3.1. Chemistry
Melting points (mp) were determined in a Büchi apparatus in open capillaries and were uncorrected. All commercial chemicals were used as purchased and solvents purified by the standard procedures prior to use [
24]. Thin-layer chromatography was performed on Merck 60 silica gel GF-254 precoated plates and the identification was done with UV light and colorization with 10% phosphomolybdic acid or ninhydrin spray followed by heating. Flash column chromatography was performed on Merck 60 silica gel (0.063–0.2 mesh). Infrared spectra were recorded using neat samples, without solvent or KBr, on a FT-IR spectrometer Nicolet Impact 410 model. NMR spectra were recorded on Bruker AC 200 (200 MHz) and Bruker DRX 400 (400 MHz) instruments. Chemical shifts (δ) are expressed in parts per million (ppm) relative to the residual solvent peak: CDCl3 7.26 ppm/77.0 ppm and coupling constants (
J) are reported in Hertz (Hz). High-resolution mass spectra (HRMS) were recorded on a QSTAR XL mass spectrometer, by electron spray ionisation (ESI-MS) technique (5 kV).
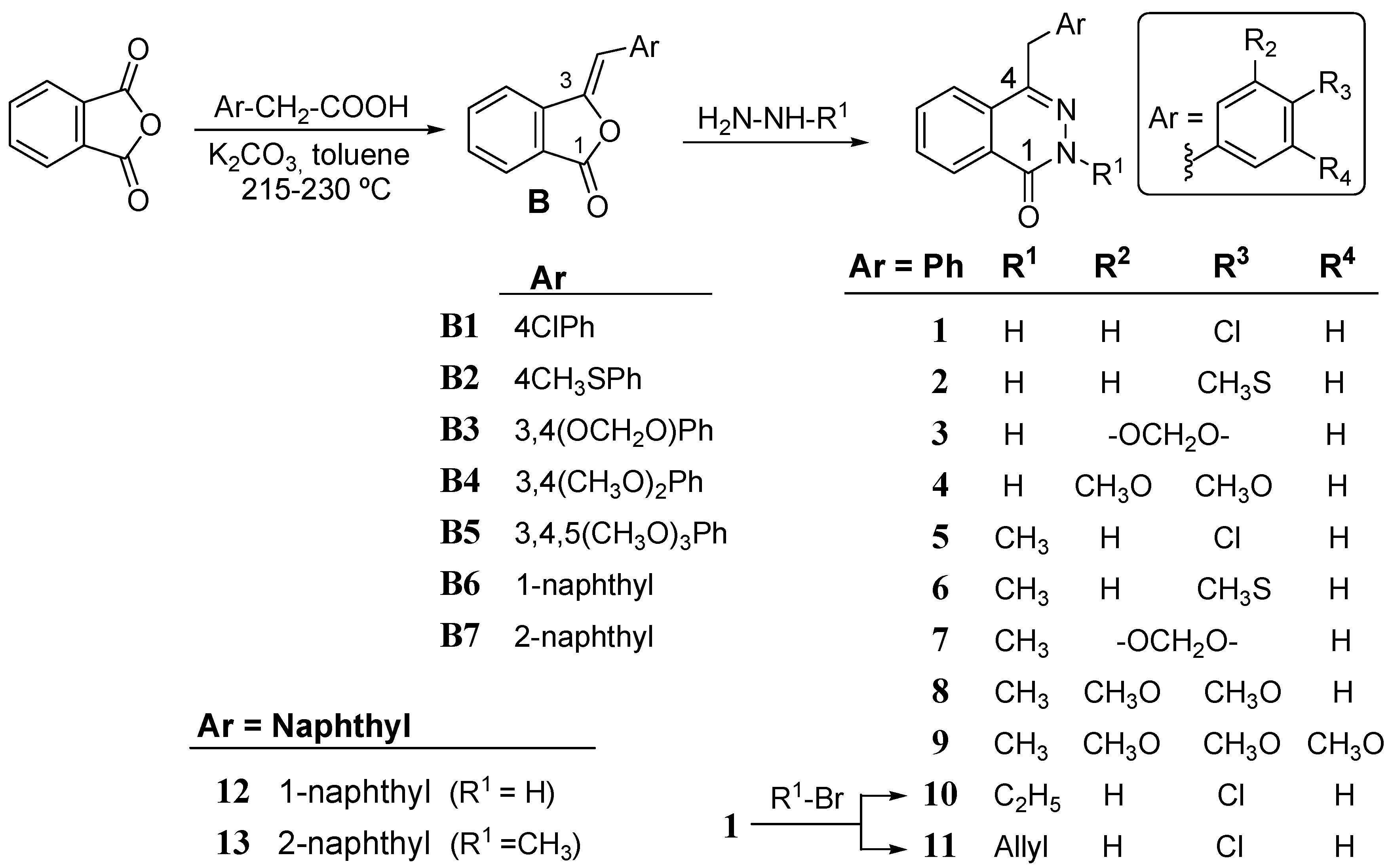
3.1.1. General Procedure for the Synthesis of Benzalphthalides B1–B16
Phthalic anhydride (2.2 mmol), the corresponding phenylacetic (naphthylacetic) acid (2.7 mmol), sodium acetate (0.26 mmol) and toluene (5 mL) were placed in a round-bottom flask to which a Dean-Stark separator was adapted. The mixtures were maintained at 210–245 °C under nitrogen and with magnetic stirring for 9–33 h. After cooling, the reaction mixtures were dissolved with ethyl acetate and washed with aqueous Na2CO3 (sat.), brine and water, dried over Na2SO4 and concentrated under reduced pressure to give the crude reaction products. Solid products were purified by crystallization and oily products chromatographed over silica gel; yields ranged from 40–95%. All the benzalphthalides were obtained as the Z isomer, and the configuration was confirmed through NOE-difference and/or 2D-ROESY experiments.
(Z)-3-(4-Chlorobenzylidene)isobenzofuran-1-one (B1). Yield 75%. Yellow crystals; mp 172–174 °C; IR (KBr), νmax: 2919, 1796, 1656, 1450, 1366, 1270, 1078, 969, 850, 825, 758, 606 cm−1. 1H-NMR δ: 6.30 (s, 1H, H-8), 7.30 (d, J = 8.8 Hz, 2H, H-3'+ H-5'), 7.53 (d, J = 7.8 Hz, 1H, H-4), 7.54 (m,1H, H-6), 7.68 (m, 1H, H-5), 7.70 (d, J = 8.8 Hz, 2H, H-2'+ H-6'), 7.87 (d, J = 7.8 Hz, 1H, H-7) ppm. 13C-NMR δ: 105.7 (C-8), 119.9 (C-4), 123.3 (C-7a), 125.6 (C-7), 129.0 (C-3' + C-5'), 130.0 (C-6), 131.3 (C-2' + C-6'), 131.6 (C-4'), 134.2 (C-1'), 134.7 (C-5), 140.3 (C-3a), 144.9 (C-3), 166.9 (C-1) ppm. ESI-MS: m/z 257.0291 [M+H]+; Anal. Calcd for C15H9ClO2: C, 70.19; H, 3.53. Found: C, 70.20; H, 3.49.
3.1.2. General Procedure for the Synthesis of Phthalazinones 1–4 and 12
Benzalphthalides B (1 mol) were mixed with an excess of hydrazine hydrate (4 mL), and few drops of toluene, and the mixture maintained at 70–80 °C under stirring for 3–12 h. After cooling reaction mixtures were extracted with ethyl acetate and washed with water, dried over Na2SO4 and concentrated under reduced pressure to give crude products that were purified by flash chromatography on silica gel and/or crystallisation.
4-(4-Chlorobenzyl)phthalazin-1(2H)-one (1). Yield 77%. Colourless oil. IR (NaCl), νmax: 3159, 2902, 1664, 1609, 1488, 1258, 815, 798, 684 cm−1. 1H-NMR δ: 4.28 (s, 2H, H-9), 7.21 (d, J = 8.8 Hz, 2H, H-3' + H-5'), 7.27 (d, J = 8.8 Hz, 2H, H-2' + H-6'), 7.75 (m, 3H, H-5 + H-6 + H-7), 8.47 (dd, J = 7.5, 2.5 Hz, 1H, H-8), 11.74 (br s, 1H, NH) ppm. 13C-NMR δ: 38.2 (C-9), 125.2 (C-7), 127.1 (C-8), 128.3 (C-8a), 128.9 (C-4a + C-3' + C-5'), 129.9 (C-2' + C-6'), 131.5 (C-5); 132.7 (C-1'), 133.6 (C-6), 136.0 (C-4'), 146.0 (C-4), 160.6 (C-1) ppm. ESI-MS: m/z 271.0560 [M+H]+; Anal. Calcd for C15H11ClN2O: C, 66.55; H, 4.10; N, 10.35. Found: C, 66.49; H, 4.11; N, 10.30.
4-(4-Methylsulfanylbenzyl)phthalazin-1(2H)-one (2). Yield 50%. Colourless oil. IR (NaCl), νmax: 3188, 2920, 1657, 1492, 1260, 1017, 966, 793, 770 cm−1. 1H-NMR δ: 2.43 (s, 3H, SCH3), 4.26 (s, 2H, H-9), 7.18 (d, J = 8.0 Hz, 2H, H-3' + H-5'), 7.26 (d, J = 8.0 Hz, 2H, H-2' + H-6'), 7.73 (m, 3H, H-5 + H-6 + H-7), 8.46 (m, 1H, H-8), 11.48 (br s, 1H, NH) ppm. 13C-NMR δ: 15.7 (SCH3), 38.3 (C-9), 125.2 (C-7), 126.9 (C-8 + C-3' + C-5'), 128.2 (C-8a), 128.9 (C-2' + C-6'), 129.7 (C-4a), 131.3 (C-5); 133.4 (C-6), 134.4 (C-1'), 136.7 (C-4'), 146.2 (C-4), 160.8 (C-1) ppm. ESI-MS: m/z 283.0827 [M+H]+; Anal. Calcd for C16H14N2OS: C, 68.06; H, 5.00; N, 9.92. Found: C, 68.01; H, 4.96; N, 9.93.
4-(3,4-Methylenedioxybenzyl)phthalazin-1(2H)-one (3). Yield 100%. Colourless oil. IR (NaCl), νmax: 3,216, 2916, 2852, 1661, 1496, 1248, 925, 860, 764 cm−1. 1H-NMR δ: 4.20 (s, 2H, H-9), 5.90 (br s, 2H, OCH2O), 6.73 (br s, 1H, H-2'), 6.74 (br s, 2H, H-5' + H-6'), 7.74 (m, 3H, H-5 + H-6 + H-7), 8.47 (dd, J = 8.7, 2.0 Hz, 1H, H-8), 10.68 (br s, 1H, NH) ppm. 13C-NMR δ: 38.5 (C-9), 101.1 (OCH2O), 108.5 (C-5'), 108.9 (C-2'), 121.5 (C-6'), 125.4 (C-7), 127.0 (C-8), 128.3 (C-8a), 129.8 (C-4a), 131.3 (C-1'), 131.5 (C-5); 133.6 (C-6), 146.5 (C-4 + C-3'), 148.0 (C-4'), 160.2 (C-1). ESI-MS: m/z 281.0848 [M+H]+; Anal. Calcd for C16H12N2O3: C, 68.56; H, 4.32; N, 9.99. Found: C, 68.49; H, 4.26; N, 9.87.
4-(3,4-Dimethoxybenzyl)phthalazin-1(2H)-one (4). Yield 92%. Colourless oil. IR (NaCl), νmax: 3294, 2919, 1651, 1514, 1352, 1259, 1029, 860, 783, 730 cm−1. 1H-NMR δ: 3.82 (s, 6H, 2 × OCH3); 4.26 (s, 2H, H-9), 6.73 (br s, 1H, H-2'), 6.77 (d, J = 8.0 Hz, 1H, H-5'), 7.74 (m, 3H, H-5 + H-6 + H-7), 7.82 (dd, J = 8.0, 1.2 Hz, 1H, H-6'), 8.47 (m, 1H, H-8), 11.50 (bs, 1H, NH) ppm. 13C-NMR δ: 38.5 (C-9), 55.8 (2 × OCH3), 111.2 (C-5'), 115.5 (C-2'), 120.4 (C-6'), 125.4 (C-7), 126.9 (C-8), 128.2 (C-8a), 129.8 (C-4a), 130.1 (C-1'), 131.2 (C-5), 133.3 (C-6), 146.5 (C-4), 147.9 (C-4'), 149.1 (C-3'), 161.0 (C-1) ppm. ESI-MS: m/z 297.1161 [M+H]+; Anal. Calcd for C17H16N2O3: C, 68.91; H, 5.44; N, 9.45. Found: C, 68.88; H, 5.39; N, 9.46.
1-Naphthylmethylphthalazin-1-one (12). Yield 98%. Oil. IR (NaCl), νmax: 3417, 2919, 1653, 1595, 1470, 1023, 870, 787 cm−1. 1H-NMR δ: 4.18 (s, 2H, H-9), 7.12 (d, J = 7.0 Hz, 1H, H-4'), 7.53 (m, 1H, H-5'), 7.55 (m, 1H, H-9'), 7.57 (m, 1H, H-8'), 7.74 (m, 3H, H-5 + H-6 + H-7), 7.75 (m, 1H, H-10'), 7.76 (m, 1H, H-6'), 8.18 (dd, J = 8.2, 1.8 Hz, 1H, H-7'), 8.44 (dd, J = 8.4, 2.0 Hz, 1H, H-8), 11.00 (br s, 1H, NH) ppm. 13C-NMR δ: 35.3 (C-9), 123.1 (C-7'), 125.1 (C-7), 125.5 (C-5'), 126.1 (C-8'), 126.4 (C-9'), 127.2 (C-8 + C-4'), 127.7 (C-6'), 128.5 (C-8a), 128.9 (C-10'),129.8 (C-4a), 131.3 (C-5), 131.9 (C-2'), 133.3 (C-1' + C-3'), 133.4 (C-6), 146.3 (C-4), 160.7 (C-1) ppm. ESI-MS: m/z 287.1106 [M+H]+; Anal. Calcd for C19H14N2O: C, 79.70; H, 4.93; N, 9.78. Found: C, 79.71; H, 4.89; N, 9.72.
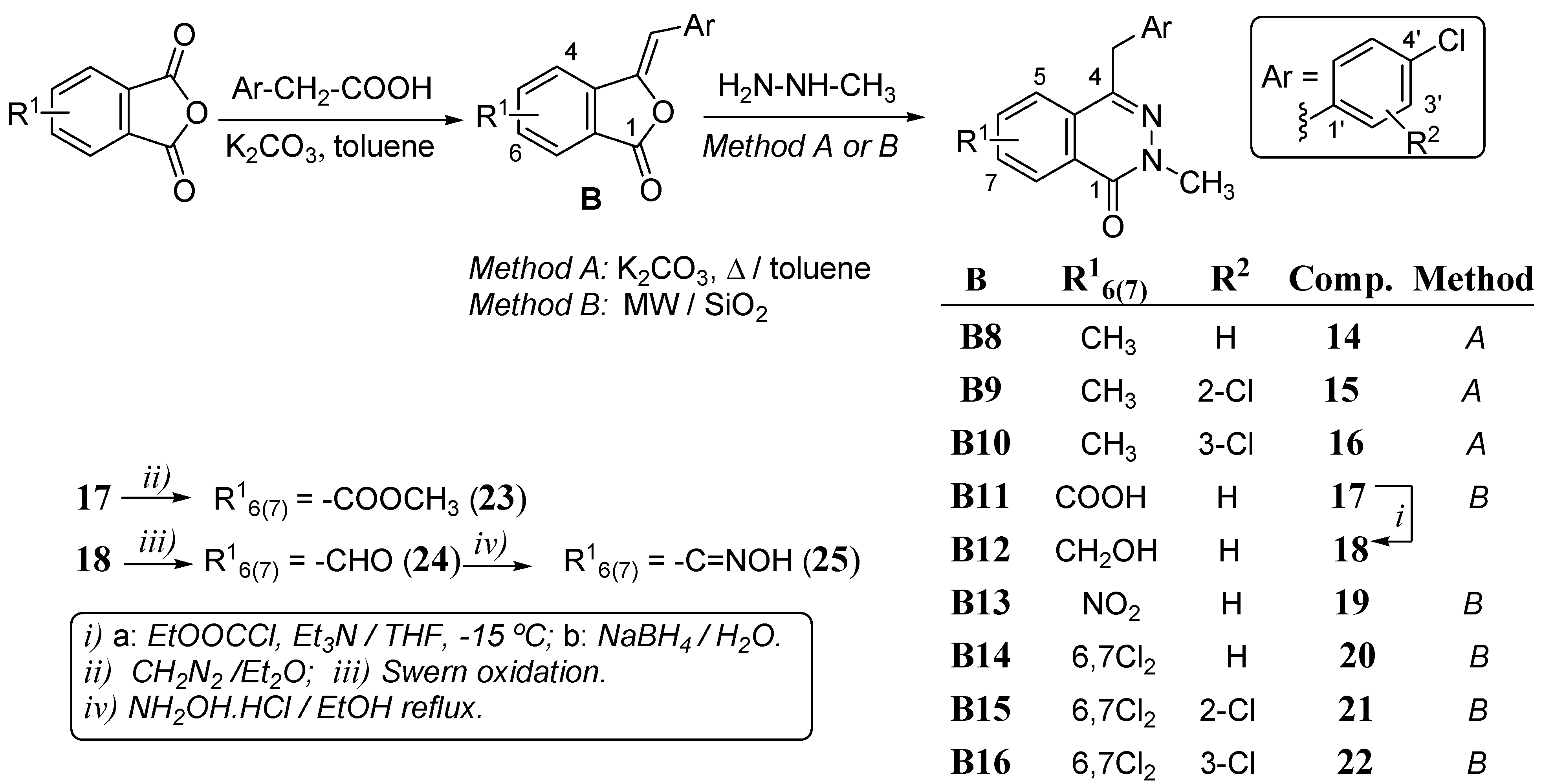
3.1.3. General Procedure for the Synthesis of Phthalazinones 5–9 and 14–16.
Benzalphthalides B (1 mol) were mixed with an excess of methylhydrazine (4 mL), the mixtures maintained at 70–80 °C under stirring for 4–11 h. After cooling reaction mixtures were extracted with ethyl acetate and washed with water, dried over Na2SO4 and concentrated under reduced pressure to give crude products that were purified by flash chromatography on silica gel. In the case of compounds 14–16 the starting benzalphthalides were 1:1 mixtures or regioisomers with the substituent at positions C-5 and C-6 and correspondingly yielded mixtures of 6(7)-substituted phthalazinones in the same proportion.
4-(4-Chlorobenzyl)-2-methylphthalazin-1(2H)-one (5). Yield 75%, oil. IR (NaCl): νmax 3068, 2920, 1650, 1587, 1489, 1262, 1093, 815, 797, 749, 700 cm−1. 1H-NMR δ: 3.87 (s, 3H, CH3), 4.25 (s, 2H, H-9), 7.20 (d, J = 8.8 Hz, 2H, H-3' + H-5'), 7.26 (d, J = 8.8 Hz, 2H, H-2' + H-6'), 7.70 (m, 3H, H-5 + H-6 + H-7), 8.42 (m, 1H, H-8) ppm. 13C-NMR δ: 38.3 (C-9), 39.4 (CH3), 125.0 (C-7), 127.2 (C-8), 128.2 (C-8a), 128.9 (C-3' + C-5'), 129.2 (C-4a), 129.8 (C-2' + C-6'), 131.3 (C-5); 132.8 (C-1' + C-6), 136.4 (C-4'), 144.5 (C-4), 159.6 (C-1) ppm. ESI-MS: m/z 285.0716 [M+H]+; Anal. Calcd for C16H13ClN2O: C, 67.49; H, 4.60; N, 9.84. Found: C, 67.39; H, 4.52; N, 9.81.
2-Methyl-4-(4-methylsulfanylbenzyl)phthalazin-1(2H)-one (6). Yield 80%, Colourless oil. IR (NaCl): νmax 2921, 2852, 1651, 1585, 1492, 1435, 1257, 1080, 810, 795, 775 cm−1. 1H-NMR δ: 2.43 (s, 3H, SCH3), 3.86 (s, 3H, NCH3), 4.23 (s, 2H, H-9), 7.17 (d, J = 8.8 Hz, 2H, H-3' + H-5'), 7.24 (d, J = 8.8 Hz, 2H, H-2' + H-6'), 7.67 (m, 3H, H-5 + H-6 + H-7), 8.43 (dd, J = 6.0, 2.9 Hz 1H, H-8) ppm. 13C-NMR δ: 15.9 (SCH3), 38.4 (C-9), 39.4 (NCH3),125.2 (C-7), 127.0 (C-8 + C-3' + C-5'), 128.2 (C-8a), 128.9 (C-2' + C-6'), 129.3 (C-4a), 131.2 (C-5); 132.8 (C-6), 134.8 (C-1'), 136.8 (C-4'), 143.9 (C-4), 159.6 (C-1) ppm. ESI-MS: m/z 297.0983 [M+H]+; Anal. Calcd for C 17H16N2OS: C, 68.89; H, 5.44; N, 9.45. Found: C, 68.81; H, 5.43; N, 9.39; S, 10.76.
2-Methyl-4-(3,4-methylenedioxybenzyl)phthalazin-1(2H)-one (7). Yield 65%, Colourless oil. IR (NaCl): νmax 2924, 2854, 1651, 1586, 1490, 1245, 1037, 925, 742, 698 cm−1. 1H-NMR δ: 3.89 (s, 3H, CH3), 4.19 (s, 2H, H-9), 5.90 (s, 2H, OCH2O), 6.70 (d, J = 8.0 Hz, 1H, H-5'), 6.71 (br s, 1H, H-2'), 6.74 (d, J = 8.0 Hz, 1H, H-6'), 7.69 (m, 3H, H-5 + H-6 + H-7), 8.41 (m, 1H, H-8) ppm. 13C-NMR δ: 38.6 (C-9), 39.4 (CH3), 100.9 (OCH2O), 108.3 (C-5'), 108.7 (C-2'), 121.3 (C-6'), 125.1 (C-7), 127.0 (C-8), 128.2 (C-8a), 129.2 (C-4a), 131.6 (C-1'), 131.1 (C-5); 132.6 (C-6), 145.1 (C-4), 146.3 (C-3'), 147.9 (C-4'), 159.6 (C-1) ppm. ESI-MS: m/z 295.1004 [M+H]+; Anal. Calcd for C17H14N2O3: C, 69.38; H, 4.79; N, 9.52. Found: C, 69.31; H, 4.77; N, 9.53.
4-(3,4-Dimethoxybenzyl)-2-methylphthalazin-1(2H)-one (8). Yield 93%, Colourless oil. IR (NaCl): νmax 2926, 1652, 1515, 1453, 1260, 1236, 1029, 791, 744 cm−1. 1H-NMR δ: 3.83 (s, 6H, 2 × OCH3), 3.89 (s, 3H, CH3), 4.24 (s, 2H, H-9), 6.77 (d, J = 7.0 Hz, 1H, H-5'), 6.78 (s, 1H, H-2'), 6.79 (d, J = 7.0 Hz, 1H, H-6'), 7.69 (m, 3H, H-5 + H-6 + H-7), 8.44 (m, 1H, H-8) ppm. 13C-NMR δ: 38.6 (C-9), 39.4 (CH3), 55.9 (2 × OCH3), 111.3 (C-5'), 111.6 (C-2'), 120.5 (C-6'), 125.2 (C-7), 127.0 (C-8), 128.2 (C-8a), 128.4 (C-4a), 130.4 (C-1'), 131.2 (C-5), 132.7 (C-6), 145.2 (C-4), 147.9 (C-4'), 149.1 (C-3'), 159.6 (C-1) ppm. ESI-MS: m/z 311, 1317 [M+H]+; Anal. Calcd for C18H18N2O3: C, 69.66; H, 5.85; N, 9.03. Found: C, 69.59; H, 5.81; N, 9.04.
4-(3,4,5-Trimethoxybenzyl)-2-methylphthalazin-1(2H)-one (9). Yield 70%, oil. IR (NaCl): νmax: 2937, 2837, 1651, 1587, 1330, 804, 776, 743 cm−1. 1H-NMR 3.77 (s, 9H, 3 × OCH3), 3.89 (s, 3H, CH3), 4.21 (s, 2H, H-9), 6.44 (s, 2H, H-2' + H-6'), 7.68 (m, 3H, H-5 + H-6 + H-7), 8.42 (m, 1H, H-8) ppm. 13C-NMR δ: 39.2 (C-9), 39.4 (CH3), 56.1 (2 × OCH3), 60.8 (OCH3), 105.4 (C-2' + C-6'), 125.2 (C-7), 127.0 (C-8), 128.1 (C-8a), 129.4 (C-4a), 131.3 (C-5), 132.8 (C-6), 136.8 (C-1'), 145.0 (C-4), 153.4 (C-3' + C-4' + C-5'), 159.6 (C-1) ppm. ESI-MS: m/z 341.1423 [M+H]+; Anal. Calcd for C19H20N2O4: C, 67.05; H, 5.92; N, 8.23. Found: C, 67.01; H, 5.88; N, 8.24.
2-Methyl-4-(naphthalen-2-ylmethyl)phthalazin-1(2H)-one (13). Yield 99%, oil. IR (NaCl): νmax 3052, 2926, 1651, 1584, 1257, 1033, 806, 785, 740, 691 cm−1. 1H-NMR δ: 3.91 (CH3) 4.39 (s, 2H, H-9), 7.38 (m, 1H, H-5), 7.40 (m, 1H, H-7), 7.52 (m, 1H, H-6), 7.60 (m, 3H, H-7' + H-8' + H-9'), 7.65 (m, 1H, H-6'), 7.75 (m, 1H, H-10'), 7.77 (br s, 1H, H-2'), 7.78 (m, 1H, H-5'), 8.46 (m, 1H, H-8) ppm. 13C-NMR δ: 39.5 (C-9), 39.8 (CH3), 125.6 (C-7), 126.1 (C-10'), 126.6 (C-7'), 127.0 (C-9'), 127.3 (C-8), 127.4 (C-6'), 128.0 (C-8'), 128.1 (C-8a), 128.5 (C-5'), 128.8 (C-2'), 129.7 (C-4a + C-5), 132.7 (C-1' + C-6), 133.9 (C-3'), 135.9 (C-4'), 145.3 (C-4), 160.0 (C-1) ppm. ESI-MS: m/z 301.1263 [M+H]+; Anal. Calcd for C20H16N2O: C, 79.98; H, 5.37; N, 9.33. Found: C, 79.91; H, 5.39; N, 9.30.
4-(4-Chlorobenzyl)-2,6(7)-dimethylphthalazin-1(2H)-one (14). Yield 93%, oil. IR (NaCl): νmax 2922, 1651, 1618, 1490, 1091, 1015, 838 cm−1. ESI-MS: m/z 299.0873 [M+H]+; Anal. Calcd for C17H15ClN2O: C, 68.34; H, 5.06; N, 9.38. Found: C, 68.19; H, 4.95; N, 9.40.
4-(4-Chlorobenzyl)-2,6-dimethylphthalazin-1(2H)-one (14a). 1H-NMR δ: 2.42 (s, 3H, CH3), 3.84 (s, 3H, NCH3), 4.21 (s, 2H, H-9), 7.24–7.22 (m, 4H, H-2' + H-6' and H-3' + H-5'), 7.40 (br s, 1H, H-5), 7.52 (d, J = 8.4, 1H, H-7), 8.30 (d, J = 8.4, 1H, H-8) ppm. 13C-NMR δ: 21.8 (CH3), 37.7 (C-9), 39.1 (NCH3), 124.3 (C-5), 126.7 (C-8), 128.5 (C-3'+ C-5'), 128.6 (C-8a), 128.9 (C-4a), 129.5 (C-2' + C-6'), 132.2 (C-4'), 132.4 (C-7), 136.2 (C-1'), 144.1 (C-6), 143.3 (C-4), 159.2 (C-1) ppm.
4-(4-Chlorobenzyl)-2,7-dimethylphthalazin-1(2H)-one (14b). 1H-NMR δ: 2.45 (s, 3H, CH3), 3.85 (s, 3H, NCH3), 4.20 (s, 2H, H-9), 7.24–7.22 (m, 4H, H-2' + H-6' and H-3' + H-5'), 7.45 (d, J = 8.4 Hz, 2H, H-5 + H-6), 8.30 (br s, 1H, H-8) ppm. 13C-NMR δ: 21.4 (CH3), 37.7 (C-9), 39.1 (NCH3), 124.7 (C-5), 126.4 (C-8), 126.6 (C-8a), 127.7 (C-4a), 128.5 (C-3'+ C-5'), 129.5 (C-2' + C-6'), 132.2 (C-4'), 136.2 (C-1'), 138.3 (C-6), 141.8 (C-7), 143.3 (C-4), 159.2 (C-1).
4-(2,4-Dichlorobenzyl)-2,6(7)-dimethylphthalazin-1(2H)-one (15). Yield 94%, oil. IR (NaCl): νmax 2921, 1653, 1618, 1472, 1347, 1048, 860, 837 cm−1. ESI-MS: m/z 333, 0483 [M+H]+; Anal. Calcd for C17H14Cl2N2O: C, 61.28; H, 4.23; N, 8.41. Found: C, 61.30; H, 4.11; N, 8.30.
4-(2,4-Dichlorobenzyl)-2,6-dimethylphthalazin-1(2H)-one (15a). 1H-NMR δ: 2.46 (s, 3H, CH3), 3.81 (s, 3H, NCH3), 4.29 (s, 2H, H-9), 7.00 (d, J = 8.4 Hz, H-6'), 7.05 (dd, J = 8.4, 1.8 Hz, H-5'), 7.38 (d, J = 1.8 Hz, H-3'), 7.48 (s, 1H, H-5), 7.51 (d, J = 8.0 Hz, 1H, H-7), 8.32 (d, J = 8.0 Hz, 1H, H-8) ppm. 13C-NMR δ: 21.9 (CH3), 34.9 (C-9), 39.1 (NCH3), 124.0 (C-5), 126.7 (C-8), 127.0 (C-5'), 127.8 (C-8a), 129.1 (C-4a + C-6'), 130.7 (C-3'), 132.7 (C-7), 132.9 (C-2'), 134.1 (C-1' + C-4'), 143.1 (C-4), 143.6 (C-6), 159.3 (C-1) ppm.
4-(2,4-Dichlorobenzyl)-2,7-dimethylphthalazin-1(2H)-one (15b). 1H-NMR δ: 2.49 (s, 3H, CH3), 3.82 (s, 3H, NCH3), 4.29 (s, 2H, H-9), 6.97 (d, J = 8.4 Hz, H-6'), 7.10 (dd, J = 8.4, 1.8 Hz, H-5'), 7.38 (d, J = 1.8 Hz, H-3'), 7.38 (d, 1H, J = 7.7 Hz, H-6), 7.50 (d, 1H, J = 7.7 Hz, H-5), 8.32 (br s, 1H, H-8) ppm. 13C-NMR δ: 21.6 (CH3), 34.9 (C-9), 39.1 (NCH3), 124.4 (C-5), 126.6 (C-8), 127.0 (C-5'), 125.6 (C-8a), 129.1 (C-4a + C-6'), 130.7 (C-3'), 142.1 (C-7), 132.9 (C-2'), 134.1 (C-1' + C-4'), 143.4 (C-4), 134.1 (C-6), 159.3 (C-1) ppm.
4-(3,4-Dichlorobenzyl)-2,6(7)-dimethylphthalazin-1(2H)-one (16). Yield 86%, oil. IR (NaCl): νmax 2921, 1651, 1618, 1470, 1347, 1031, 823 cm−1. ESI-MS: m/z 333.0483 [M+H]+; Anal. Calcd for C17H14Cl2N2O2: C, 61.28; H, 4.23; N, 8.41. Found: C, 61.17; H, 4.12; N, 8.49.
4-(3,4-Dichlorobenzyl)-2,6-dimethylphthalazin-1(2H)-one (16a). 1H-NMR δ: 2.45 (s, 3H, CH3), 3.84 (s, 3H, NCH3), 4.20 (s, 2H, H-9), 7.10 (dd, J = 8.6, 2.0 Hz, H-6'), 7.33 (d, J = 8.6 Hz, H-5'), 7.35 (d, J = 2.0 Hz, H-2'), 7.39 (s, 1H, H-5), 7.51 (d, J = 8.0 Hz, 1H, H-7), 8.32 (d, J = 9.0 Hz, 1H, H-8) ppm. 13C-NMR δ: 22.0 (CH3), 37.6 (C-9), 39.2 (NCH3), 124.3 (C-5), 125.8 (C-8a), 127.1 (C-8), 128.0 (C-4a + 5'), 130.2 (C-2'), 130.4 (C-6'), 130.7 (C-3'), 132.5 (C-4'), 132.8 (C-7), 138.1 (C-1'), 143.5 (C-4), 143.6 (C-6), 159.4 (C-1) ppm.
4-(3,4-Dichlorobenzyl)-2,7-dimethylphthalazin-1(2H)-one (16b). 1H-NMR δ: 2.48 (s, 3H, CH3), 3.86 (s, 3H, NCH3), 4.20 (s, 2H, H-9), 7.11 (dd, J = 8.0, 2.0 Hz, H-6'), 7.32 (d, J = 8.0, 1.8 Hz, H-5'), 7.35 (d, J = 2.0 Hz, H-2'), 7.48 (dd, 1H, J = 7.7,1.5 Hz, H-6), 7.50 (d, 1H, J = 7.7 Hz, H-5), 8.23 (br s, 1H, H-8) ppm. 13C-NMR δ: 21.6 (CH3), 37.8 (C-9), 39.2 (NCH3), 124.6 (C-5), 125.8 (C-8a), 126.8 (C-8), 128.0 (C-5'), 129.1 (C-4a), 130.4 (C-6'), 130.7 (C-3'), 130.2 (C-2'), 132.5 (C-4'), 134.1 (C-6), 138.1 (C-1'), 142.2 (C-7), 143.8 (C-4), 159.4 (C-1) ppm.
3.1.4. Procedure for the Synthesis of Compounds 10 and 11
A mixture of phthalazinone 1 (0.20 mmol), ethyl bromide or allyl bromide (0.22 mmol), potassium carbonate (33 mg) and acetonitrile (5 mL) were maintained under reflux for 25 h. Solvent was removed under vacuum and the crude mixture dissolved in ethyl acetate, washed with water, dried over Na2SO4 and concentrated under reduced pressure to give crude products that were purified by flash chromatography on silica gel.
4-(4-Chlorobenzyl)-2-ethylphthalazin-1(2H)-one (10). Yield 89%, Colourless oil. IR (NaCl): νmax 2930, 1650, 1585, 1350, 1262, 1090, 830, 798, 691 cm−1. 1H-NMR δ: 1.43 (t, J = 7.3 Hz, 3H, CH3), 4.26 (s, 2H, H-9), 4.33 (q, J = 7.3 Hz, 2H, CH2), 7.18 (d. J = 8.5 Hz, H-3' + H-5'), 7.26 (d, J = 8.5 Hz, 2H, H-2' + H-6'), 7.66 (m, 3H, H-5 + H-6 + H-7), 8.45 (m, 1H, H-8) ppm. 13C-NMR δ: 13.6 (CH3), 38.3 (C-9), 46.2 (CH2), 124.9 (C-7), 127.3 (C-8), 128.4 (C-8a), 128.8 (C-3' + C-5'), 129.0 (C-4a), 129.7 (C-2' + C-6'), 131.2 (C-5); 132.7 (C-1'), 132.8 (C-6), 136.5 (C-4'), 144.6 (C-4), 159.0 (C-1) ppm. ESI-MS: m/z 299.0873 [M+H]+; Anal. Calcd for C17H15ClN2O: C, 68.34; H, 5.06; N, 9.38. Found: C, 68.27; H, 5.04; N, 9.30.
2-Allyl-4-(4-Chlorobenzyl)phthalazin-1(2H)-one (11). Yield 73%, oil. IR (NaCl): νmax 3073, 2930, 1655, 1586, 1490, 1092, 810, 796 cm−1. 1H-NMR δ: 4.25 (s, 2H, H-9), 4.85 (m, 2H, CH2), 5.20/5.27 (m, 2H, =CH2), 6.06 (m, 1H, CH=), 7.18 (d. J = 8.2 Hz, H-3' + H-5'), 7.22 (d, J = 8.2 Hz, 2H, H-2' + H-6'), 7.67 (m, 3H, H-5 + H-6 + H-7), 8.42 (m, 1H, H-8) ppm. 13C-NMR δ: 38.2 (C-9), 53.4 (CH2), 117.8 (=CH2), 124.8 (C-7), 127.3 (C-8), 128.3 (C-8a), 128.7 (C-3' + C-5'), 129.1 (C-4a), 129.6 (C-2' + C-6'), 130.1 (C-1'), 131.2 (C-5); 132.5 (CH=), 132.8 (C-6), 136.2 (C-4'), 144.8 (C-4), 158.9 (C-1) ppm. ESI-MS: m/z 311.0873 [M+H]+; Anal. Calcd for C18H15ClN2O: C, 69.57; H, 4.86; N, 9.01. Found: C, 69.48; H, 4.80; N, 9.02.
3.1.5. General Procedure for the Synthesis of Phthalazinones 17–22
A solution of the corresponding benzalphthalide B (1 mol), methylhydrazine (3 mL) in dichloromethane (6 mL) was absorbed in silica gel (10:1 respecting the benzalphthalide). The solvent was removed under vacuum and the mixture MW irradiated (350 W) for 1–6 minutes. Then, 3 drops of water were added and stirred for 20 min at room temperature. Ethyl acetate was added to the mixture and the silica gel filtered out. The solvent was removed under vacuum and the crude mixture purified by flash chromatography on silica gel. Phthalazinones 17–19 were obtained as 1:1 mixtures of regioisomers at the 6/7 positions.
4-(4-Chlorobenzyl)-6(7)-hydroxycarbonyl-2-methylphthalazin-1(2H)-one (17). Yield 90%, oil. IR (NaCl): νmax 3430–2715, 1720, 1645, 1614, 1352, 1088, 803, 720 cm−1. ESI-MS: m/z 330.0611 [M+H]+; Anal. Calcd for C17H13ClN2O3: C, 62.11; H, 3.99; N, 8.52. Found: C, 62.15; H, 3.90; N, 8.50.
4-(4-Chlorobenzyl)-6-hydroxycarbonyl-2-methylphthalazin-1(2H)-one (17a). 1H-NMR (CD3OD + CDCl3) δ: 3.88 (s, 3H, NCH3), 4.29 (s, 2H, H-9), 7.18–7.30 (m, 4H, H-2' + H-6' and H-3'+ H-5'), 8.45 (br s, 1H, H-5), 8.33 (d, J = 8.4 Hz, 1H, H-7), 7.73 (d, J = 8.4 Hz, 1H, H-8) ppm. 13C-NMR (CD3OD + CDCl3) δ: 38.0 (C-9), 39.4 (NCH3), 125.3 (C-8), 127.7 (C-8a), 128.7 (C-3' + H-5'), 128.9 (C-5), 129.7 (C-2' + H-6'), 130.2 (C-4a), 131.6 (C-4'), 132.6 (C-6), 133.4 (C-7), 135.7 (C-1'), 144.8 (C-4), 159.5 (C-1), 166.7 (COOH) ppm.
4-(4-Chlorobenzyl)-7-hydroxycarbonyl-2-methylphthalazin-1(2H)-one (17b). 1H-NMR (CD3OD + CDCl3) δ: 3.88 (s, 3H, NCH3), 4.31 (s, 2H, H-9), 7.18–7.30 (m, H-2' + H-6' and H-3' + H-5'), 8.33 (d, J = 8.0 Hz, 1H, H-5), 8.49 (d, J = 8.0 Hz, 1H, H-6), 9.09 (s, 1H, H-8) ppm. 13C-NMR (CD3OD + CDCl3) δ: 37.8 (C-9), 39.4 (NCH3), 127.0 (C-8), 127.2 (C-5), 127.7 (C-8a), 128.7 (C-3' + H-5'), 129.7 (C-2' + H-6'), 130.3 (C-4a), 131.6 (C-6), 131.7 (C-4'), 135.7 (C-1'), 145.5 (C-4), 134.8 (C-7), 159.2 (C-1), 166.5 (COOH) ppm.
4-(4-Chlorobenzyl)-6(7)-hydroxymethyl-2-methylphthalazin-1(2H)-one (18). Yield 91%, Colourless oil. IR (NaCl): νmax 3306, 1632, 1617, 1582, 1356, 1060, 844, 821 cm−1. ESI-MS: m/z 313.0611 [M+H]+; Anal. Calcd for C17H15ClN2O3: C, 64.87; H, 4.80; N, 8.90. Found: C, 64.76; H, 4.70; N, 8.87.
4-(4-Chlorobenzyl)-6-hydroxymethyl-2-methylphthalazin-1(2H)-one (18a). 1H-NMR δ: 3.84 (s, 3H, NCH3), 4.22 (s, 2H, H-9), 4.80 (s, 2H, CH2OH), 7.16–7.22 (m, 4H, H-2'+ H-6'and H-3' + H-5'), 7.60 (d, J = 8.0 Hz, 1H, H-7), 7.66 (s, 1H, H-5), 8.31 (d, J = 8.0 Hz, 1H, H-8) ppm. 13C-NMR δ: 38.1 (C-9), 39.5 (NCH3), 64.3 (CH2), 122.1 (C-5), 127.2 (C-8a), 127.3 (C-8), 128.9 (C-7 + C-3' + H-5'), 129.2 (C-4a), 129.8 (C-2' + H-6'), 132.6 (C-4'), 136.3 (C-1'), 144.8 (C-4), 146.6 (C-6), 159.6 (C-1) ppm.
4-(4-Chlorobenzyl)-7-hydroxymethyl-2-methylphthalazin-1(2H)-one (18b). 1H-NMR δ: 3.84 (s, 3H, NCH3), 4.23 (s, 2H, H-9), 4.82 (s, 2H, CH2OH), 7.16–7.22 (m, 4H, H-2' + H-6' and H-3' + H-5'), 7.64 (d, J = 8.4 Hz, 1H, H-5), 7.72 (dd, J = 8.4, 1.5 Hz, 1H, H-6), 8.36 (br s, 1H, H-8) ppm. 13C-NMR δ: 38.3 (C-9), 39.5 (NCH3), 64.3 (CH2), 124.5 (C-5), 125.3 (C-8), 127.2 (C-8a), 128.3 (C-4a), 128.9 (C-3' + H-5'), 129.8 (C-2' + H-6'), 131.5 (C-6), 132.6 (C-4'), 136.3 (C-1), 144.6 (C-4), 145.2 (C-7), 159.6 (C-1) ppm.
4-(4-Chlorobenzyl)-6(7)-nitro-2-methylphthalazin-1(2H)-one (19). Yield 53%, yellowish oil. IR (NaCl): νmax 2918, 1662, 1618, 1531, 1344, 1090, 794 cm−1. ESI-MS: m/z 329.0567 [M+H]+; Anal. Calcd for C16H12ClN3O3: C, 58.28; H, 3.67; N, 12.74. Found: C, 58.18; H, 3.72; N, 12.50.
4-(4-Chlorobenzyl)-6-nitro-2-methylphthalazin-1(2H)-one (19a). 1H-NMR (400 MHz) δ: 3.82 (s, 3H, CH3), 4.23 (s, 2H, H-9), 7.08–7.18 (m, 4H, H-3' + H-5' + H-2' + H-6'), 8.47 (s, 1H, H-5), 7.72 (d, J = 8.7 Hz, 1H, H-7), 8.53 (d, J = 8.7Hz, 1H, H-8) ppm. 13C-NMR (100 MHz) δ: 38.5 (C-9), 39.8 (CH3), 123.3 (C-8), 125.1 (C-5); 129.0 (C-8a), 129.3 (C-3' + C-5'), 129.8 (C-7 + C-2' + C-6'), 131.9 (C-4'), 132.8 (C-4a), 135.3 (C-1'), 143.4 (C-4), 149.0 (C-6), 158.4 (C-1) ppm.
4-(4-Chlorobenzyl)-7-nitro-2-methylphthalazin-1(2H)-one (19b). 1H-NMR (400 MHz) δ: 3.82 (s, 3H, CH3), 4.23 (s, 2H, H-9), 7.08–7.18 (m, 4H, H-3' + H-5' + H-2'+ H-6'), 8.37 (d, J = 8.4 Hz, 1H, H-6), 8.49 (d, J = 8.4 Hz, 1H, H-5), 9.18 (s, 1H, H-8) ppm. 13C-NMR (100 MHz) δ: 38.3 (C-9), 39.8 (CH3), 120.7 (C-8), 126.9 (C-6), 129.0 (C-8a), 129.3 (C-3' + C-5'), 129.8 (C-5 + C-2' + C-6'), 131.9 (C-4'), 133.8 (C-4a), 135.5 (C-1'), 143.1 (C-4), 150.2 (C-7), 158.1 (C-1) ppm.
6,7-Dichloro-4-(4-chlorobenzyl)-phthalazin-1(2H)-one (20). Yield 64%, Colourless oil. IR (NaCl): νmax 2943, 1652, 1490, 1090, 1015, 804, 732 cm−1. 1H-NMR δ: 3.85 (s, 3H, CH3), 4.20 (s, 2H, H-9), 7.18 (d, J = 8.4, 2H, H-2' + H-6'), 7.28 (d, J = 8.4 Hz, 2H, H-3' + H-5'), 7.71 (s, 1H, H-5), 8.49 (s, 1H, H-8) ppm. 13C-NMR δ: 38.1 (C-9), 39.6 (CH3), 126.7 (C-8), 127.5 (C-8a), 128.4 (C-1'), 129.1 (C-5 + C-3' + C-5'), 129.7 (C-2' + C-6'), 133.0 (C-4'), 135.5 (C-7), 136.5 (C-4a), 138.0 (C-6), 143.0 (C-4), 157.9 (C-1) ppm. ESI-MS: m/z 352.9937 [M+H]+; Anal. Calcd for C16H11Cl3N2O: C, 54.34; H, 3.14; N, 7.92. Found: C, 54.40; H, 3.07; N, 7.79.
6,7-Dichloro-4-(2,4-dichlorobenzyl)-phthalazin-1(2H)-one (21). Yield 86%, oil. IR (NaCl): νmax 2923, 1660, 1581, 1471, 1128, 1101, 850 cm−1. 1H-NMR (400 MHz) 3.80 (s, 3H, CH3), 4.20 (s, 2H, H-9), 7.03 (d, J = 8.0 Hz, 1H, H-6'), 7.15 (dd, J = 8.0, 1.7 Hz, 1H, H-5'), 7.47 (d, J = 1.7 Hz, 1H, H-3'), 7.76 (s, 1H, H-5), 8.53 (s, 1H, H-8) ppm. 13C-NMR δ: 35.0 (C-9), 39.5 (CH3), 126.3 (C-8), 127.3 (C-8a), 127.4 (C-5'), 128.4 (C-1'), 129.1 (C-5), 129.5 (C-3'), 131.0 (C-6'), 133.3 (C-2'), 133.6 (C-4'), 134.3 (C-7), 136.6 (C-4a), 138.2 (C-6), 142.0 (C-4), 158.0 (C-1) ppm. ESI-MS: m/z 386.9547 [M+H]+; Anal. Calcd for C16H10Cl4N2O: C, 49.52; H, 2.60; N, 7.22. Found: C, 49.43; H, 2.71; N, 7.14.
6,7-Dichloro-4-(3,4-dichlorobenzyl)-phthalazin-1(2H)-one (22). Yield 89%, oil. IR (NaCl): νmax 2921, 1651, 1618, 1470, 1347, 1031, 823 cm−1. 1H-NMR δ: 3.81 (s, 3H, CH3), 4.25 (s, 2H, H-9), 7.09 (d, J = 8.6 Hz, 1H, H-6'), 7.10 (d, J = 8.6 Hz, 1H, H-5'), 7.40 (br s, 1H, H-2'), 7.80 (s, 1H, H-5), 8.49 (s, 1H, H-8) ppm. 13C-NMR δ: 37.8 (C-9), 39.6 (CH3), 126.1 (C-8), 127.3 (C-8a), 128.1 (C-5'), 129.0 (C-5), 130.8 (C-6'), 131.9 (C-2'), 132.3 (C-3'), 133.5 (C-4'), 133.9 (C-7), 136.3 (C-4a), 137.5 (C-1'), 138.3 (C-6), 143.1 (C-4), 158.2 (C-1) ppm. ESI-MS: m/z 386.9547 [M+H]+; Anal. Calcd for C16H10Cl4N2O: C, 49.52; H, 2.60; N, 7.22. Found: C, 49.61; H, 2.53; Cl, 36.57; N, 7.17.
3.1.6. Synthesis of Phthalazinone Carboxymethyl ester 23
The phthalazinone 17 (20 mg, 0,06 mmoles) was treated with a saturated solution diazomethane in ether (2 mL), and maintaind in darkness at room temperature overnight. The solvent was removed to give 22 mg (99%) of the ester 23, as a regioisomeric mixture.
4-(4-Chlorobenzyl)-6(7)-methoxycarbonyl-2-methylphthalazin-1(2H)-one (23). Oil. IR (NaCl): νmax 2928, 1704, 1652, 1614, 1490, 1347, 1090, 1015, 845 cm−1. ESI-MS: m/z 343.0771 [M+H]+; Anal. Calcd for C18H15ClN2O3: C, 63.07; H, 4.41; N, 8.17. Found: C, 62.97; H, 4.51; N, 8.22.
4-(4-Chlorobenzyl)-6-methoxycarbonyl-2-methylphthalazin-1(2H)-one (23a). 1H-NMR δ: 3.88 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 4.28 (s, 2H, H-9), 7.19 (d, J = 8.8 Hz, 2H, H-3' + H-5'), 7.27 (d, J = 8.8 Hz, 2H, H-2' + H-6'), 7.70 (d, J = 8.8 Hz, 1H, H-7), 8.29 (d, J = 8.8 Hz, 1H, H-8), 8.40 (s, 1H, H-5) ppm. 13C-NMR δ: 38.2 (C-9), 39.6 (CH3), 52.7 (OCH3), 125.4 (C-5); 128.2 (C-8a), 129.0 (C-8 + C-3' + C-5'), 129.8 (C-2' + C-6'), 131.3 (C-7), 132.0 (C-4'), 133.0 (C-4a), 133.1 (C-6), 136.0 (C-1'), 144.1 (C-4), 159.1 (C-1), 165.6 (COO) ppm.
4-(4-Chlorobenzyl)-7-methoxycarbonyl-2-methylphthalazin-1(2H)-one (23b). 1H-NMR δ: 3.88 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 4.30 (s, 2H, H-9), 7.19 (d, J = 8.8 Hz, 2H, H-3' + H-5'), 7.27 (d, J = 8.8 Hz, 2H, H-2' + H-6'), 8.29 (d, J = 8.4 Hz, 1H, H-6), 8.51 (d, J = 8.4 Hz, 1H, H-5), 9.03 (s, 1H, H-8). 13C-NMR δ: 38.4 (C-9), 39.6 (CH3), 52.7 (OCH3), 126.9 (C-5); 127.8 (C-8), 128.2 (C-8a), 129.0 (C-3' + C-5'), 129.8 (C-2' + C-6'), 132.0 (C-4'), 132.6 (C-4a), 132.9 (C-6), 134.0 (C-7), 136.0 (C-1'), 144.8 (C-4), 159.1 (C-1), 165.6 (COO).
3.1.7. Synthesis of the Phthalazinone Aldehyde 24
To a three-neck round-bottom flask filled with dichloromethane (15 mL) and a stirring bar, two compensated pressure addition funnels were adapted. Air was removed, the system filled with Ar and taken to −55 °C, then a solution of 2M oxallyl chloride in dichloromethane (1.10 mL, 2.20 mmol) was added. Five min later a mixture of dimethylsulfoxide (0.4 mL, 4.44 mmol) in dichloromethane (2.3 mL) was added dropwise. After 5 min a solution of phthalazinone 18 (230 mg, 0.73 mmol) in dichloromethane (6.5 mL) was added slowly. The mixture was maintained with stirring for 30 min at −55 °C. Then, triethylamine (1.0 mL, 7.20 mmol) was added and the mixture taken to 0 °C for 60 min. Then, water (5 mL) was added to the mixture, which was transferred to a separatory funnel, where it was washed with aqueous solutions of 2N HCl, NaHCO3 (saturated) and NaCl to pH = 7. The organic layer was dried over Na2SO4, concentrated under reduced pressure to give a crude mixture, that was purified by flash chromatography on silica gel in CH2Cl2/AcOEt (9:1) to provide 138 mg (61%) of aldehyde 24.
4-(4-Chlorobenzyl)-6(7)-formyl-2-methylphthalazin-1(2H)-one (24). Oil. IR (NaCl): νmax 2928, 1704, 1652, 1614, 1490, 1347, 1090, 1015, 845 cm−1. ESI-MS: m/z 313.0666 [M+H]+; Anal. Calcd. for C17H13ClN2O2: C, 65.29; H, 4.19; N, 8.96. Found: C, 65.31; H, 4.12; N, 8.83.
4-(4-Chlorobenzyl)-6-formyl-2-methylphthalazin-1(2H)-one (24a). 1H-NMR δ: 3.89 (s, 3H, CH3), 4.32 (s, 2H, H-9), 7.19 (d, J = 8.8 Hz, 2H, H-3' + H-5'), 7.29 (d, J = 8.8 Hz, 2H, H-2' + H-6'), 8.17 (s, 1H, H-5), 8.18 (d, J = 8.8 Hz, 1H, H-7), 8.61 (d, J = 8.8 Hz, 1H, H-8), 10.10 (s, 1H, CHO) ppm. 13C-NMR δ: 38.3 (C-9), 39.7 (CH3), 127.2 (C-5); 128.8 (C-8a), 128.6 (C-8), 129.1 (C-3' + C-5'), 129.8 (C-2 '+ C-6'), 130.7 (C-7), 131.9 (C-4a), 133.0 (C-4'), 135.8 (C-1'), 138.9 (C-6), 144.8 (C-4), 158.8 (C-1), 190.8 (CHO) ppm.
4-(4-Chlorobenzyl)-7-formyl-2-methylphthalazin-1(2H)-one (24b). 1H-NMR δ: 3.90 (s, 3H, CH3), 4.29 (s, 2H, H-9), 7.19 (d, J = 8.8 Hz, 2H, H-3'+ H-5'), 7.29 (d, J = 8.8 Hz, 2H, H-2'+ H-6'), 7.77 (d, J = 8.4 Hz, 1H, H-6), 8.17 (d, J = 8.4 Hz, 1H, H-5), 8.90 (br s, 1H, H-8), 10.17 (s, 1H, CHO) ppm. 13C-NMR δ: 38.3 (C-9), 39.7 (CH3), 126.1 (C-5); 128.8 (C-8a), 129.1 (C-3'+C-5'), 129.6 (C-8), 129.8 (C-2' + C-6'), 131.3 (C-6), 133.0 (C-4'), 135.8 (C-1'), 137.8 (C-4a), 138.9 (C-7), 144.1 (C-4), 159.0 (C-1), 190.7 (CHO) ppm.
3.1.8. Synthesis of the 6(7)hydroxylimino-phthalazinone 25
To a solution of 24 (100 mg, 0.32 mmol) in ethanol (5 mL), dry pyridine (83 μL, 1.03 mmol) and hydroxylamine clorhydrate (25 mg, 0.35 mmol) were added. The mixture was refluxed under stirring for 2 hours. Solvents were removed under vacuum and the mixture dissolved in ethyl acetate. The organic layer was washed with solutions of 2N HCl and NaCl to pH = 7, dried over Na2SO4, and taken do dryness to give 95 mg (92%) of the regioisomers 25.
4-(4-Chlorobenzyl)-6(7)-hydroxylimino-2-methylphthalazin-1(2H)-one (25). Oil. IR (NaCl): νmax 3441, 2927, 1632, 1579, 1111, 995, 796, 674 cm−1. ESI-MS: m/z 328.0775 [M+H]+; Anal. Calcd for C17H14ClN3O2: C, 62.30; H, 4.31; N, 12.82. Found: C, 62.35; H, 4.38; N, 12.83.
4-(4-Chlorobenzyl)-6-hydroxylimino-2-methylphthalazin-1(2H)-one (25a). 1H-NMR (400 MHz, DMSO-d6) δ: 3.72 (s, 3H, CH3), 4.29 (s, 2H, H-9), 7.33-7.34 (m, 4H, H-2' + H-6' and H-3' + H-5'), 7.90 (d, J = 8.5 Hz, 1H, H-8), 8.04 (d, J = 8.5 Hz, 1H, H-7), 8.33 (s, 1H, HC=N), 8.41 (s, 1H, H-5) ppm. 13C-NMR (100 MHz) δ: 36.8 (C-9), 39.1 (CH3), 124.3 (C-5); 126.2 (C-8), 127.8 (C-4a), 128.5 (C-3' + C-5'), 128.7 (C-8a), 130.0 (C-7), 130.3 (C-2' + C-6'), 131.2 (C-4'), 136.2 (C-6), 144.3 (C-4), 137.0 (C-1'), 147.1 (HC=N) 158.2 (C-1), ppm.
4-(4-Chlorobenzyl)-7-hydroxylimino-2-methylphthalazin-1(2H)-one (25b). 1H-NMR (400 MHz, MDSO-d6) δ: 3.71 (s, 3H, CH3), 4.29 (s, 2H, H-9), 7.33-7.34 (m, 4H, H-2' + H-6' and H-3' + H-5'), 8.04 (d, J = 8.4 Hz, 1H, H-6), 8.33 (s, 1H, HC=N), 8.50 (d, J = 8.4 Hz, 1H, H-5), 8.60 (d, J = 8.5 Hz, 1H, H-8 ppm. 13C-NMR (100 MHz) δ: 38.8 (C-9), 39.1 (CH3), 123.9 (C-5); 127.6 (C-8), 127.9 (C-8a), 128.0 (C-4a), 128.5 (C-3' + C-5'), 130.3 (C-6 + C-2'+C-6'), 131.2 (C-4'), 137.0 (C-7 + C-1'), 144.3 (C-4), 147.1 (HC=N) 158.2 (C-1) ppm.