Fibrous Polymeric Composites Based on Alginate Fibres and Fibres Made of Poly-ε-caprolactone and Dibutyryl Chitin for Use in Regenerative Medicine
Abstract
:1. Introduction
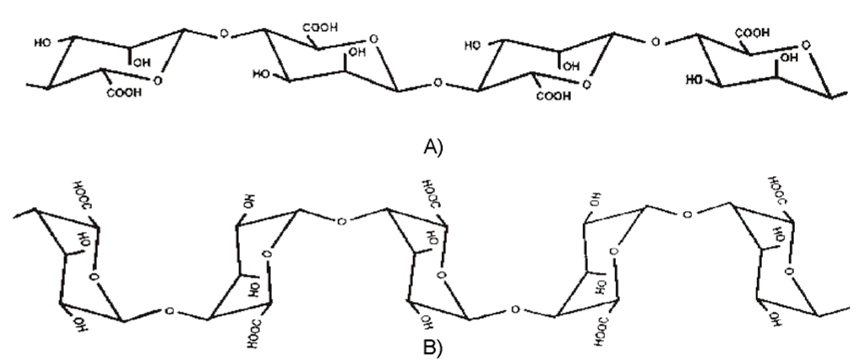
- alginate fibres containing TCP nanoadditive (ZA).
- alginate fibres containing TCP nanoadditive (CA).
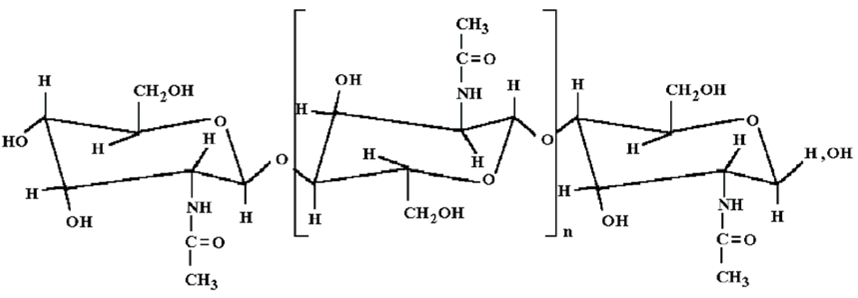
- made from PCL and DBC using the electrospinning method.
2. Results and Discussion
2.1. Properties of Alginate Fibres
| Type of fibres | As-spun draw ratio [%] | Total draw ratio [%] | Total pores volume, [cm3/g] | Tenacity [cN/tex] | Elongation at break [%] |
|---|---|---|---|---|---|
| Zinc alginate with TCP | +70 | 224.20 | 0.31 | 23.99 ± 0.80 | 5.57 ± 0.48 |
| Calcium alginate with TCP | +70 | 89.27 | 0.38 | 24.39 ± 0.71 | 10.39 ± 0.41 |
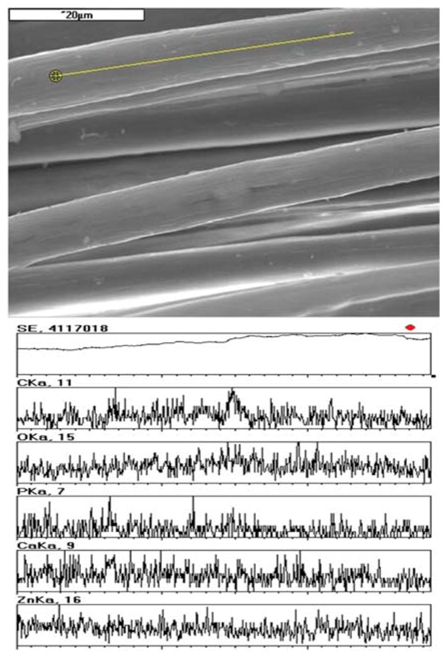
| Type of fabric | Needle type | Number of needling operations [1/cm2] | Depth of needling [mm] | Area density [g/m2] | Fabric thickness [mm] | Pore area [m2/g] | Average pore size [µm] |
|---|---|---|---|---|---|---|---|
| Zinc alginate fibres with TCP | 15 × 18 × 40 × 3.5 RB | 30 | 12 | 56 | 1.36 | 0.353 | 74.8 |
2.2. Properties of Fibrous Nonwoven Fabrics Obtained by Electrospinning
| Type of polymer | Type of solvent | Cp [%] | T [°C] | Φ [%] | U [kV] | h [cm] | ω [1/min] | P [obr/min] |
|---|---|---|---|---|---|---|---|---|
| PCL | dichloromethane | 7.5 | 13.6 | 48 | 35 | 50 | 20 | 1.1 |
| DBC | ethyl alcohol | 6.0 | 23.0 | 48 | 28 | 15 | 20 | 5.5 |
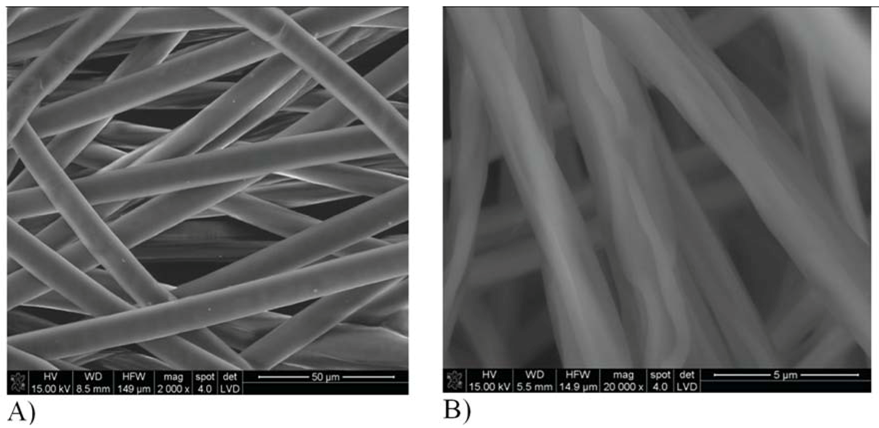
| Type of fabric | Wetting angle [°] | Surface roughness in central part | Surface roughness in extreme part | ||||
|---|---|---|---|---|---|---|---|
| Ra [µm] | Rt [µm] | Rz [µm] | Ra [µm] | Rt [µm] | Rz [µm] | ||
| PCL | 131 ± 2.24 | 1.08 ± 0.12 | 0.83 ± 0.02 | 0.64 ± 0.06 | 1.84 ± 0.45 | 1.31 ± 0.29 | 1.46 ± 0.81 |
| DBC | 98 ± 1.78 | 0.96 ± 0.16 | 0.98 ± 0.09 | 0.54 ± 0.08 | 0.61 ± 0.02 | 0.87 ± 0.03 | 0.64 ± 0.12 |

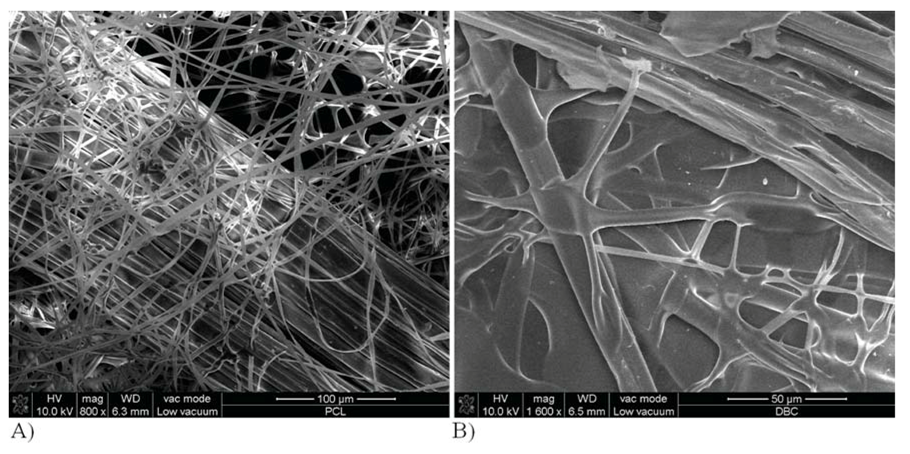
2.3. Properties of Variant I Composites
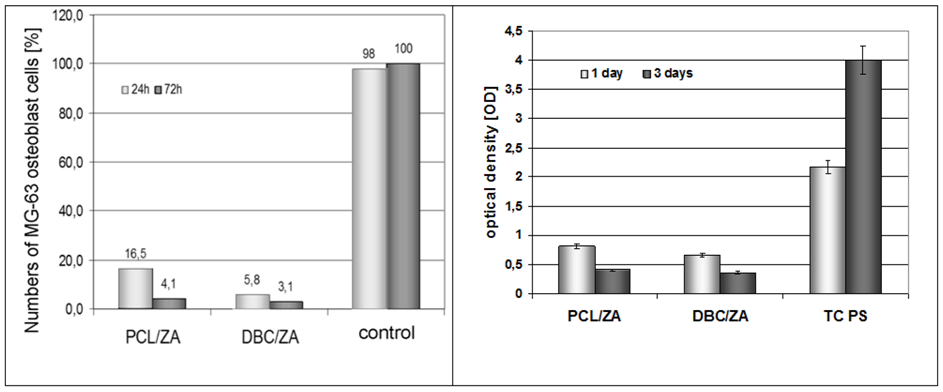
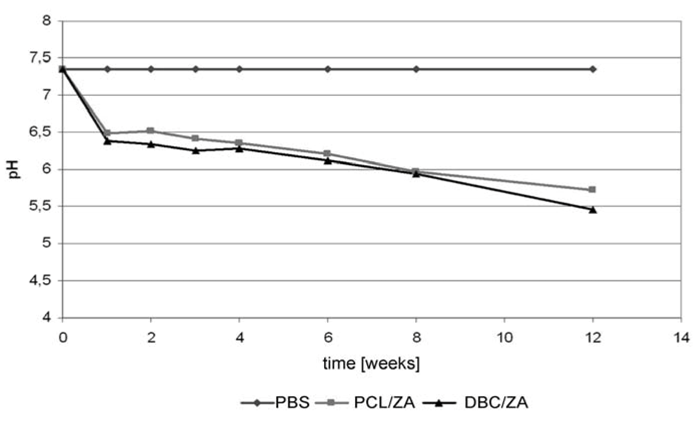
2.4. Properties of Variant II Composites
- nonwoven fabric (electrospinning) with the addition of micrometric fibres in a ZA:CA ratio of 5:95, proportion of micro fibres 20%.
- nonwoven fabric (electrospinning) with the addition of micrometric fibres in a ZA:CA ratio of 5:95, proportion of micro fibres 28%.
| Type of fabric | pH of immersion medium | Wetting angle [°] | Surface roughness | |||
|---|---|---|---|---|---|---|
| water | PBS | Ra [µm] | Rt [µm] | Rz [µm] | ||
| PCL/ZA:CA | 6.58 | 7.38 | 78.4 ± 2.3 | 8.85 ± 0.26 | 61.39 ± 1.84 | 47.12 ± 1.31 |
| DBC/ZA:CA | 6.59 | 7.39 | 58.2 ± 1.4 | 3.47 ± 0.14 | 46.65 ± 0.86 | 20.18 ± 0.62 |
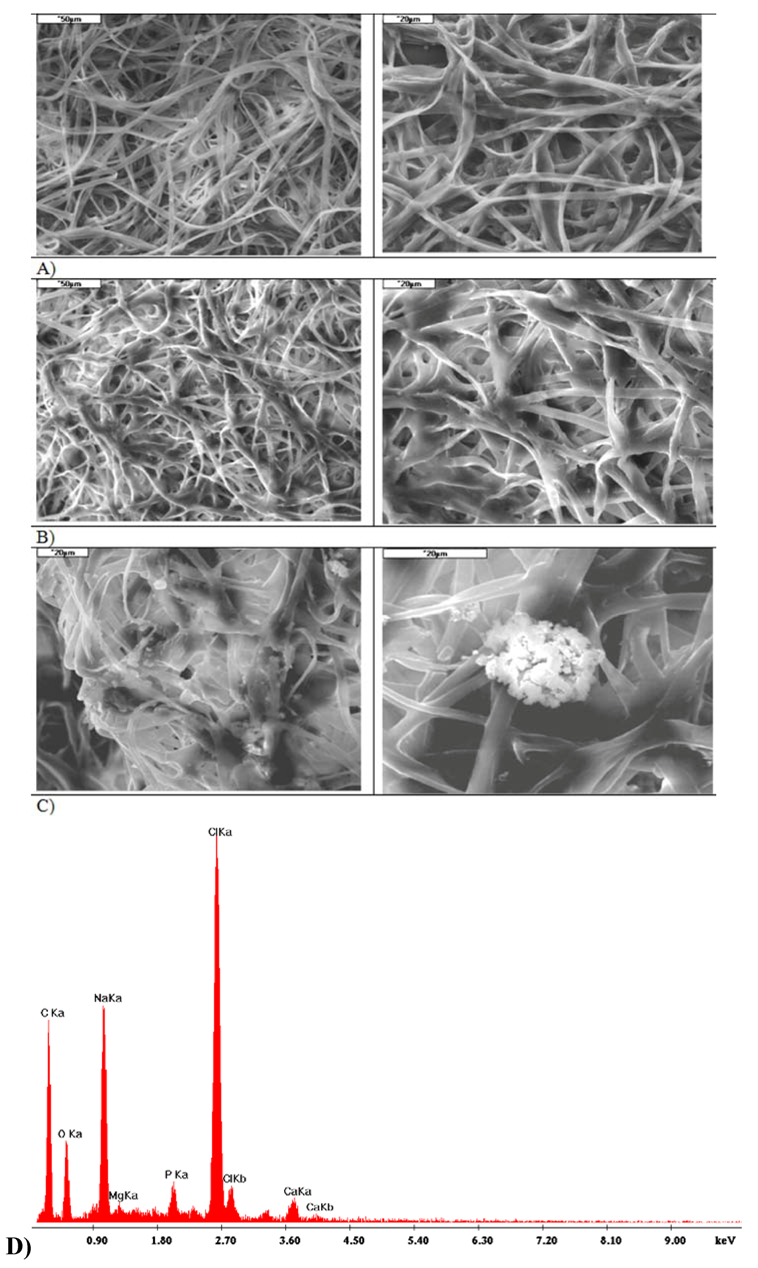
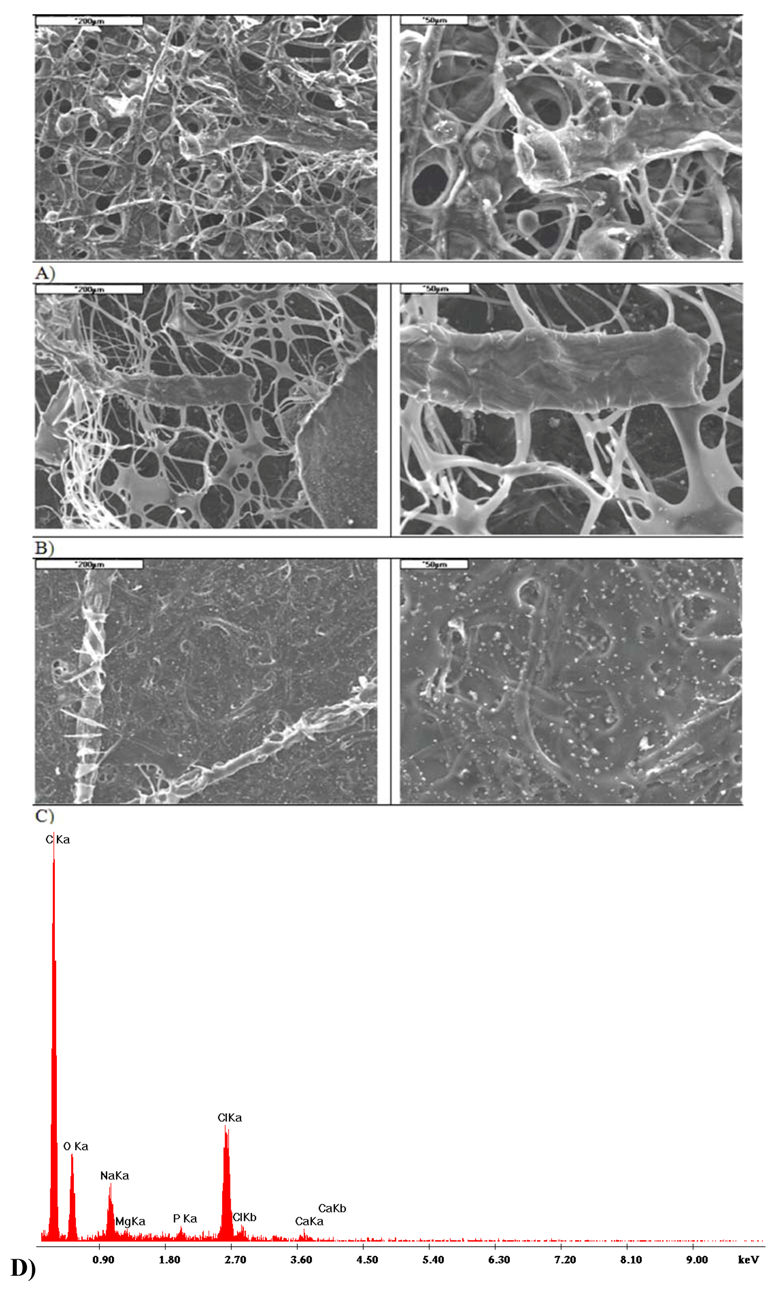

2.5. Comparative Analysis of I and II Variant Composites
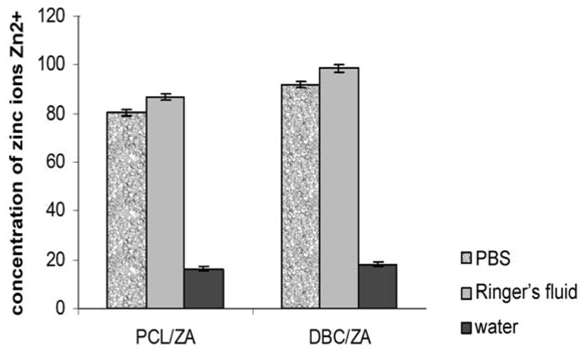
3. Experimental
3.1. Raw Materials
3.2. Types of Composites

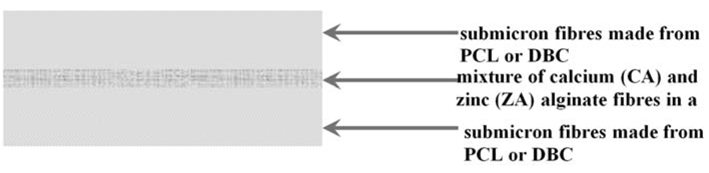
3.3. Production of Alginate Fibres
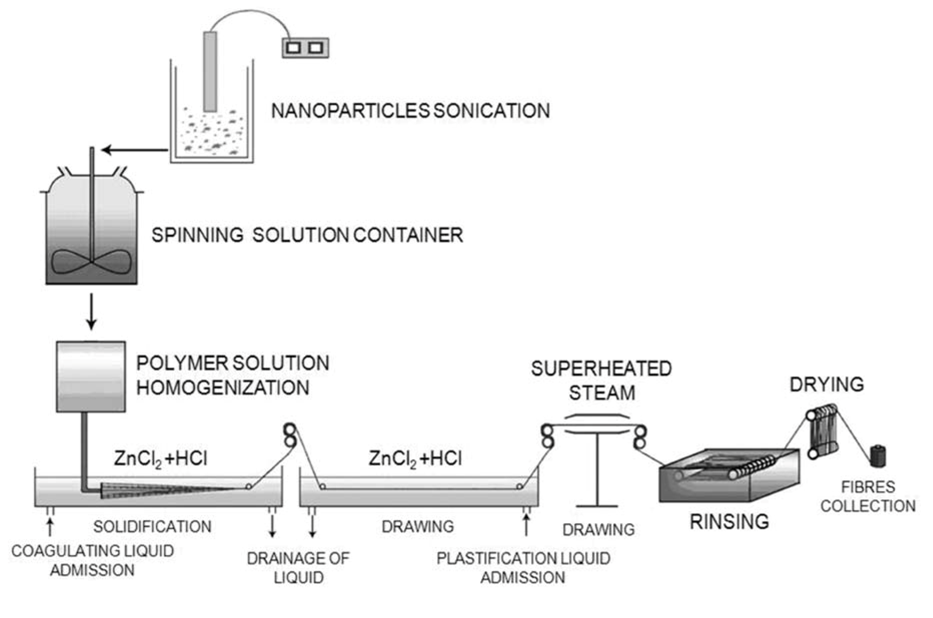
3.4. Production of Fabrics from DBC and PCL

3.5. Testing Methods
4. Conclusions
Acknowledgments
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Composite Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysacharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Moe, S.; Draget, K.; Skjåk-Bræk, G.; Smidsrød, O. Alginates. In Food Polysaccharides and Their Applications; Stephen, A.M., Ed.; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Fenton, J.C.; Keys, A.F.; Mahoney, P.M.J. Alginate fabric, its use in wound dressings and surgical haemostats and a process for its manufacture. U.S. Patent 5,714,232, 1998. [Google Scholar]
- Hench, L.L; Jones, J.R. Biomaterials, Artificial Organs and Tissue Engineering; Woodhead Publishing Limited: Cambridge, UK, 2005. [Google Scholar]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S., II; Cho, Ch.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef]
- Draczynski, Z. Honeybee corpses as an available source of chitin. J. Appl. Polym. Sci. 2008, 109, 1974–1981. [Google Scholar] [CrossRef]
- Draczynski, Z. Synthesis and solubility properties of chitin acetate/butyrate copolymers. J. Appl. Polym. Sci. 2008, 122, 175–182. [Google Scholar] [CrossRef]
- Stawski, D.; Rabiej, S.; Herczyńska, L.; Draczynski, Z. Thermogravimetric analysis of chitins of different origin. J. Therm. Anal. Cal. 2008, 93, 489–494. [Google Scholar] [CrossRef]
- Krucińska, I.; Szosland, L.; Cisło, R.; Błasińska, A.; Komisarczyk, A.; Chilarski, A.; Bilska, J.; Pilas, B. A wound dressing material of dibutyrylchityn reconstituted therefrom. Patent No 2005.
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.Ch.; Lee, H.-S.; Oh, J.-S.; Akaike, T.; Cho, Ch.-S. A nowel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, L.; Shen, X.; Tan, Z. Preparation of polycaprolactone tissue engineering scaffolds by improved solvent casting/particulate leaching method. J. Macromol. Sci. B 2006, 45, 1171–1181. [Google Scholar] [CrossRef]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite/poly(e-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef]
- Low, S.W.; Ng, Y.J.; Yeo, T.T.; Chou, N. Use of Osteoplug polycaprolactone implants as novel burr-hole covers. Singapore Med. J. 2009, 50, 777–780. [Google Scholar]
- Vollath, D. Nanomaterials. In An Introduction to Synthesis, Properties and Applications; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Boguń, M.; Mikołajczyk, T.; Rabiej, S. Effect of fomation conditions on the structure and properties of nanocomposite alginate fibers. J. Appl. Polym. Sci. 2009, 114, 70–82. [Google Scholar] [CrossRef]
- Mikołajczyk, T.; Bogun, M.; Kurzak, A.; Szparaga, G. Zinc alginate fibres with a tricalcium phosphate (TCP) nanoadditive. Fibres Text. East. Eur. 2009, 17, 12–18. [Google Scholar]
- Boguń, M.; Rabiej, S. The influence of fiber formation conditions on the structure and properties of nanocomposite alginate fibers containing tricalcium phosphate or montmorillonite. Polym. Compos. 2010, 31, 1321–1331. [Google Scholar]
- Boguń, M. Nanokompozytowe włókna alginianowe i kompozyty z ich udziałem do zastosowań w inżynierii biomateriałowej; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2010. [Google Scholar]
- Bogun, M.; Stodolak, E.; Mikolajczyk, T.; Krucinska, I.; Błazewicz, M. Biodegradable Composites Based on the Alginate Fibres and Polycaprolactone, and Dibutyrylchitin for Applications in Regeneration of the Osseous Tissue. In Proceedings of FIBREMED 2011, Tampere, Finland, 28–30 June 2011.
- Beachleya, V.; Wen, X. Polymer nanofibrous structures: Fabrication, Biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef]
- Xiang, P.; Li, M.; Zhang, Ch.-Y.; Chen, D.-L.; Zho, Z.-H. Cytocompatibility of electrospun nanofiber tubular scaffolds for small diameter tissue engineering blood vessels. Inter. J. Biol. Macrom. 2011, 49, 281–288. [Google Scholar] [CrossRef]
- Krucinska, I.; Gliscinska, E.; Chrzanowski, M. System for electrospinning fibres. Patent No 2010.
- Krucinska, I.; Komisarczyk, A.; Chrzanowski, M.; Gliscinska, E.; Wrzosek, H. Electrostatic field in electrospinning with a multicapillary head-modeling and experiment. Fibres Text. East. Eur. 2009, 17, 38–44. [Google Scholar]
- Krucinska, I.; Gliscinska, E.; Chrzanowski, M.; Komisarczyk, A. Multi-nozzle Laboratory Stand for Electrospinning Process. In Proceedings of 10th World Textile Conference, AUTEX 2010, Vilnius, Lithuania, 20–23 June 2010.
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone activity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boguń, M.; Krucińska, I.; Kommisarczyk, A.; Mikołajczyk, T.; Błażewicz, M.; Stodolak-Zych, E.; Menaszek, E.; Ścisłowska-Czarnecka, A. Fibrous Polymeric Composites Based on Alginate Fibres and Fibres Made of Poly-ε-caprolactone and Dibutyryl Chitin for Use in Regenerative Medicine. Molecules 2013, 18, 3118-3136. https://doi.org/10.3390/molecules18033118
Boguń M, Krucińska I, Kommisarczyk A, Mikołajczyk T, Błażewicz M, Stodolak-Zych E, Menaszek E, Ścisłowska-Czarnecka A. Fibrous Polymeric Composites Based on Alginate Fibres and Fibres Made of Poly-ε-caprolactone and Dibutyryl Chitin for Use in Regenerative Medicine. Molecules. 2013; 18(3):3118-3136. https://doi.org/10.3390/molecules18033118
Chicago/Turabian StyleBoguń, Maciej, Izabella Krucińska, Agnieszka Kommisarczyk, Teresa Mikołajczyk, Marta Błażewicz, Ewa Stodolak-Zych, Elżbieta Menaszek, and Anna Ścisłowska-Czarnecka. 2013. "Fibrous Polymeric Composites Based on Alginate Fibres and Fibres Made of Poly-ε-caprolactone and Dibutyryl Chitin for Use in Regenerative Medicine" Molecules 18, no. 3: 3118-3136. https://doi.org/10.3390/molecules18033118
APA StyleBoguń, M., Krucińska, I., Kommisarczyk, A., Mikołajczyk, T., Błażewicz, M., Stodolak-Zych, E., Menaszek, E., & Ścisłowska-Czarnecka, A. (2013). Fibrous Polymeric Composites Based on Alginate Fibres and Fibres Made of Poly-ε-caprolactone and Dibutyryl Chitin for Use in Regenerative Medicine. Molecules, 18(3), 3118-3136. https://doi.org/10.3390/molecules18033118






