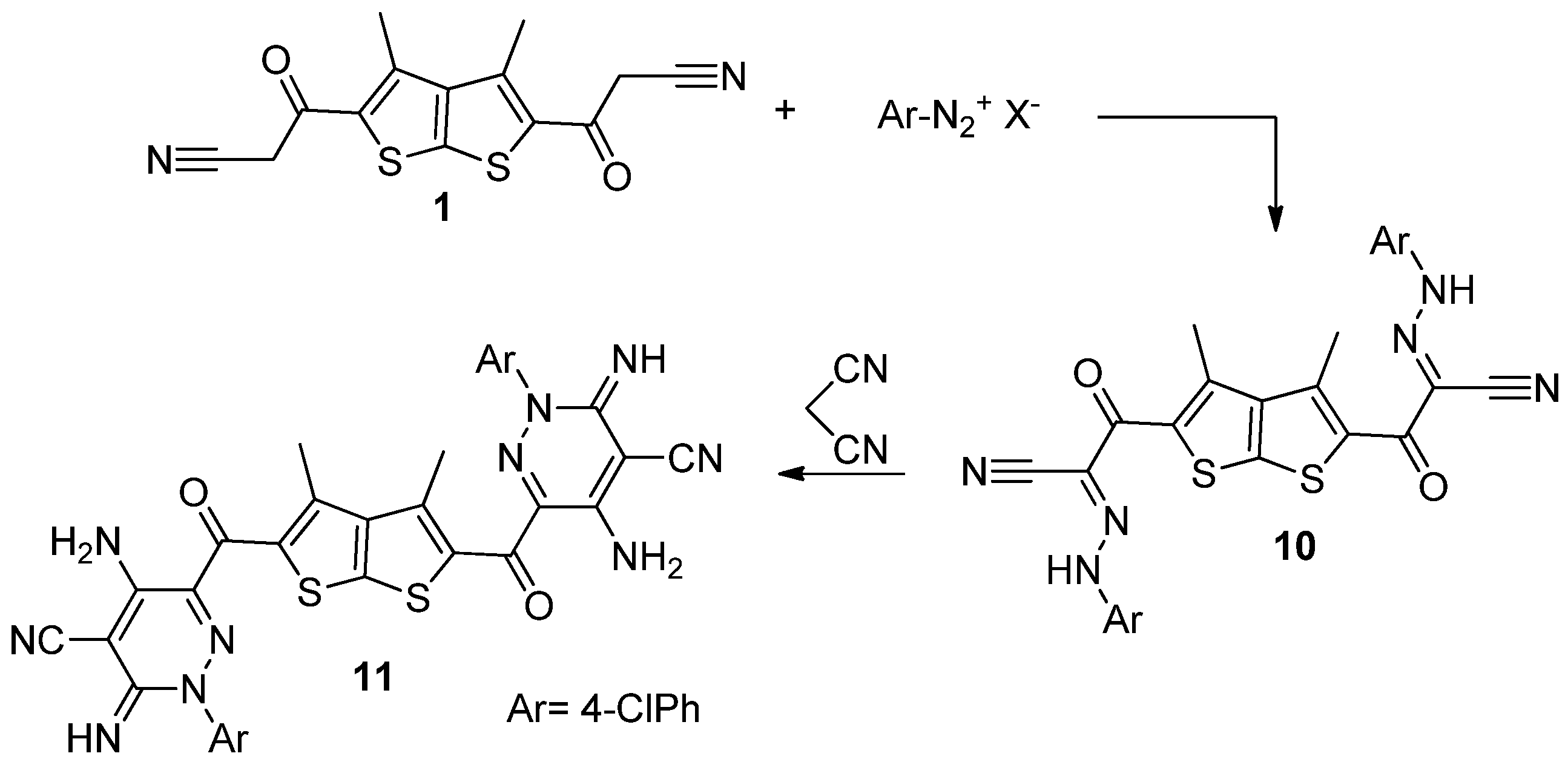
3.1. Reaction of Thieno[2,3-b]thiophene 1 with Hydrazonoyl Halides
General Procedure
3.2. Reaction of Hydrazone 10 with Malononitrile
4. Conclusions
Acknowledgments
References
- El-Kerdawy, M.M.; Yousif, M.Y.; El-Emam, A.A.; Moustafa, M.A.; El-Sherbeny, M.A. Synthesis and antiinflammatory activity of certain thienopyrimidine derivatives. Boll. Chim. Farm. 1996, 135, 301–305. [Google Scholar]
- Modica, M.; Santagati, M.; Santagati, A.; Cutuli, V.; Mangano, N.; Caruso, A. Synthesis of new [1,3,4]thiadiazolo[3,2-a]thieno[2,3-d]pyrimidinone derivatives with antiinflammatory activity. Pharmazie 2000, 55, 500–502. [Google Scholar]
- Chambhare, R.V.; Khadse, B.G.; Bobde, A.S.; Bahekar, R.H. Synthesis and preliminary evaluation of some N-[5-(2-furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d]pyrimidin-3-yl]-carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3H-thieno[2,3-d]pyrimidin-4-ones asantimicrobial agents. Eur. J. Med. Chem. 2003, 38, 89–100. [Google Scholar] [CrossRef]
- Santagati, N.A.; Caruso, A.; Cutuli, V.M.; Caccamo, F. Synthsis and phamacological evaluation of thieno[2,3-d]pyrimidin-2,4-dione and 5H-pyrimido[5,4-b]indol-2,4-dione derivatives. Farmaco 1995, 50, 689–695. [Google Scholar]
- Egbertson, M.S.; Cook, J.J.; Bednar, B.; Prugh, J.D.; Bednar, R.A.; Gaul, S.L.; Gould, R.J.; Hartman, G.D.; Homnick, C.F.; Holahan, M.A.; et al. Non-peptide GPIIb/IIIa inhibitors. 20. Centrally constrained thienothiophene alpha-sulfonamides are potent, long acting in vivo inhibitors of platelet aggregation. J. Med. Chem. 1999, 42, 2409–2421. [Google Scholar] [CrossRef]
- Meyer, M.D.; Altenbach, R.J.; Basha, F.Z.; Carroll, W.A.; Condon, S.; Elmore, S.W.; Kerwin, J.F.; Sippy, K.B.; Tietje, K.; Wendt, M.D.; et al. Structure-activity studies for a novel series of tricyclic substituted hexahydrobenz[e]isoindole α1A adrenoceptor antagonists as potential agents for the symptomatic treatment of benign prostatic hyperplasia (BPH). J. Med. Chem. 2000, 43, 1586–1603. [Google Scholar] [CrossRef]
- Cardile, A.P.V.; Santagati, A.; Gentile, B. Thienopyrimidine derivatives prevent cartilage destruction in articular disease. Farmaco 2001, 56, 959–964. [Google Scholar] [CrossRef]
- Lee, K.; Sotzing, G.A. Poly(thieno[3,4-b]thiophene). A new stable low band gap conducting polymer. Macromolecules 2001, 34, 5746–5747. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Genevicius, K.; Shkunov, M.; Sparrowe, D.; Tierney, S.; McCulloch, U. Stable polythiophene semiconductors incorporating thieno[2,3-b]thiophene. J. Am. Chem. Soc. 2005, 127, 1078–1079. [Google Scholar] [CrossRef]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Katritzky, A., Ed.; Pergamon Press: Oxford, UK, 1984; Volume 5, p. 277. [Google Scholar]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Shintai, I., Ed.; Elsevier: Oxford, UK, 1986; Volume 5, p. 3. [Google Scholar]
- Butnariu, R.M.; Caprosu, M.D.; Bejan, V.; Angalagiu, I.I.; Ungureanu, M.; Poiata, A.; Tuchilus, C.; Florescu, M. Pyridazine and phthalazine derivatives with potential antimicrobial activity. J. Heterocycl. Chem. 2007, 44, 1149–1152. [Google Scholar] [CrossRef]
- Caprosu, M.; Butnariu, R.; Mangalagiu, I.I. Synthesis and antimicrobial activity of some new pyridazine derivatives. Heterocycles 2005, 65, 1871–1879. [Google Scholar] [CrossRef]
- Dima, S.; Caprosu, M.; Ungureanu, M.; Grosu, G.; Petrovanu, M. New derivatives of 1-methyl-phthalazine with antimicrobial and fungistatic action. Ann. Pharm. Fr. 1999, 5, 415–416. [Google Scholar]
- Dodge, D. Herbicides and Plant Metabolism; Cambridge University Press: Cambridge, UK, 1989; p. 114. [Google Scholar]
- Drochioiu, G.; Sunel, V.; Oniscu, C.; Basu, C.; Murariu, M. The breakdown of plant biostructure followed by amino acids determination. Roum. Biotechnol. Lett. 2001, 6, 155–165. [Google Scholar]
- Drochioiu, G.; Strajeriu, S.; Petrovanu, M.; Druta, I. Plant genetic resources newsletter. 2002, 129, 47–51. [Google Scholar]
- Druta, I.; Cuciac, C.; Danac, R.; Avram, E.; Rotaru, A.; Drochioiu, G. The phytotoxic effect of some new monoquaternary salts of 4,4'-bipyridyl and 1,10-phenanthroline. Pak. J. Appl. Sci. 2002, 2, 145–150. [Google Scholar] [CrossRef]
- Druta, I.; Danac, R.; Ungureanu, M.; Drochioiu, G. Antimicrobial activity of new derivatives of 1,10-phenanthroline, Ann. Pharm. Fr. 2002, 60, 348–351. [Google Scholar]
- Gokçe, M.; Dogruer, D.; Sahin, M.F. Synthesis and antinociceptive activity of 6-substituted-3-pyridazinone derivatives. Farmaco 2001, 56, 233–237. [Google Scholar] [CrossRef]
- Mabkhoot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar] [CrossRef]
- Mabkhoot, Y.N.; Kheder, N.A.; Al-Majid, A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 9418–9426. [Google Scholar] [CrossRef]
- Kheder, N.A.; Mabkhoot, Y.N.; Farag, A.M. Synthesis and antimicrobial evaluation of some bis(thioxopyridine), bis(pyrazolo[3,4-b]pyridine), bis(thieno[2,3-b]pyridine), bis(1,3,4-thiadiazole) and bis(thiophene) derivatives. Heterocycles 2008, 75, 2937–2948. [Google Scholar] [CrossRef]
- Kheder, N.A. Convenient synthesis of novel bis(hydrazone) and bis(indole) derivatives. Heterocycles 2009, 78, 1281–1288. [Google Scholar] [CrossRef]
- Kheder, N.A. Synthesis of some novel bis(pyrazole), bis(pyridine) and bis pyrazolo[5,1-c]-1,2,4-triazine derivatives. Heterocycles 2009, 78, 1815–1822. [Google Scholar] [CrossRef]
- Wolkoff, P. A new method of preparing hydrazonyl halides. Can. J. Chem. 1975, 53, 1333–1335. [Google Scholar] [CrossRef]
- Dieckmann, W.; Platz, L. By a new methode of formation of Osotetrazonen. Chem. Ber. 1905, 38, 2986–2990. [Google Scholar] [CrossRef]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles. Part II. New routes to acetyl thiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Eweiss, N.F.; Hassaneen, H.M.; Al-gharib, M.S. Synthesis and rearrangement of ethyl aryloxyglyoxalate arylhydrazones. Bull. Chem. Soc. Jpn. 1975, 48, 365–366. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5–11 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mabkhot, Y.N.; Kheder, N.A.; Farag, A.M. Synthesis and Antimicrobial Activity of Some New Thieno[2,3-b]thiophene Derivatives. Molecules 2013, 18, 4669-4678. https://doi.org/10.3390/molecules18044669
Mabkhot YN, Kheder NA, Farag AM. Synthesis and Antimicrobial Activity of Some New Thieno[2,3-b]thiophene Derivatives. Molecules. 2013; 18(4):4669-4678. https://doi.org/10.3390/molecules18044669
Chicago/Turabian StyleMabkhot, Yahia Nasser, Nabila Abdelshafy Kheder, and Ahmad M. Farag. 2013. "Synthesis and Antimicrobial Activity of Some New Thieno[2,3-b]thiophene Derivatives" Molecules 18, no. 4: 4669-4678. https://doi.org/10.3390/molecules18044669
APA StyleMabkhot, Y. N., Kheder, N. A., & Farag, A. M. (2013). Synthesis and Antimicrobial Activity of Some New Thieno[2,3-b]thiophene Derivatives. Molecules, 18(4), 4669-4678. https://doi.org/10.3390/molecules18044669





