High Pressure Extraction of Antioxidants from Solanum stenotomun Peel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Supercritical Fluid Extraction (SFE)
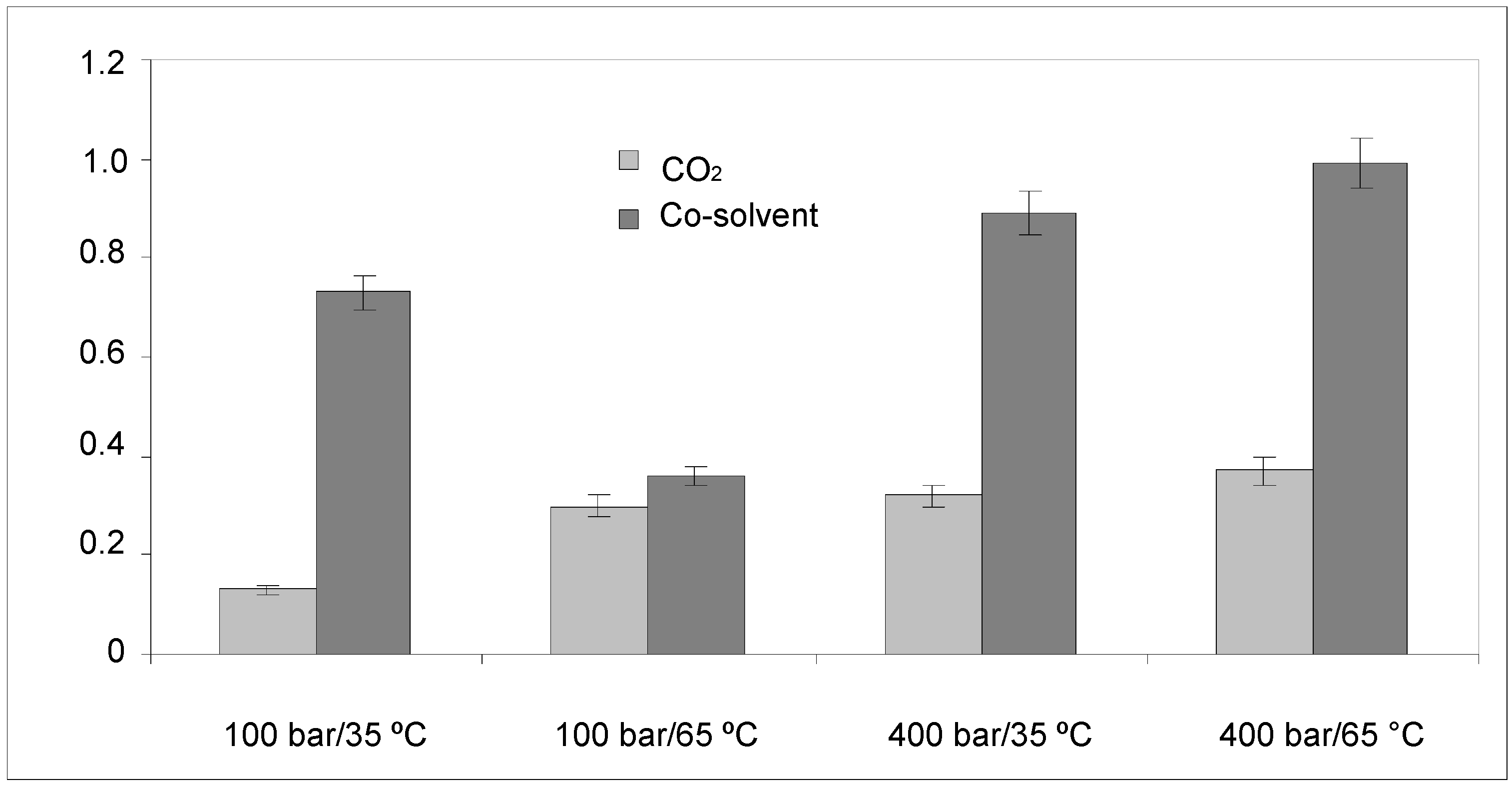
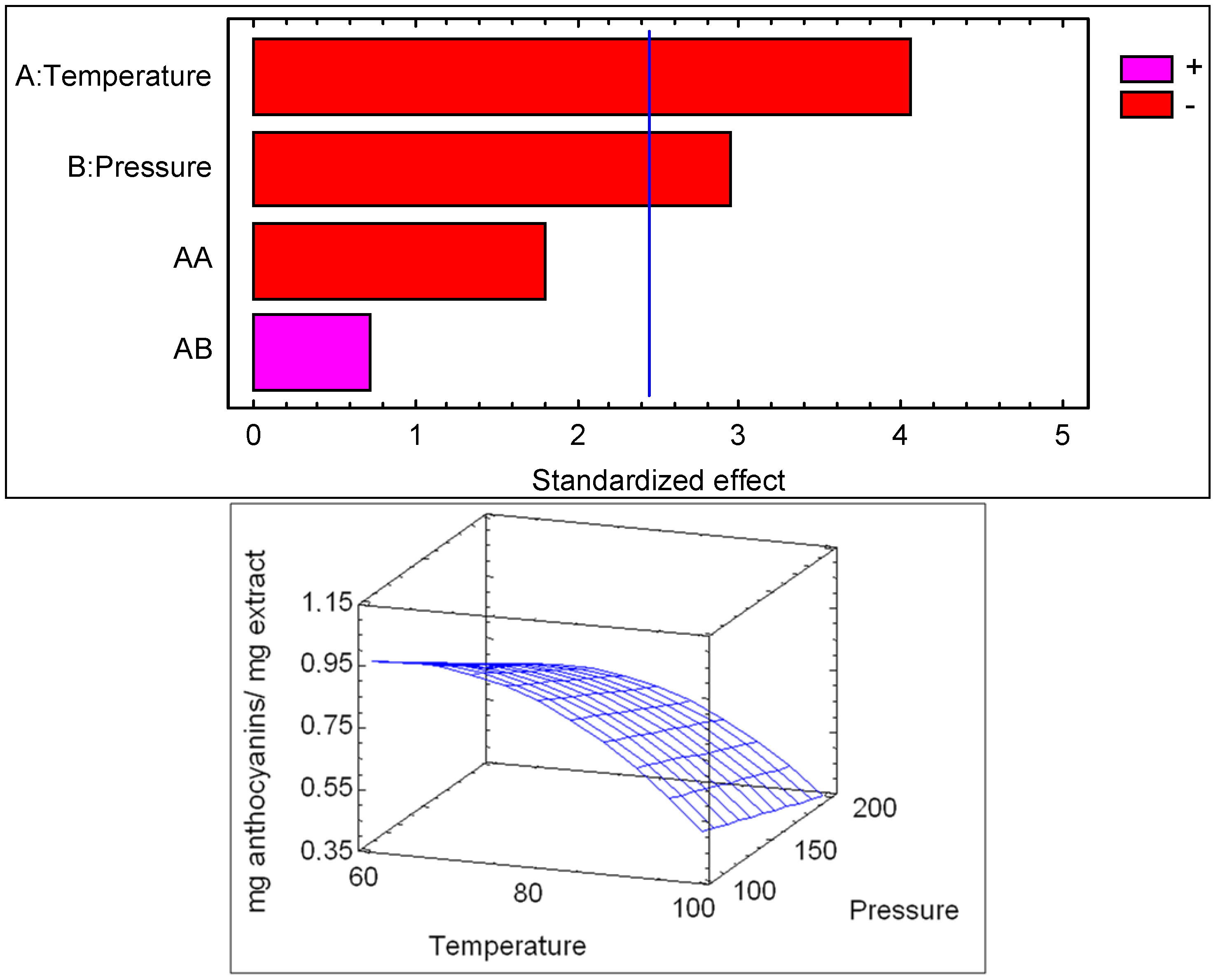
2.1.1. Anthocyanin Contents
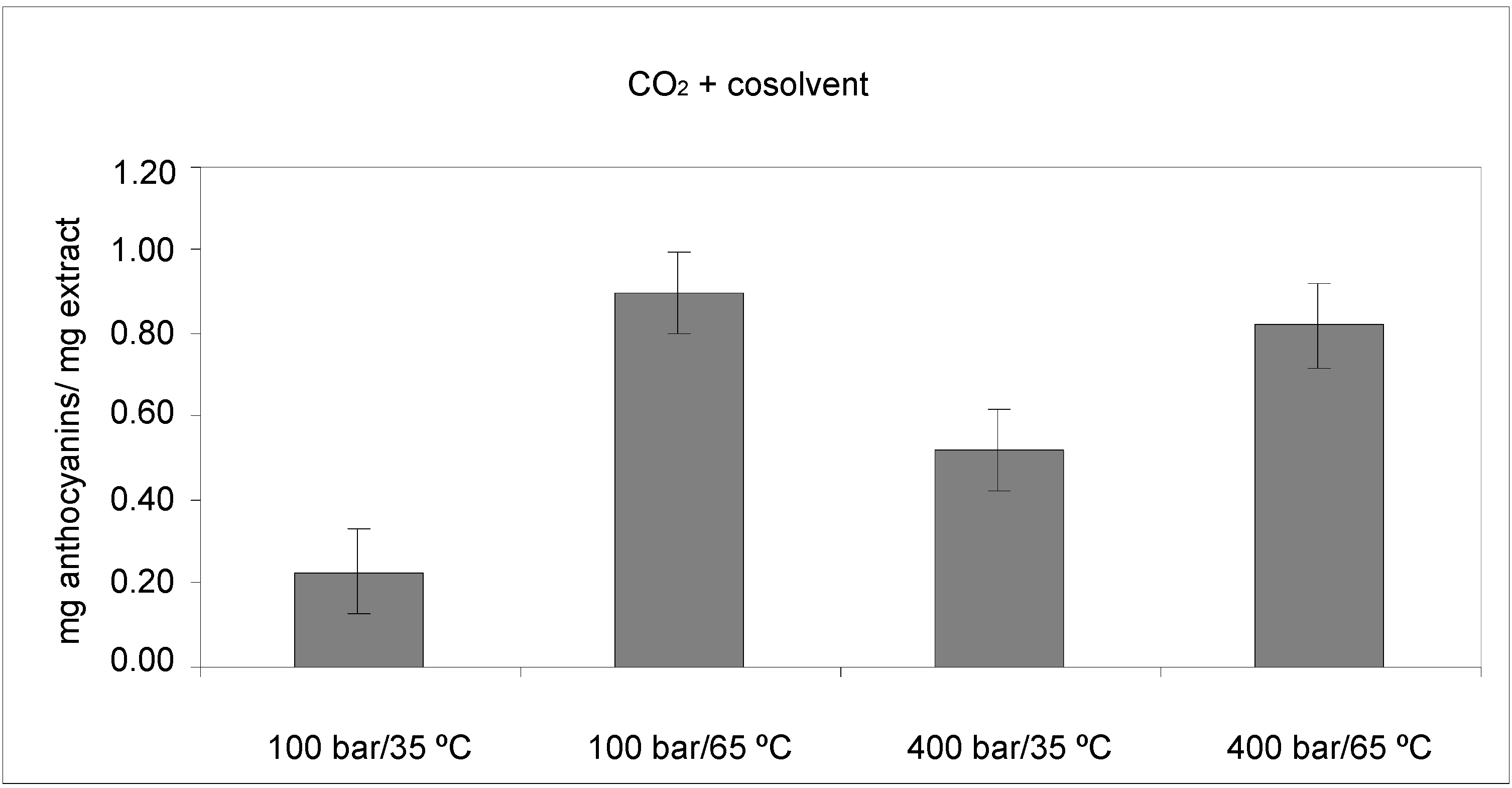
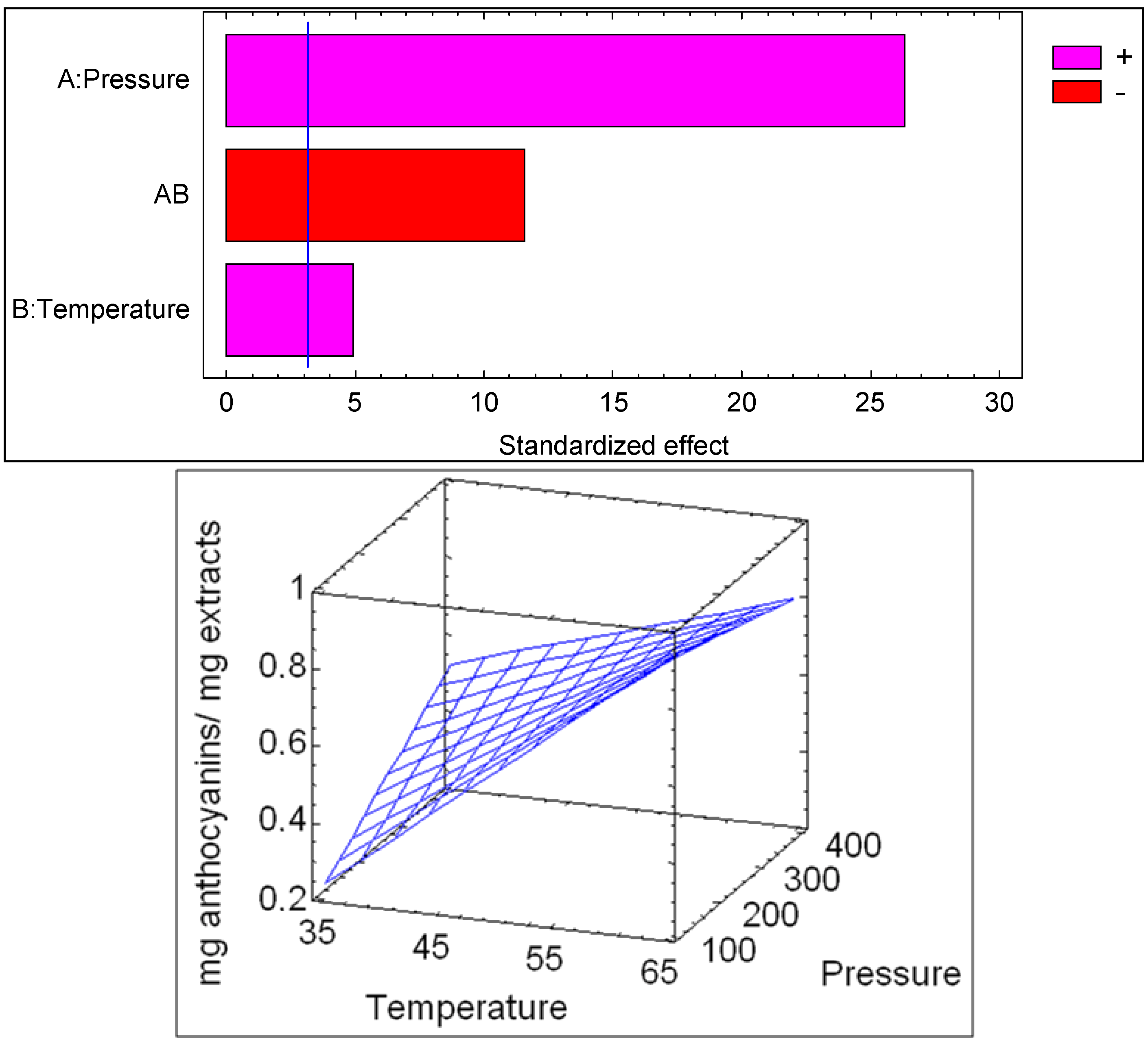
| Source | SS | DF | MS | F-Value | p-value |
|---|---|---|---|---|---|
| P | 0.0153125 | 1 | 0.0153125 | 23.71 | 0.0165 |
| T | 0.446512 | 1 | 0.446512 | 691.37 | 0.0001 |
| PT | 0.0861125 | 1 | 0.0861125 | 133.34 | 0.0014 |
| Blok | 0.0003125 | 1 | 0.0003125 | 0.48 | 0.5367 |
| Total error | 0.0019375 | 3 | 0.000645833 | ||
| Total (corr.) | 0.550188 | 7 |
2.2. Pressurized Liquid Extraction (PLE)
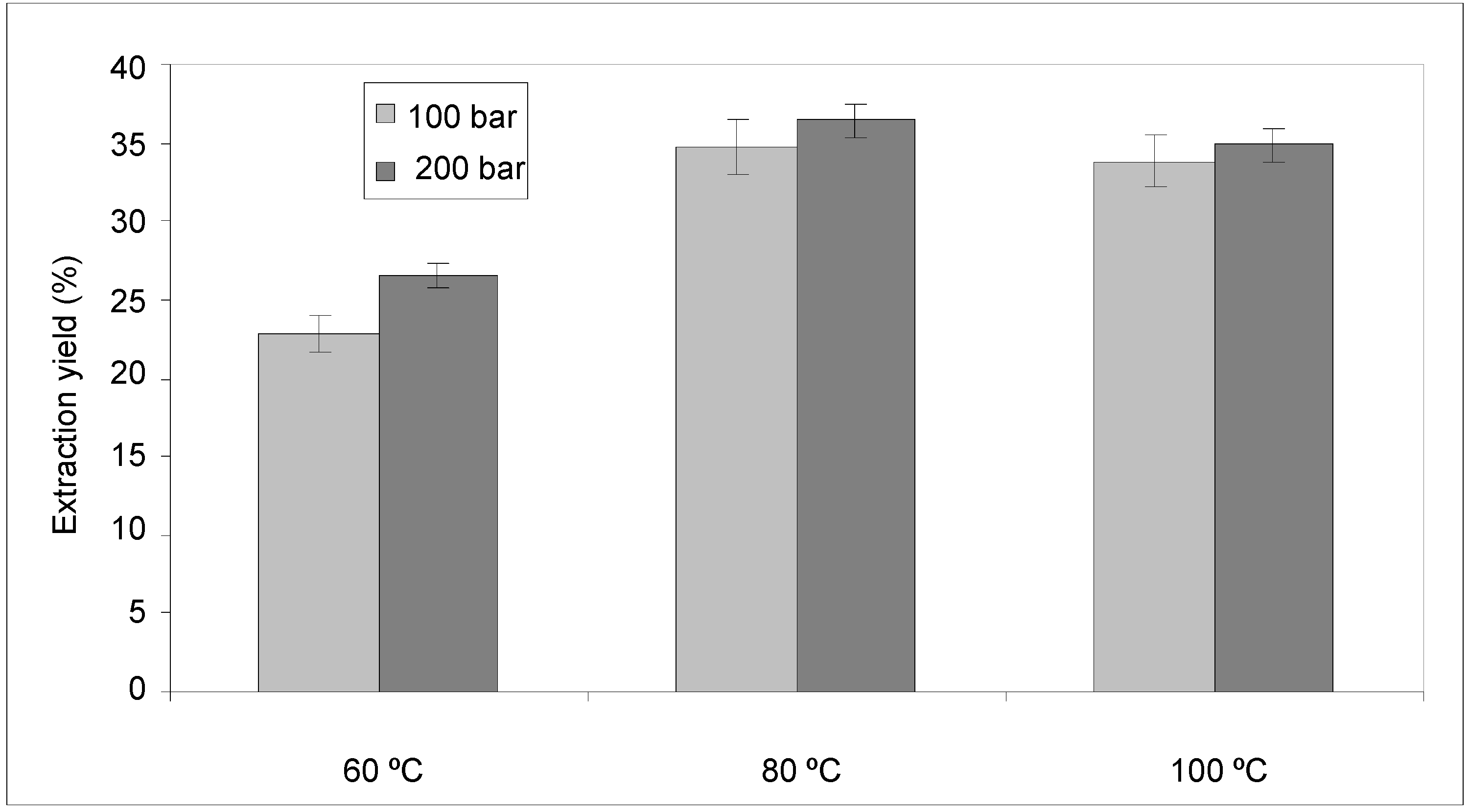
2.2.1. Anthocyanin and Phenolic Contents
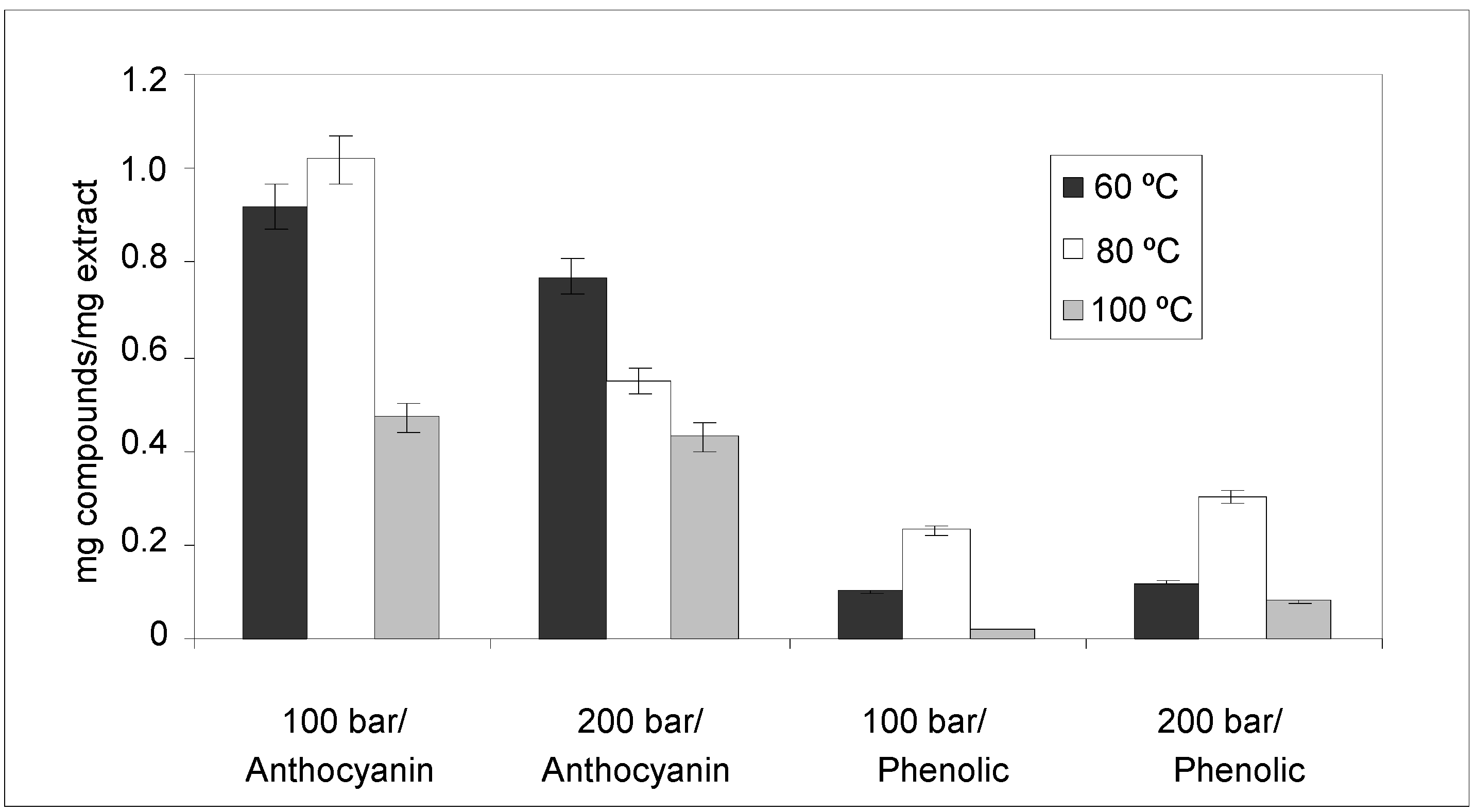
| Source | SS | DF | MS | F-Value | p-value |
|---|---|---|---|---|---|
| P | 0.154133 | 1 | 0.154133 | 8,61 | 0.0261 |
| T | 0.292613 | 1 | 0.292613 | 16,35 | 0.0068 |
| PT | 0.0091125 | 1 | 0.0091125 | 0,51 | 0.5023 |
| T2 | 0.0570375 | 1 | 0.0570375 | 3.19 | 0.1245 |
| Blok | 0.0003 | 1 | 0.0003 | 0.02 | 0.9012 |
| Total error | 0.107404 | 6 | 0.0179007 | ||
| Total (corr.) | 0.6206 | 11 |
2.3. Antiradical Activities of the Extracts
| Pressure | 60 °C | 80 °C | 100 °C |
|---|---|---|---|
| 100 bar | 0.91 | 1.66 | 0.72 |
| 200 bar | 1.05 | 1.53 | 0.80 |
3. Experimental
3.1. Samples and Chemicals
3.2. Extractions with Solvents at High Pressures
3.3. Anthocyanins Analysis
3.4. Phenolic Compounds Analysis
3.5. Antioxidant Assay with DPPH
3.6. Experimental Design
| Extraction method | Experimental variable | Factor | Levels | |||
|---|---|---|---|---|---|---|
| SFE | Pressure | P | 100 bar | 400 bar | ||
| Temperatue | T | 35 °C | 65 °C | |||
| PLE | Pressure | P | 100 bar | 200 bar | ||
| Temperatue | T | 60 °C | 80°C | 100 °C | ||
4. Conclusions
References
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cumium nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picryhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Lurton, L. Grape polyphenols: New powerful health ingredients. Innov. Food Tech. 2003, 18, 28–33. [Google Scholar]
- Fiori, L. Supercritical extraction of grape seed oil at industrial-scale: Plant and process design, modeling, economic feasibility. Chem. Eng. Process. 2010, 49, 866–872. [Google Scholar] [CrossRef]
- King, J.W.; Srinivas, K. Multiple unit processing using sub and supercritical fluids. J. Supercrit. Fluids 2009, 47, 598–610. [Google Scholar]
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2006, 70, 270–276. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Extraction of anthocyanins and other phenolics from black currants with sulfured water. J. Agric. Food Chem. 2002, 50, 5939–5946. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Gizir, A.M.; Turker, N.; Artuvan, E. Pressurized acidified water extraction of black carrot (Daucus carota ssp. sativus var. artrorubens Alef.) anthocyanins. Eur. Food Res. Technol. 2008, 226, 363–370. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xi, G.S.; Zheng, Y.C.; Miao, F.S. Microwave-assisted extraction of flavonoids from Chinese herb Radix puerariae (Ge Gen). J. Med. Plant Res. 2010, 4, 304–308. [Google Scholar]
- Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.; Gariepy, Y.; Orsat, V.; Raghavan, G. Microwave-assisted extraction of phenolic antioxidants from potato peels. Molecules 2011, 16, 2218–2232. [Google Scholar] [CrossRef]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.; Akber Aisa, H.; Tsao, R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Pereira, C.; Meireles, M. Economic analysis of rosemary, fennel and anise essential oils obtained by supercritical fluid extraction. Flavour Fragr. J. 2007, 22, 407–413. [Google Scholar] [CrossRef]
- Fernández, M.; Rodríguez, J.; García, M.; De Lucas, A.; Gracia, I. Application of supercritical fluid extraction to Brewer’s spent grain management. Ind. Eng. Chem. Res. 2008, 47, 1614–1619. [Google Scholar]
- Schutz, E. Supercritical fluids and applications—A patent review. Chem. Eng. Technol. 2007, 30, 685–688. [Google Scholar] [CrossRef]
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimisation of supercritical fluid extraction of bioactive compounds from grape (Vitis Labrusca B.) peel by using response superface methodology. Innov. Food Sci. Emerg. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Wijngaard, H.; Bronton, N. The optimisation of extraction of antioxidants from apple pomace by pressurized liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruits and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.; Aliberti, L.; Rastrelli, L. Application of pressurized liquid extraction in the analysis of aflatoxins B1, B2, G1, and G2 in nuts. J. Sep. Sci. 2009, 32, 3837–3844. [Google Scholar]
- Srinivas, K.; King, J.W.; Howard, L.H.; Monrad, J.K. Binary diffusion coefficients of phenolic compounds in subcritical water using a chromatographic peak broadening technique. Fluid Phase Equilib. 2011, 301, 234–243. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinium crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 7, 745–552. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Luthria, D.L.; Robbins, R.J. Optimization of extraction process for phenolic acids from black cohosh (Cimicifuga racemosa) by pressurized liquid extraction. J. Sci. Food Agric. 2006, 86, 156–162. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Sarneckis, C.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimized tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds extracted are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cardoso, L.C.; Serrano, C.M.; Quintero, E.T.; López, C.P.; Antezana, R.M.; Martínez de la Ossa, E.J. High Pressure Extraction of Antioxidants from Solanum stenotomun Peel. Molecules 2013, 18, 3137-3151. https://doi.org/10.3390/molecules18033137
Cardoso LC, Serrano CM, Quintero ET, López CP, Antezana RM, Martínez de la Ossa EJ. High Pressure Extraction of Antioxidants from Solanum stenotomun Peel. Molecules. 2013; 18(3):3137-3151. https://doi.org/10.3390/molecules18033137
Chicago/Turabian StyleCardoso, Lourdes Casas, Casimiro Mantell Serrano, Edwin Torrez Quintero, Clara Pereyra López, Ruder Medrano Antezana, and Enrique J. Martínez de la Ossa. 2013. "High Pressure Extraction of Antioxidants from Solanum stenotomun Peel" Molecules 18, no. 3: 3137-3151. https://doi.org/10.3390/molecules18033137
APA StyleCardoso, L. C., Serrano, C. M., Quintero, E. T., López, C. P., Antezana, R. M., & Martínez de la Ossa, E. J. (2013). High Pressure Extraction of Antioxidants from Solanum stenotomun Peel. Molecules, 18(3), 3137-3151. https://doi.org/10.3390/molecules18033137






