α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Separation and Structural Elucidation

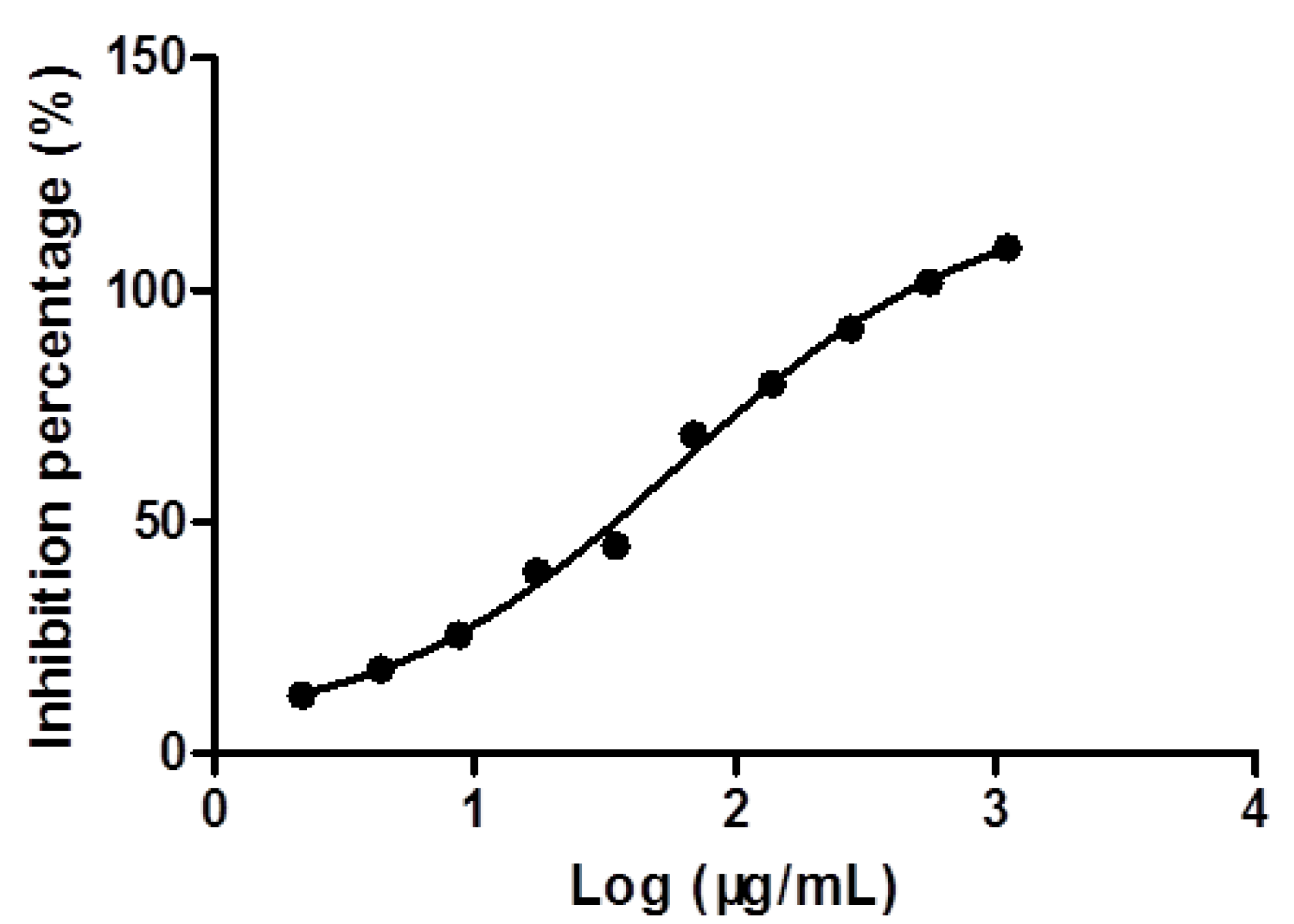
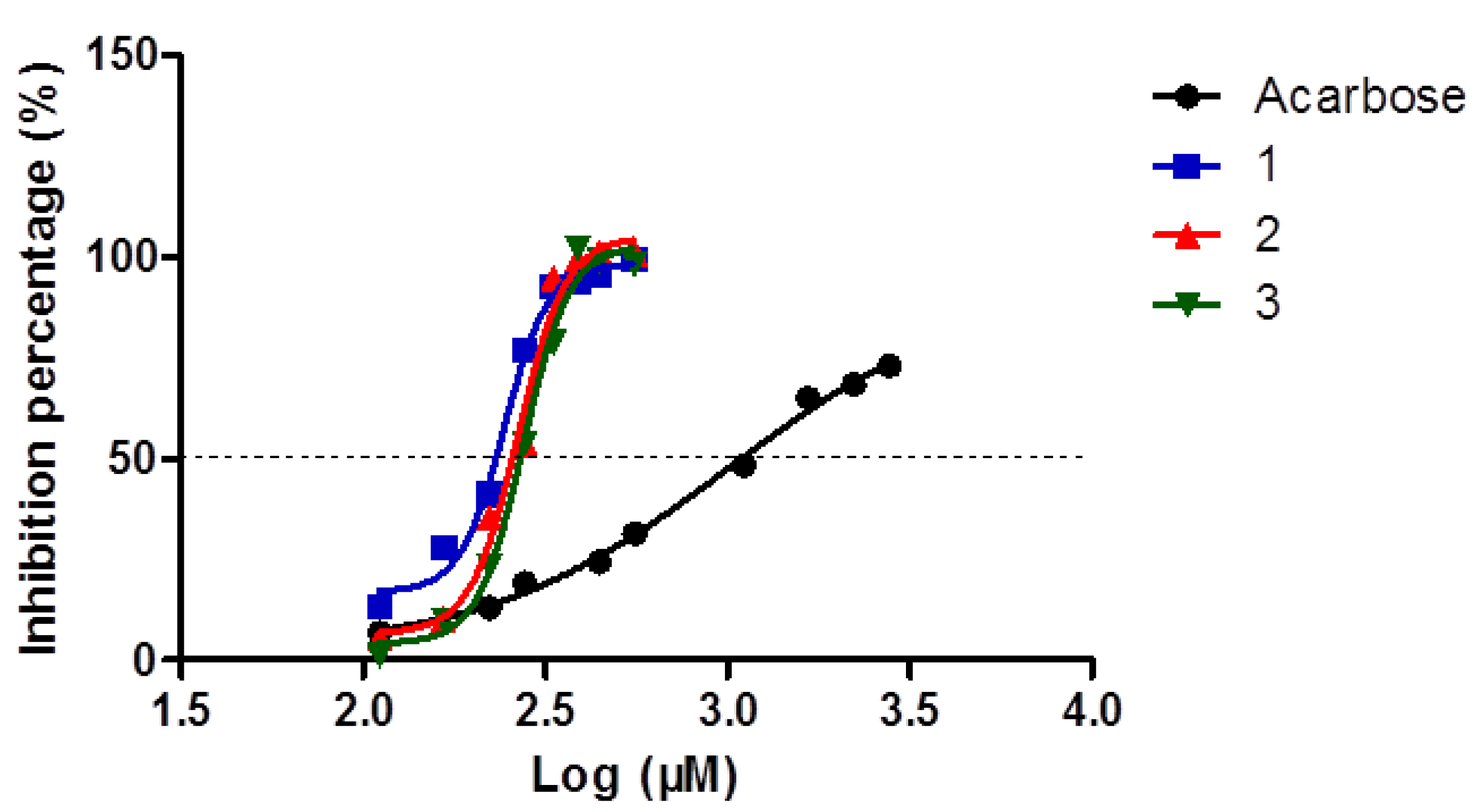
2.3. Optimization of HPLC Condition
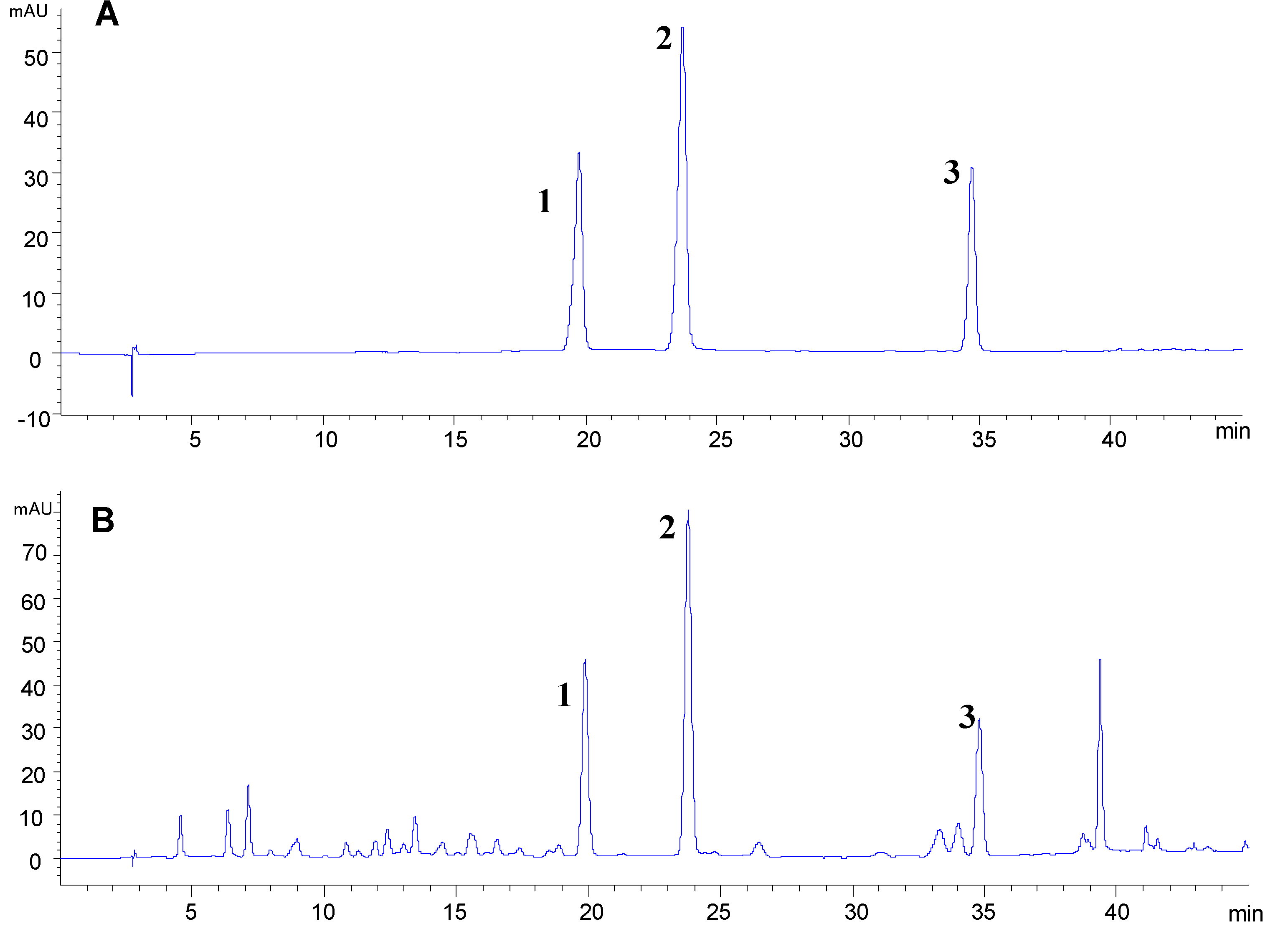
2.4. Optimization of Sample Extraction for HPLC Analysis
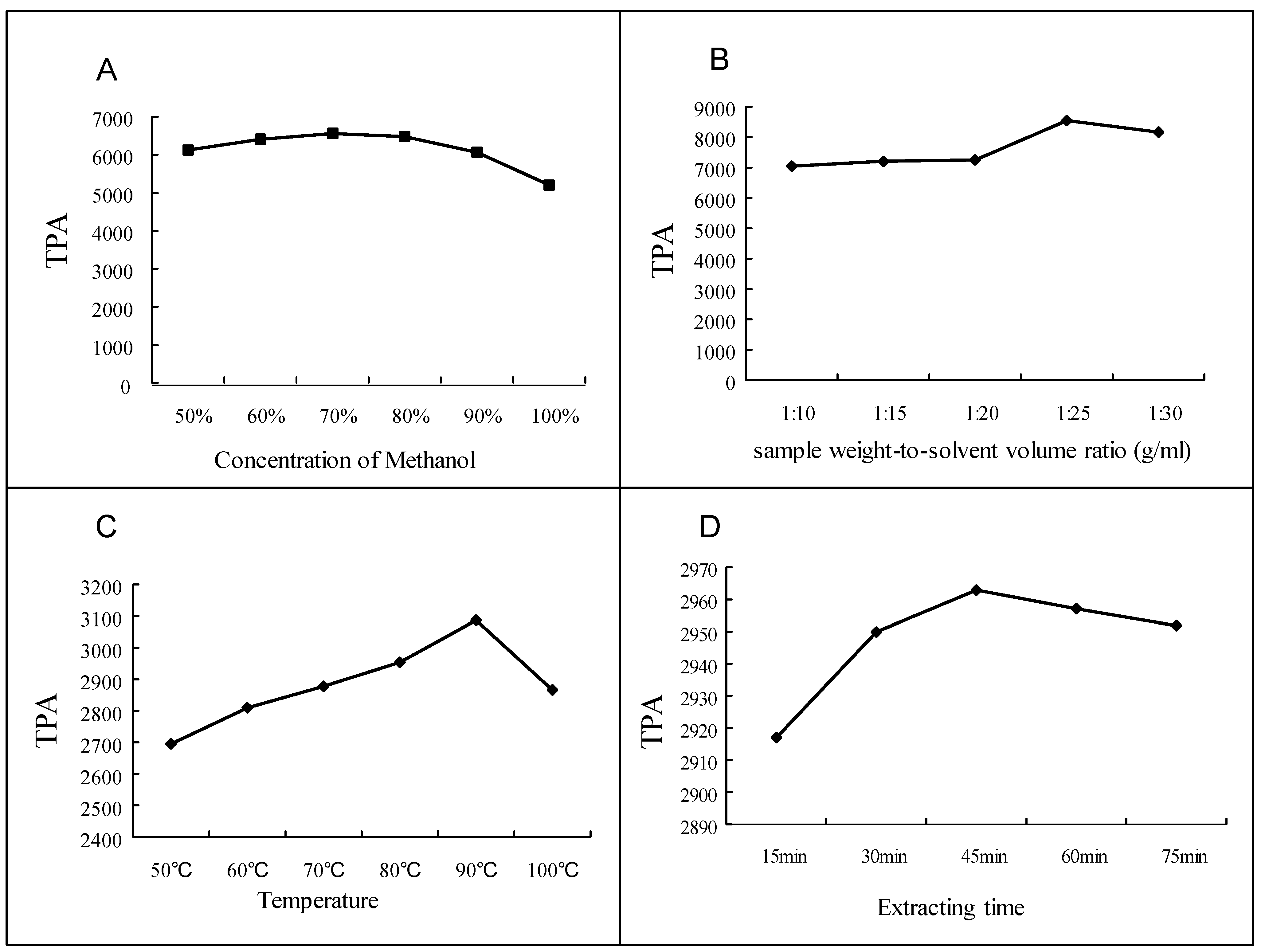
2.5. Method Validation
| Flavonoid glycosides | RT (min) | Calibration curve a | R2 | Test range (µg/mL) | LOD b (ng) | LOQ c (ng) |
|---|---|---|---|---|---|---|
| Vitexin (1) | 19.8 | y = 27.707x + 29.277 | 0.9999 | 3.2–206.0 | 0.6 | 1.9 |
| Isovitexin (2) | 23.7 | y = 21.677x + 20.783 | 0.9999 | 3.3–214.0 | 0.7 | 2.5 |
| Isorhamnetin 3-O-β-d-rutinoside (3) | 34.7 | y = 12.942x + 14.820 | 0.9999 | 3.3–212.0 | 0.5 | 1.5 |
| Original (mg) | Spiked (mg) | Found (mg) | Recovery a (%) | R.S.D (%) | |
|---|---|---|---|---|---|
| Vitexin (1) | 0.55 | 1.03 | 103.6 | 1.98 | |
| 0.46 | 0.50 | 0.94 | 96.0 | 1.19 | |
| 0.38 | 0.83 | 97.4 | 0.98 | ||
| Isovitexin (2) | 1.35 | 2.51 | 102.2 | 1.09 | |
| 1.13 | 1.10 | 2.24 | 103.6 | 0.99 | |
| 0.85 | 1.96 | 97.6 | 1.27 | ||
| Isorhamnetin 3-O-β-d-rutinoside (3) | 0.88 | 1.59 | 98.9 | 2.21 | |
| 0.72 | 0.74 | 1.44 | 97.3 | 1.00 | |
| 0.59 | 1.30 | 98.3 | 1.28 |
2.6. Quantitative Analysis of Three Flavonoid Glycosides
| Vitexin | Isovitexin | Isorhamnetin 3-O-β-d-Rutinoside | Total contents of 3 flavonoid glycosides | |
|---|---|---|---|---|
| BZY1 | 0.34 ± 0.00 | 1.80 ± 0.03 | 3.15 ± 0.03 | 5.30 ± 0.05 |
| BZY2 | 0.20 ± 0.01 | 1.48 ± 0.03 | 3.18 ± 0.08 | 4.87 ± 0.11 |
| BZY3 | 0.10 ± 0.00 | 1.17 ± 0.01 | 5.16 ± 0.01 | 6.42 ± 0.02 |
| BZY4 | 0.61 ± 0.02 | 2.13 ± 0.05 | 2.55 ± 0.07 | 5.29 ± 0.13 |
| BZY5 | 0.53 ± 0.00 | 1.97 ± 0.02 | 2.40 ± 0.01 | 4.90 ± 0.03 |
| BZY6 | 0.30 ± 0.00 | 1.96 ± 0.02 | 4.01 ± 0.05 | 6.27 ± 0.07 |
| BZY7 | 0.29 ± 0.01 | 1.43 ± 0.04 | 2.69 ± 0.06 | 4.41 ± 0.11 |
| BZY8 | 1.32 ± 0.01 | 3.30 ± 0.02 | 3.06 ± 0.03 | 7.68 ± 0.06 |
| BZY9 | 2.31 ± 0.04 | 5.65 ± 0.08 | 3.60 ± 0.05 | 11.55 ± 0.17 |
| BZY10 | 0.46 ± 0.02 | 2.43 ± 0.04 | 2.39 ± 0.09 | 5.28 ± 0.14 |
| BZY11 | 0.85 ± 0.04 | 1.73 ± 0.06 | 1.66 ± 0.06 | 4.23 ± 0.16 |
3. Experimental
3.1. Chemicals
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Samples Preparation for α-Glucosidase Inhibitory Assay
3.5. Assay of α-Glucosidase Inhibitory Activity
3.6. Samples Preparation for HPLC Analysis
3.7. Chromatographic Conditions
3.8. Method Validation of HPLC Analysis
4. Conclusions
Acknowledgments
References
- WHO Diabetes Programme. Diabetes. Available online: http://www.who.int/diabetes/en/index.html (accessed on 8 March 2013).
- Goodarz, D.; Mariel, M.F.; Yuan, L.; Gitanjali, M.S.; Melanie, J.C.; Christopher, J.P.; John, K.L.; Farshad, F.; Young-Ho, K.; Gretchen, A.S.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Ren, S.; Xu, D.D.; Pan, Z.; Gao, Y.; Jiang, Z.G.; Gao, Q.P. Two flavanone compounds from litchi (Litchi chinensis Sonn.) seeds, One previously unreported, and appraisal of their α-glucosidase inhibitory activities. Food Chem. 2011, 127, 1760–1763. [Google Scholar] [CrossRef]
- Hollander, P. Safety profile of acarbose, an alpha-glucosidsase inhibitor. Drugs 1992, 44 (Suppl. 3), 47–53. [Google Scholar] [CrossRef]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. Alpha-Glucosidase inhibitory action of natural acylated anthocyanins.1. Survey of natural pigment with potent inhibitory activity. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar] [CrossRef]
- Mcdougall, G.J.; Shpiro, F.; Doboson, P.; Smith, P.; Black, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Niwa, T.; Doi, U.; Osawa, T. Inhibitory activity of corn-derived bisamide compounds against α-glucosidase. J. Agric. Food Chem. 2003, 51, 90–94. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Matsuda, H.; Tanabe, G.; Muraoka, O. Absolute stereostructure of potent a-glucosidase inhibitor, salacinol, with unique thiosugar sulfate inner salt structure from Salacia reticulata. Bioorgan. Med. Chem. 2002, 10, 1547–1554. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.N.; Xu, P.Y.; Kawabata, J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007, 105, 628–634. [Google Scholar] [CrossRef]
- Li, H.L.; Song, F.R.; Xing, J.P.; Tsao, R.; Liu, Z.Q.; Liu, S.Y. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J. Am. Soc. Mass Spectr. 2009, 20, 1496–1503. [Google Scholar] [CrossRef]
- Jiangsu New Medical School, Chinese Medicine Dictionary; Shanghai Science and Technology Publishing Club: Shanghai, China, 1986.
- The State Pharmacopoeia Commission of P.R. China, Microctis Folium. In Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume I, p. 88.
- Zeng, C.Y.; Mei, Q.X.; Dai, W.B. New Research Progress in Pharmacological Activities of Microcos Paniculata. Chin. Arch. Tradit. Chin. Med. 2010, 28, 1927–1929. [Google Scholar]
- Zhang, Z.Z. Effects of Jian Pi Shu Wei Tang on antidiatebtes in clinic. J. Hubei Chin. Med. 2004, 26, 20. [Google Scholar]
- Li, K.P.; Bi, Y.G.; Gao, C.K.; Li, W.M. Enrichment and purification characteristics of macroporous resins to total flavonoids of Microcos paniculata L. J. Guangdong Coll. Pharm. 2008, 24, 539–542. [Google Scholar]
- Feng, S.X.; Liu, M.F.; Wei, X.Y.; Lin, L.D. Triterpenoids and Flavonoids from the Leaves of Microcos paniculata. J. Trop. Subtrop. Bot. 2008, 16, 51–56. [Google Scholar]
- Luo, J.P.; Zhang, L.P.; Yang, S.L.; Roberts, M.F.; Phillipson, J.D. Seperation and structure elucidation of alkaloids from Chinese drug buzhaye, Folium Microcos. Acta Pharm. Sin. 2009, 44, 150–153. [Google Scholar]
- Feng, J.; Yang, X.; Wang, R. Bio-assay guided isolationan didentification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef]
- Choo, C.Y.; Sulong, N.Y.; Mana, F.; Wong, T.W. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in vivo α-glucosidase inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef]
- Makio, S.; Koji, K.; Masahiko, T.; Masahide, Y.; Kimiye, B. Antioxidant constituents in the dayflower (Commelina communis L.) and their α-glucosidase-inhibitory activity. J. Nat. Med. 2008, 62, 349–353. [Google Scholar] [CrossRef]
- Du, G.; Zhao, H.; Song, Y.; Zhang, Q.; Wang, Y. Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography–triple quadrupole mass spectrometry with novel shell-type column. J. Sep. Sci. 2011, 34, 2576–2585. [Google Scholar] [CrossRef]
- Li, G.H.; Zhang, Q.W.; Wang, L.; Zhang, X.Q.; Ye, W.C.; Wang, Y.T. New Isoflavone C-glycosides from Pueraria lobata. Helv. Chim. Acta 2011, 94, 423–428. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Q.W.; Li, S.L.; Wang, Y.; Ye, W.C.; Wang, Y.T. Simultaneous quantification of major flavonoids in Bawanghua, the edible flower of Hylocereus undatus using pressurized liquid extraction and high-performance liquid chromatography. Food Chem. 2012, 125, 528–533. [Google Scholar]
- Zhou, Y.Q.; Zhang, Q.W.; Li, S.L.; Yin, Z.Q.; Zhang, X.Q.; Ye, W.C. Quantitative determination of 13 flavonoids in seed of Oroxylum indicum by high performance liquid chromatography. Curr. Pharm. Anal. 2012, 8, 206–213. [Google Scholar] [CrossRef]
- Li, D.Q.; Qian, Z.M.; Li, S.P. Inhibition of Three Selected Beverage Extracts on α-Glucosidase and Rapid Identification of Their Active Compounds Using HPLC-DAD-MS/MS and Biochemical Detection. J. Agric. Food Chem. 2010, 58, 6608–6613. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-G.; Li, P.; Li, P.; Yan, R.; Zhang, X.-Q.; Wang, Y.; Zhang, X.-T.; Ye, W.-C.; Zhang, Q.-W. α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium. Molecules 2013, 18, 4221-4232. https://doi.org/10.3390/molecules18044221
Chen Y-G, Li P, Li P, Yan R, Zhang X-Q, Wang Y, Zhang X-T, Ye W-C, Zhang Q-W. α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium. Molecules. 2013; 18(4):4221-4232. https://doi.org/10.3390/molecules18044221
Chicago/Turabian StyleChen, Yan-Gan, Ping Li, Peng Li, Ru Yan, Xiao-Qi Zhang, Ying Wang, Xian-Tao Zhang, Wen-Cai Ye, and Qing-Wen Zhang. 2013. "α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium" Molecules 18, no. 4: 4221-4232. https://doi.org/10.3390/molecules18044221
APA StyleChen, Y.-G., Li, P., Li, P., Yan, R., Zhang, X.-Q., Wang, Y., Zhang, X.-T., Ye, W.-C., & Zhang, Q.-W. (2013). α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium. Molecules, 18(4), 4221-4232. https://doi.org/10.3390/molecules18044221





