Design, Synthesis and Biological Evaluation of N-Sulfonyl Homoserine Lactone Derivatives as Inhibitors of Quorum Sensing in Chromobacterium violaceum
Abstract
:1. Introduction
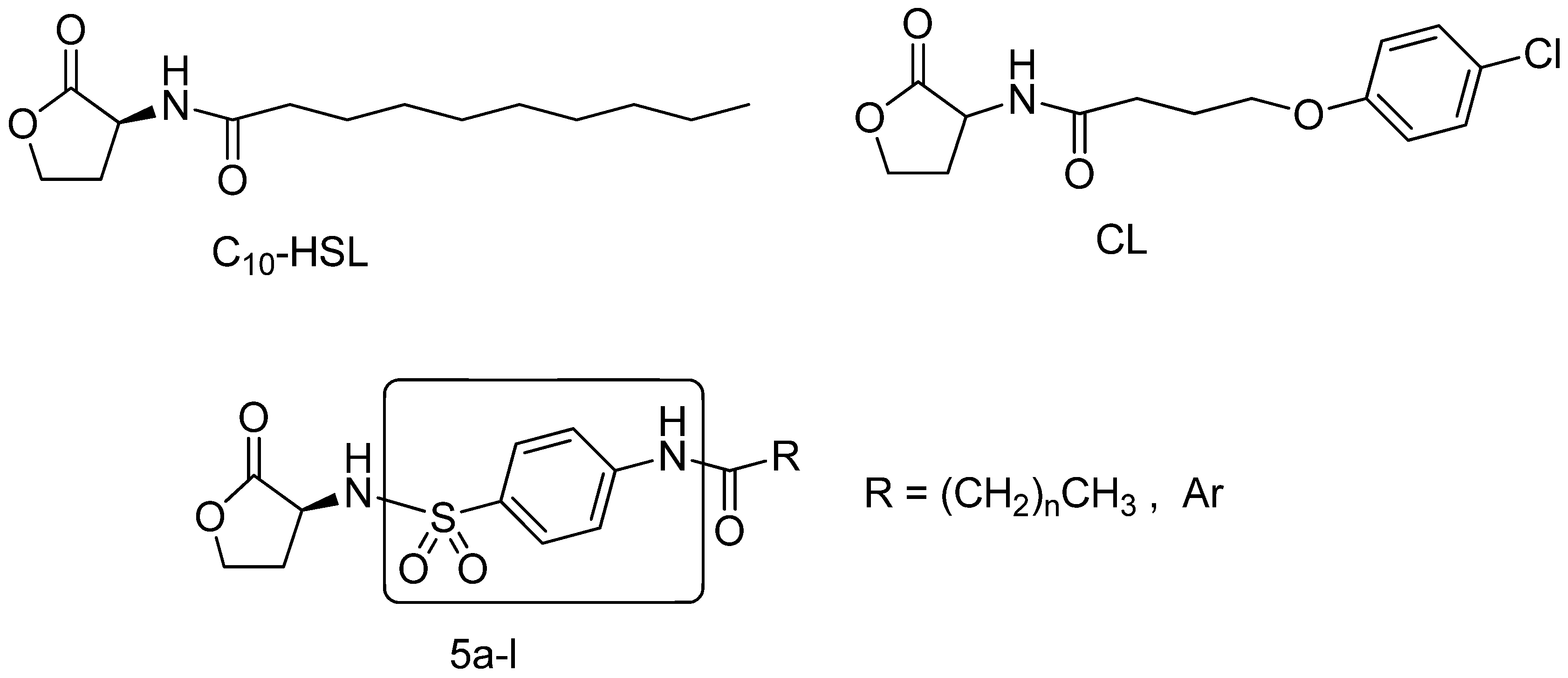
2. Results and Discussion
2.1. Chemistry
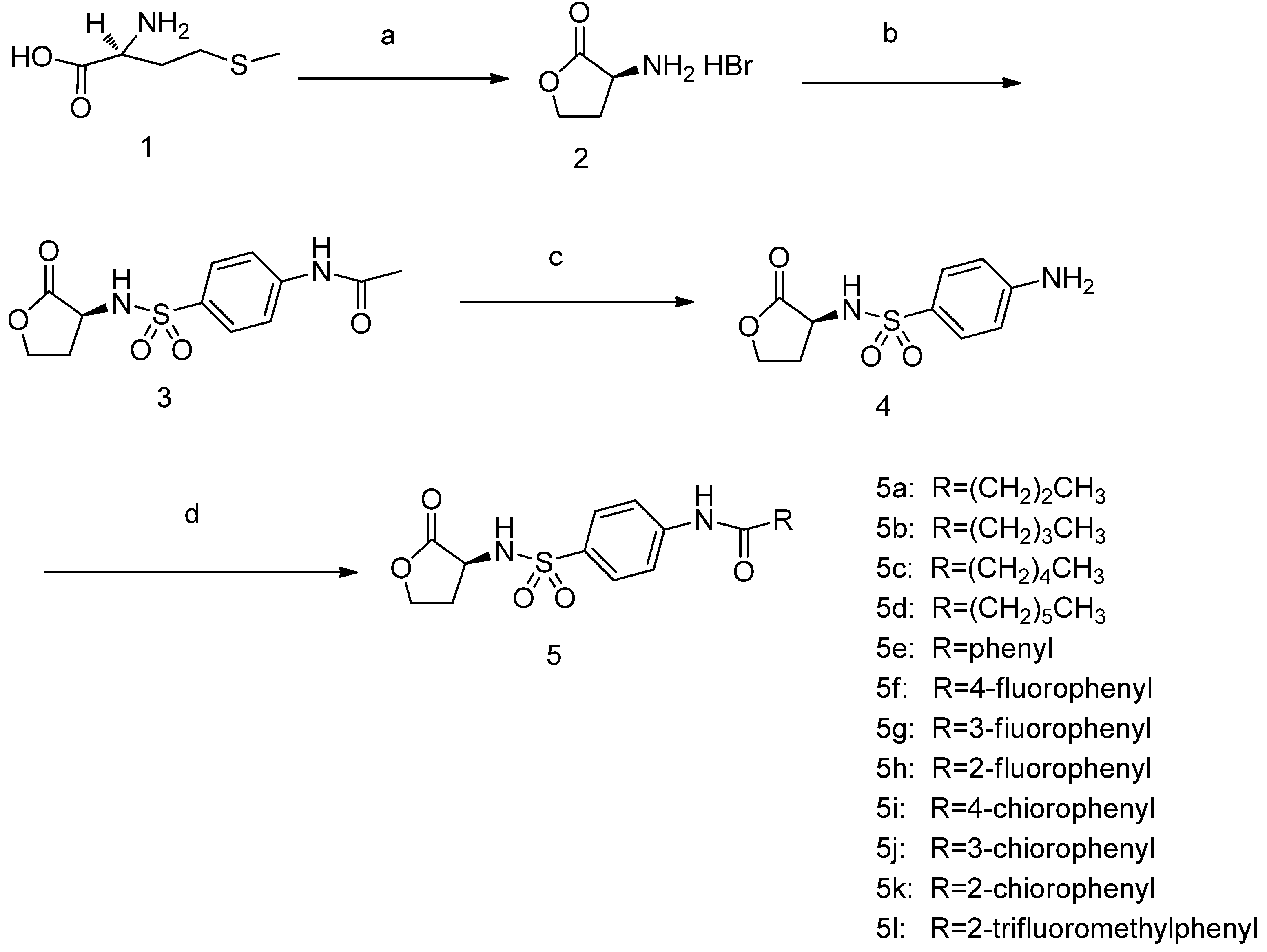
2.2. Biological Results and Discussion
2.2.1. Antibacterial Activity

2.2.2. Quorum Sensing Inhibition
| Compound | R | IC50(μM) |
|---|---|---|
| 5a | (CH2)2CH3 | NA a |
| 5b | (CH2)3CH3 | 48.49 ± 2.68 |
| 5c | (CH2)4CH3 | 29.00 ± 2.41 |
| 5d | (CH2)5CH3 | NA a |
| 5e |  | 17.25 ± 2.60 |
| 5f | 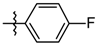 | 14.72 ± 2.58 |
| 5g |  | 7.79 ± 2.68 |
| 5h | 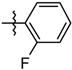 | 1.64 ± 0.27 |
| 5i | 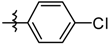 | 15.86 ± 2.35 |
| 5j | 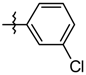 | 8.56 ± 1.25 |
| 5k | 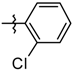 | 1.66 ± 0.33 |
| 5l | 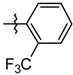 | 4.91 ± 0.97 |
| C10-HSL | 0.32 ± 0.07 |
2.2.3. Molecular Docking Studies
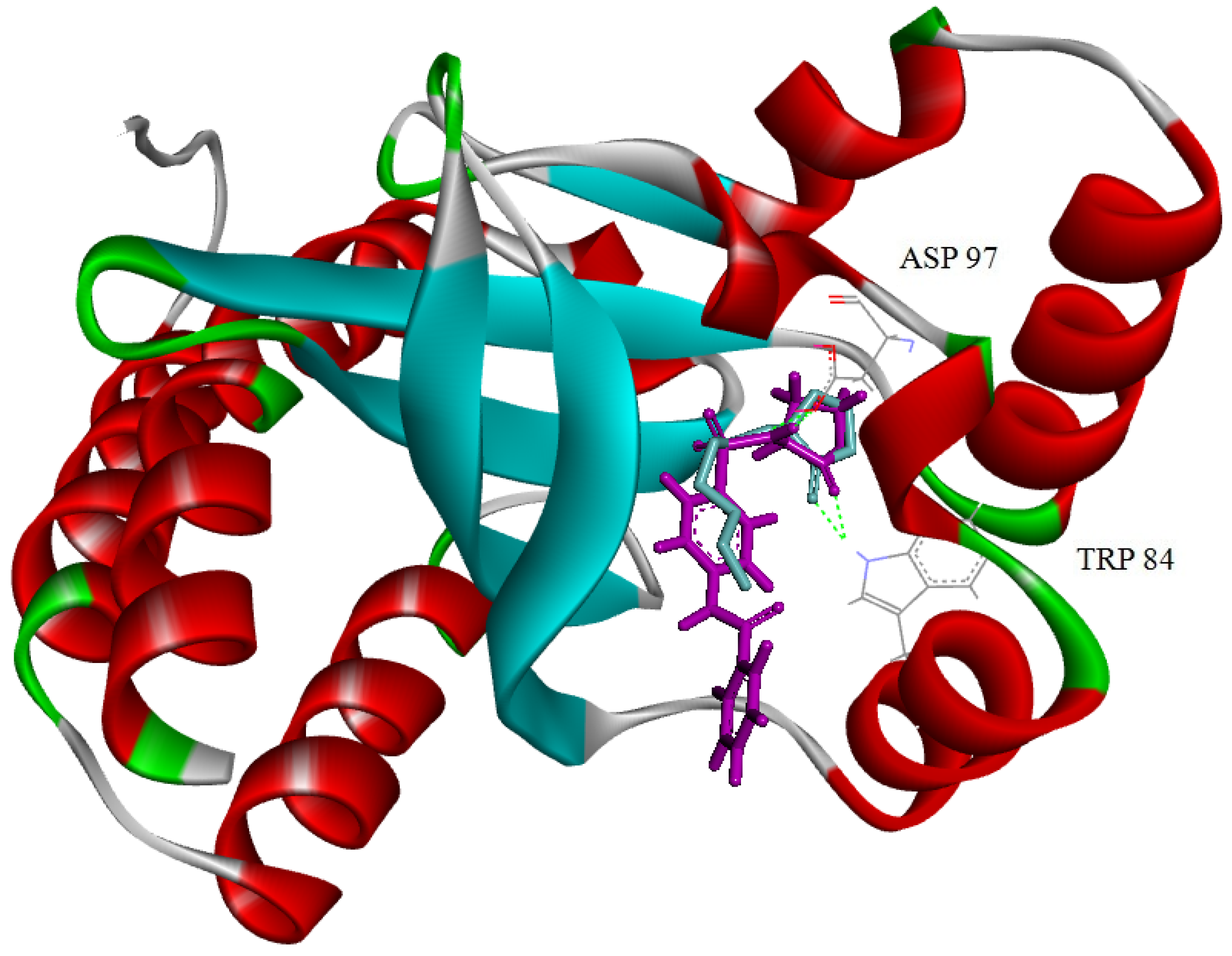
3. Experimental
3.1. General
3.2. Chemical Synthesis
3.2.1. Synthesis of Compounds 2–5
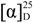 = −24.5° (c 0.10, H2O); lit. ref. [21] = −25.3° (c 0.087, H2O); 1H-NMR (DMSO-d6): δ 8.76 (3H, s), 4.45 (1H, t, J = 8.0 Hz, J = 7.6 Hz), 4.34–4.31 (2H, m), 2.55–2.51 (1H, m), 2.30–2.28 (1H, m); EI-MS: m/z = 102.1 [M+H]+.
= −24.5° (c 0.10, H2O); lit. ref. [21] = −25.3° (c 0.087, H2O); 1H-NMR (DMSO-d6): δ 8.76 (3H, s), 4.45 (1H, t, J = 8.0 Hz, J = 7.6 Hz), 4.34–4.31 (2H, m), 2.55–2.51 (1H, m), 2.30–2.28 (1H, m); EI-MS: m/z = 102.1 [M+H]+.3.2.2. General Procedure for of the Preparation of Compounds 5–10
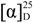 = +4.5° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.28 (1H, s), 8.17 (1H, d, J = 8.0 Hz), 7.77–7.74 (4H, m), 4.34–4.32 (1H, m), 4.21–4.19 (1H, m), 4.10–4.07 (1H, m), 2.35–2.32 (2H, m), 2.11–2.08 (1H, m), 1.81–1.79 (1H, m), 1.64–1.58 (2H, m), 0.91(3H, t, J = 7.3 Hz, J = 7.6 Hz); 13C-NMR (DMSO-d6): δ 174.5, 171.9, 142.9, 134.9, 127.7, 118.6, 65.1, 51.3, 38.4, 30.8, 29.4, 18.4, 13.6; EI-MS: m/z = 327.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C14H19N2O5S: 327.1015; Found: 327.1013.
= +4.5° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.28 (1H, s), 8.17 (1H, d, J = 8.0 Hz), 7.77–7.74 (4H, m), 4.34–4.32 (1H, m), 4.21–4.19 (1H, m), 4.10–4.07 (1H, m), 2.35–2.32 (2H, m), 2.11–2.08 (1H, m), 1.81–1.79 (1H, m), 1.64–1.58 (2H, m), 0.91(3H, t, J = 7.3 Hz, J = 7.6 Hz); 13C-NMR (DMSO-d6): δ 174.5, 171.9, 142.9, 134.9, 127.7, 118.6, 65.1, 51.3, 38.4, 30.8, 29.4, 18.4, 13.6; EI-MS: m/z = 327.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C14H19N2O5S: 327.1015; Found: 327.1013.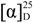 = +5.7° (c = 0.08, CH3OH); 1H-NMR (DMSO-d6): δ 10.28 (1H, s), 8.17 (1H, d, J = 9.6 Hz), 7.76–7.73 (4H, m), 4.34–4.33 (1H, m), 4.21–4.19 (1H, m), 4.10–4.07 (1H, m), 2.35–2.30 (2H, m), 2.09–2.08 (1H, m), 1.81–1.79 (1H, m), 1.59–1.55 (1H, m), 1.33–1.31 (1H, m), 0.89 (3H, t, J = 8.0 Hz, J = 8.0 Hz); 13C-NMR (DMSO-d6): δ 174.5, 172.2, 143.0, 134.9, 127.7, 118.6, 65.1, 51.3, 36.2, 30.8, 29.4, 27.1, 21.8, 13.8; EI-MS: m/z = 341.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C15H21N2O5S: 341.1171; Found: 341.1176.
= +5.7° (c = 0.08, CH3OH); 1H-NMR (DMSO-d6): δ 10.28 (1H, s), 8.17 (1H, d, J = 9.6 Hz), 7.76–7.73 (4H, m), 4.34–4.33 (1H, m), 4.21–4.19 (1H, m), 4.10–4.07 (1H, m), 2.35–2.30 (2H, m), 2.09–2.08 (1H, m), 1.81–1.79 (1H, m), 1.59–1.55 (1H, m), 1.33–1.31 (1H, m), 0.89 (3H, t, J = 8.0 Hz, J = 8.0 Hz); 13C-NMR (DMSO-d6): δ 174.5, 172.2, 143.0, 134.9, 127.7, 118.6, 65.1, 51.3, 36.2, 30.8, 29.4, 27.1, 21.8, 13.8; EI-MS: m/z = 341.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C15H21N2O5S: 341.1171; Found: 341.1176.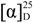 = +6.4° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.24 (1H, s), 8.13 (1H, d, J = 9.2 Hz), 7.76–7.73 (4H, m), 4.34–4.31 (1H, m), 4.21–4.19 (1H, m), 4.10–4.09 (1H, m), 2.36–2.32 (2H, m), 2.11–2.09 (1H, m), 1.81–1.79 (1H, m) 1.61–1.57 (2H, m), 1.30–1.29 (4H, m), 0.89–0.86 (3H, m); 13C-NMR (DMSO-d6): δ 174.5, 171.9, 142.9, 134.9, 127.6, 118.5, 65.1, 51.3, 36.4, 30.8, 29.4, 24.6, 21.9; EI-MS: m/z 355.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C16H23N2O5S: 355.1328; Found: 355.1326.
= +6.4° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.24 (1H, s), 8.13 (1H, d, J = 9.2 Hz), 7.76–7.73 (4H, m), 4.34–4.31 (1H, m), 4.21–4.19 (1H, m), 4.10–4.09 (1H, m), 2.36–2.32 (2H, m), 2.11–2.09 (1H, m), 1.81–1.79 (1H, m) 1.61–1.57 (2H, m), 1.30–1.29 (4H, m), 0.89–0.86 (3H, m); 13C-NMR (DMSO-d6): δ 174.5, 171.9, 142.9, 134.9, 127.6, 118.5, 65.1, 51.3, 36.4, 30.8, 29.4, 24.6, 21.9; EI-MS: m/z 355.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C16H23N2O5S: 355.1328; Found: 355.1326.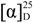 = +4.7° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.26 (1H, s), 8.16 (1H, d, J = 9.2 Hz), 7.76–7.73 (4H, m), 4.34–4.31 (1H, m), 4.22–4.21 (1H, m), 4.11–4.07 (1H, m), 2.35–2.32 (2H, m), 2.11–2.09 (1H, m), 1.81–1.79 (1H, m), 1.60–1.57 (2H, m), 1.32–1.27 (6H, m), 0.88–0.84 (3H, m); 13C-NMR (DMSO-d6): δ 174.5, 171.9, 142.9, 134.9, 127.7, 118.5, 65.1, 51.3, 36.5, 31.0, 29.4, 28.3, 24.9, 21.9, 13.9; EI-MS: m/z = 369.5 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H25N2O5S: 369.1484; Found: 369.1477.
= +4.7° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.26 (1H, s), 8.16 (1H, d, J = 9.2 Hz), 7.76–7.73 (4H, m), 4.34–4.31 (1H, m), 4.22–4.21 (1H, m), 4.11–4.07 (1H, m), 2.35–2.32 (2H, m), 2.11–2.09 (1H, m), 1.81–1.79 (1H, m), 1.60–1.57 (2H, m), 1.32–1.27 (6H, m), 0.88–0.84 (3H, m); 13C-NMR (DMSO-d6): δ 174.5, 171.9, 142.9, 134.9, 127.7, 118.5, 65.1, 51.3, 36.5, 31.0, 29.4, 28.3, 24.9, 21.9, 13.9; EI-MS: m/z = 369.5 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H25N2O5S: 369.1484; Found: 369.1477.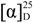 = +9.1° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.62 (1H, s), 8.20 (1H, d, J = 8.0 Hz), 8.01–7.95 (4H, m), 7.83–7.81 (2H, d, J = 8.8 Hz), 7.57–7.55 (3H, m), 4.38–4.35 (1H, m), 4.23–4.21 (1H, m), 4.12–4.10 (1H, m), 2.14–2.10 (1H, m), 1.86–1.84 (1H, m); 13C-NMR (DMSO-d6): δ 174.5, 166.7, 142.9, 135.6, 134.5, 131.9, 128.5, 127.8, 127.5, 119.8, 65.1, 51.3, 29.4; EI-MS: m/z = 361.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H17N2O5S: 361.0858; Found: 361.0855.
= +9.1° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.62 (1H, s), 8.20 (1H, d, J = 8.0 Hz), 8.01–7.95 (4H, m), 7.83–7.81 (2H, d, J = 8.8 Hz), 7.57–7.55 (3H, m), 4.38–4.35 (1H, m), 4.23–4.21 (1H, m), 4.12–4.10 (1H, m), 2.14–2.10 (1H, m), 1.86–1.84 (1H, m); 13C-NMR (DMSO-d6): δ 174.5, 166.7, 142.9, 135.6, 134.5, 131.9, 128.5, 127.8, 127.5, 119.8, 65.1, 51.3, 29.4; EI-MS: m/z = 361.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H17N2O5S: 361.0858; Found: 361.0855.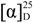 = +10.6° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.63 (1H, s), 8.23 (1H, d, J = 8.0 Hz), 8.05–7.97 (4H, m), 7.83–7.81 (2H, d, J = 8.0 Hz), 7.40 (2H, t, J = 9.2 Hz, J = 8.4 Hz), 4.38–4.36 (1H, m), 4.23–4.20 (1H, m), 4.12–4.10 (1H, m), 2.14–2.12 (1H, m), 1.85–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 174.4, 165.6, 163.4, 142.6, 135.5, 130.5, 127.4, 119.7, 115.4, 65.0, 51.2, 29.3; EI-MS m/z = 379.3 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16FN2O5S: 379.0764; Found: 379.0764.
= +10.6° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.63 (1H, s), 8.23 (1H, d, J = 8.0 Hz), 8.05–7.97 (4H, m), 7.83–7.81 (2H, d, J = 8.0 Hz), 7.40 (2H, t, J = 9.2 Hz, J = 8.4 Hz), 4.38–4.36 (1H, m), 4.23–4.20 (1H, m), 4.12–4.10 (1H, m), 2.14–2.12 (1H, m), 1.85–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 174.4, 165.6, 163.4, 142.6, 135.5, 130.5, 127.4, 119.7, 115.4, 65.0, 51.2, 29.3; EI-MS m/z = 379.3 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16FN2O5S: 379.0764; Found: 379.0764.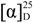 = +9.3° (c = 0.11, CH3OH); 1H-NMR (DMSO-d6): δ 10.67 (1H, s), 8.24 (1H, d, J = 8.8 Hz), 8.00 (2H, d, J = 8.0 Hz), 7.85–7.82 (4H, m), 7.65–7.62 (1H, m), 7.51–7.49 (1H, m), 4.39–4.36 (1H, m), 4.24–4.22 (1H, m), 4.11–4.09 (1H, m), 2.15–2.12 (1H, m), 1.86–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 174.4, 164.6, 163.2, 142.5, 136.7, 135.8, 130.7, 127.5, 123.9, 119.9, 118.7, 114.7, 65.0, 51.2, 29.4; EI-MS: m/z = 379.2 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16FN2O5S: 379.0764; Found: 379.0763.
= +9.3° (c = 0.11, CH3OH); 1H-NMR (DMSO-d6): δ 10.67 (1H, s), 8.24 (1H, d, J = 8.8 Hz), 8.00 (2H, d, J = 8.0 Hz), 7.85–7.82 (4H, m), 7.65–7.62 (1H, m), 7.51–7.49 (1H, m), 4.39–4.36 (1H, m), 4.24–4.22 (1H, m), 4.11–4.09 (1H, m), 2.15–2.12 (1H, m), 1.86–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 174.4, 164.6, 163.2, 142.5, 136.7, 135.8, 130.7, 127.5, 123.9, 119.9, 118.7, 114.7, 65.0, 51.2, 29.4; EI-MS: m/z = 379.2 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16FN2O5S: 379.0764; Found: 379.0763.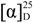 = +11.7° (c = 0.12, CH3OH) for 99.2% e.e. purity material. HPLC (chiral sample) DAICEL AYH Chiralpak, 70:30 hexane/ EtOH, flow rate of 1 mL/min; retention time of the major enantiomer 41.545 min, minor enantiomer 25.533 min; HPLC (Racemic sample) DAICEL AYH Chiralpak, 70:30 hexane/EtOH, flow rate of 1 mL/min; retention times of 25.475 and 41.422 min; 1H-NMR (DMSO-d6): δ 10.84 (1H, s), 8.24 (1H, d, J = 8.4 Hz), 7.93 (2H, d, J = 8.0 Hz), 7.83 (2H, d, J = 8.8 Hz), 7.71–7.69 (1H, m), 7.60–7.59 (1H, m), 7.37–7.35 (2H, m) 4.38–4.35 (1H, m), 4.23–4.21 (1H, m), 4.11–4.09 (1H, m), 2.14–2.12 (1H, m), 1.83–1.82 (1H, m); 13C-NMR (DMSO-d6): δ 174.6, 163.4, 160.1, 142.7, 135.9, 133.0, 130.0, 128.5, 127.7, 124.7, 124.5, 119.4, 116.4, 65.2, 51.4, 29.5; EI-MS: m/z = 379.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16FN2O5S: 379.0764; Found: 379.0762.
= +11.7° (c = 0.12, CH3OH) for 99.2% e.e. purity material. HPLC (chiral sample) DAICEL AYH Chiralpak, 70:30 hexane/ EtOH, flow rate of 1 mL/min; retention time of the major enantiomer 41.545 min, minor enantiomer 25.533 min; HPLC (Racemic sample) DAICEL AYH Chiralpak, 70:30 hexane/EtOH, flow rate of 1 mL/min; retention times of 25.475 and 41.422 min; 1H-NMR (DMSO-d6): δ 10.84 (1H, s), 8.24 (1H, d, J = 8.4 Hz), 7.93 (2H, d, J = 8.0 Hz), 7.83 (2H, d, J = 8.8 Hz), 7.71–7.69 (1H, m), 7.60–7.59 (1H, m), 7.37–7.35 (2H, m) 4.38–4.35 (1H, m), 4.23–4.21 (1H, m), 4.11–4.09 (1H, m), 2.14–2.12 (1H, m), 1.83–1.82 (1H, m); 13C-NMR (DMSO-d6): δ 174.6, 163.4, 160.1, 142.7, 135.9, 133.0, 130.0, 128.5, 127.7, 124.7, 124.5, 119.4, 116.4, 65.2, 51.4, 29.5; EI-MS: m/z = 379.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16FN2O5S: 379.0764; Found: 379.0762.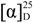 = +11.3° (c = 0.11, CH3OH); 1H-NMR (DMSO-d6): δ 10.67 (1H, s), 8.20 (1H, d, J = 8.0 Hz), 8.01–7.98 (4H, m), 7.84 (4H, d, J = 9.2 Hz), 7.65 (2H, d, J = 8.4 Hz), 4.39–4.36 (1H, m), 4.23–4.21 (1H, m), 4.11–4.09 (1H, m), 2.14–2.13 (1H, m), 1.86–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 175.1, 165.5, 143.2, 137.4, 136.3, 133.7, 130.3, 129.1, 128.1, 120.4, 65.1, 51.8, 30.0; EI-MS: m/z = 395.2 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16ClN2O5S: 395.0468; Found: 395.0474.
= +11.3° (c = 0.11, CH3OH); 1H-NMR (DMSO-d6): δ 10.67 (1H, s), 8.20 (1H, d, J = 8.0 Hz), 8.01–7.98 (4H, m), 7.84 (4H, d, J = 9.2 Hz), 7.65 (2H, d, J = 8.4 Hz), 4.39–4.36 (1H, m), 4.23–4.21 (1H, m), 4.11–4.09 (1H, m), 2.14–2.13 (1H, m), 1.86–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 175.1, 165.5, 143.2, 137.4, 136.3, 133.7, 130.3, 129.1, 128.1, 120.4, 65.1, 51.8, 30.0; EI-MS: m/z = 395.2 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16ClN2O5S: 395.0468; Found: 395.0474.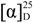 = +10.4° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.74 (1H, s), 8.25 (1H, d, J = 8.4 Hz), 8.02–8.00 (6H, m), 7.91–7.90 (1H, m), 7.85–7.82 (1H, m), 7.72–7.69 (1H, m), 7.62–7.58 (1H, m), 4.40–4.38 (1H, m), 4.24–4.22 (1H, m), 4.12–4.11 (1H, m), 2.15–2.14 (1H, m), 1.86–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 174.6, 164.7, 142.6, 136.4, 135.9, 133.3, 131.8, 130.6, 127.6, 126.7, 119.9, 65.2, 51.4, 29.5; EI-MS: m/z = 395.3 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16ClN2O5S: 395.0468; Found: 395.0468.
= +10.4° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.74 (1H, s), 8.25 (1H, d, J = 8.4 Hz), 8.02–8.00 (6H, m), 7.91–7.90 (1H, m), 7.85–7.82 (1H, m), 7.72–7.69 (1H, m), 7.62–7.58 (1H, m), 4.40–4.38 (1H, m), 4.24–4.22 (1H, m), 4.12–4.11 (1H, m), 2.15–2.14 (1H, m), 1.86–1.83 (1H, m); 13C-NMR (DMSO-d6): δ 174.6, 164.7, 142.6, 136.4, 135.9, 133.3, 131.8, 130.6, 127.6, 126.7, 119.9, 65.2, 51.4, 29.5; EI-MS: m/z = 395.3 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16ClN2O5S: 395.0468; Found: 395.0468.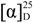 = +12.5° (c = 0.12, CH3OH); 1H-NMR (DMSO-d6) δ ppm: 10.93 (1H, s), 8.25 (1H, d, J = 8.0 Hz), 7.92 (2H, d, J = 8.0 Hz), 7.84 (2H, d, J = 8.0 Hz), 7.59–7.50 (4H, m), 4.38–4.36 (1H, m), 4.24–4.20 (1H, m), 4.11–4.09 (1H, m), 2.51–2.50 (1H, m), 1.87–1.84 (1H, m); 13C-NMR (DMSO-d6) δ: 175.0, 166.0, 142.9, 137.0, 136.4, 132.0, 130.4, 130.3, 129.5, 128.3, 127.9, 119.8, 65.7, 51.9, 30.0; EI-MS: m/z = 395.2 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16ClN2O5S: 395.0468; Found: 395.0464.
= +12.5° (c = 0.12, CH3OH); 1H-NMR (DMSO-d6) δ ppm: 10.93 (1H, s), 8.25 (1H, d, J = 8.0 Hz), 7.92 (2H, d, J = 8.0 Hz), 7.84 (2H, d, J = 8.0 Hz), 7.59–7.50 (4H, m), 4.38–4.36 (1H, m), 4.24–4.20 (1H, m), 4.11–4.09 (1H, m), 2.51–2.50 (1H, m), 1.87–1.84 (1H, m); 13C-NMR (DMSO-d6) δ: 175.0, 166.0, 142.9, 137.0, 136.4, 132.0, 130.4, 130.3, 129.5, 128.3, 127.9, 119.8, 65.7, 51.9, 30.0; EI-MS: m/z = 395.2 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C17H16ClN2O5S: 395.0468; Found: 395.0464.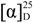 = +11.2° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.99 (1H, s), 8.26 (1H, d, J = 8.0 Hz), 7.88–7.82 (6H, m), 7.77–7.70 (2H, m), 4.39–4.36 (1H, m), 4.24–4.22 (1H, m), 4.11–4.09 (1H, m), 2.17–2.15 (1H, m), 1.88–1.83 (1H, m); 13C-NMR (DMSO-d6) δ: 174.6, 166.1, 142.4, 136.0, 136.2, 132.8, 130.4, 128.6, 127.7, 126.5, 126.0, 125.1, 122.4, 119.3, 65.2, 51.3, 29.5; EI-MS: m/z = 429.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C18H16F3N2O5S: 429.0732; Found: 429.0724.
= +11.2° (c = 0.10, CH3OH); 1H-NMR (DMSO-d6): δ 10.99 (1H, s), 8.26 (1H, d, J = 8.0 Hz), 7.88–7.82 (6H, m), 7.77–7.70 (2H, m), 4.39–4.36 (1H, m), 4.24–4.22 (1H, m), 4.11–4.09 (1H, m), 2.17–2.15 (1H, m), 1.88–1.83 (1H, m); 13C-NMR (DMSO-d6) δ: 174.6, 166.1, 142.4, 136.0, 136.2, 132.8, 130.4, 128.6, 127.7, 126.5, 126.0, 125.1, 122.4, 119.3, 65.2, 51.3, 29.5; EI-MS: m/z = 429.4 [M+H]+; ESI-HRMS: m/z [M+H]+ calcd for C18H16F3N2O5S: 429.0732; Found: 429.0724.3.3. Evaluation of the Biological Activity
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Geske, G.D.; O’Nell, J.C.; Miller, D.M.; Mattmann, M.E.; Blackwell, H.E. Modulation of Bacterial Quorum Sensing with Synthetic Ligands: Systematic Evaluation of N-Acylated Homoserine Lactones in Multiple Species and New Insights into Their Mechanisms of Action. J. Am. Chem. Soc. 2007, 129, 13613–13625. [Google Scholar]
- Yang, L.; Liu, Y.; Stemberg, C.; Molin, S. Evaluation of Enoyl-Acyl Carrier Protein Reductase Inhibitors as Pseudomonas aeruginosa Quorum-Quenching Reagents. Molecules 2010, 15, 780–792. [Google Scholar] [CrossRef]
- Amara, N.; Mashiach, R.; Amar, D.; Krief, P.; Spieser, S.A.H.; Bottomley, M.J.; Aharoni, A.; Meijler, M.M. Covalent Inhibition of Bacterial Quorum Sensing. J. Am. Chem. Soc. 2009, 131, 10610–10619. [Google Scholar] [CrossRef]
- Persson, T.; Hansen, T.H.; Rasmussen, T.B.; Skindersø, M.E.; Givskov, M.; Nielsen, J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005, 3, 253–262. [Google Scholar]
- Kim, C.; Kim, J.; Park, H.Y.; Park, H.J.; Kim, C.K.; Yoon, J.; Lee, J.H. Development of Inhibitors against TraR Quorum-Sensing System in Agrobacterium tumefaciens by Molecular Modeling of the Ligand-Receptor Interaction. Mol. Cells 2009, 28, 447–453. [Google Scholar] [CrossRef]
- Steven, A.M.; Queneau, Y.; Soulère, L.; von Bodman, S.; Doutheau, A. Mechanisms and Synthetic Modulators of AHL-Dependent Gene Regulation. Chem. Rev. 2011, 111, 4–27. [Google Scholar] [CrossRef]
- Churchill, M.E.; Chen, L. Structural Basis of Acyl-homoserine Lactone-Dependent Signaling. Chem. Rev. 2011, 111, 68–85. [Google Scholar] [CrossRef]
- Galloway, W.R.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum Sensing in Gram Negative Bacteria: Small-Molecule Modulation of AHL and AI-2 Quorum Sensing Pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef]
- Boukraa, M.; Sabbah, M.; Soulère, L.; EI Efrit, M.L.; Queneau, Y.; Doutheau, A. AHL-dependent quorum sensing inhibition: Synthesis and biological evaluation of α-(N-alkyl-carboxamide)-γ-butyrolactones andα-(N-alkyl-sulfonamide)-γ-butyrolactones. Bioorg. Med. Chem. Lett. 2011, 21, 6876–6879. [Google Scholar] [CrossRef]
- Geake, G.D.; Wezeman, R.J.; Siegel, A.P.; Blackwell, H.E. Small Molecule Inhibitors of Bacterial Quorum Sensing and Biofilm Formation. J. Am. Chem. Soc. 2005, 127, 12762–12763. [Google Scholar] [CrossRef]
- Reverchon, S.; Chanteqrel, B.; Deshayes, C.; Doutheau, A; Cotte-Pattat, N. New Synthetic Analogues of N-Acyl Homoserine Lactones as Agonists or Antagonists of Transcriptional Regulators Involved in Bacterial Quorum Sensing. Bioorg. Med. Chem. 2002, 12, 1153–1157. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chrornobacteriurn violaceurn: Exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- Swem, L.R.; Swem, D.L.; O’Loughlin, C.T.; Gatmaitan, R.; Zhao, B.; Ulrich, S.M.; Basster, B.L. A Quorum-Sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol. Cell 2009, 35, 143–153. [Google Scholar] [CrossRef]
- Chen, G.; Swem, L.R.; Swem, D.L.; O’Loughlin, C.T.; Jeffrey, P.D.; Bassler, B.L.; Hughson, F.M. A strategy for antagonizing quorum sensing. Mol. Cell 2011, 42, 199–209. [Google Scholar] [CrossRef]
- Fuqua, C.; Parsek, M.R.; Greenberg, E.P. Regulation of gene expression by cell-to-cell communication Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001, 35, 439–468. [Google Scholar] [CrossRef]
- Choudhary, S.; Schmidt-Dannert, C. Application of quorum sensing in biotechnology. Appl. Microbiol. Biot. 2010, 86, 1267–1279. [Google Scholar] [CrossRef]
- Castang, S.; Chantegrel, B.; Deshayes, C.; Dolmazon, R.; Gouet, P.; Haser, R.; Reverchon, S.; Nasser, W.; Hugouvieux-Cotte-Pattat, N.; Doutheau, A. N-Sulfonyl homoserine lactones as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. Lett. 2004, 14, 5145–5149. [Google Scholar] [CrossRef]
- Brackman, G.; Risseeuw, M.; Celen, S.; Cos, P.; Maes, L.; Neils, H.J.; Calenbergh, S.V.; Coenye, T. Synthesis and evaluation of the quorum sensing inhibitory effect of substituted triazolyldihydrofuranones. Bioorg. Med. Chem. 2012, 20, 4737–4743. [Google Scholar] [CrossRef]
- Sabbah, M.; Fanny, F.; Grand, L.; Boukraa, M.; Efrit, M.L.; Doutheau, A.; Soulère, L.; Queneau, Y. Synthesis and biological evaluation of new N-acyl-homoserine-lactone analogues, base on triazole and tetrazole scaffolds, acting as LuxR-dependent quorum sensing modulators. Bioorg. Med. Chem. 2012, 20, 4727–4736. [Google Scholar] [CrossRef]
- Nielsen, J.; Givskov, M. Compounds and Methods for controlling bacterial virulence field of the invention. WO03/106445 A1, 24 December 2013. [Google Scholar]
- McLean, R.J.; Pierson, L.S.; Fuqua, C. A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods. 2004, 58, 351–360. [Google Scholar] [CrossRef]
- Olivero, J.T.; Pájaro, N.P.; Stashenko, E. Antiquorum sensing activity of essential oils isolated from different species of the genus piper. Vitea revista de la facultad de quimica farmaceutica 2011, 18, 2145–2660. [Google Scholar]
- Sample Availability: Samples of the compounds 5a–l are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, M.; Yu, Y.; Hua, Y.; Feng, F.; Tong, Y.; Yang, X.; Xiao, J.; Song, H. Design, Synthesis and Biological Evaluation of N-Sulfonyl Homoserine Lactone Derivatives as Inhibitors of Quorum Sensing in Chromobacterium violaceum. Molecules 2013, 18, 3266-3278. https://doi.org/10.3390/molecules18033266
Zhao M, Yu Y, Hua Y, Feng F, Tong Y, Yang X, Xiao J, Song H. Design, Synthesis and Biological Evaluation of N-Sulfonyl Homoserine Lactone Derivatives as Inhibitors of Quorum Sensing in Chromobacterium violaceum. Molecules. 2013; 18(3):3266-3278. https://doi.org/10.3390/molecules18033266
Chicago/Turabian StyleZhao, Mingming, Yingying Yu, Yuhui Hua, Fan Feng, Yigang Tong, Xiaohong Yang, Junhai Xiao, and Hongrui Song. 2013. "Design, Synthesis and Biological Evaluation of N-Sulfonyl Homoserine Lactone Derivatives as Inhibitors of Quorum Sensing in Chromobacterium violaceum" Molecules 18, no. 3: 3266-3278. https://doi.org/10.3390/molecules18033266
APA StyleZhao, M., Yu, Y., Hua, Y., Feng, F., Tong, Y., Yang, X., Xiao, J., & Song, H. (2013). Design, Synthesis and Biological Evaluation of N-Sulfonyl Homoserine Lactone Derivatives as Inhibitors of Quorum Sensing in Chromobacterium violaceum. Molecules, 18(3), 3266-3278. https://doi.org/10.3390/molecules18033266




