Abstract
A single fraction of essential oil can often contain hundreds of compounds. Despite of the technical improvements and the enhanced selectivity currently offered by the state-of-the-art gas chromatography (GC) and mass spectrometry (MS) instruments, the complexity of essential oils is frequently underestimated. Comprehensive two-dimensional GC coupled to time-of-flight MS (GC×GC-TOFMS) was used to improve the chemical characterization of ylang-ylang essential oil fractions recently reported in a previous one-dimensional (1D) GC study. Based on both, the enhanced chromatographic separation and the mass spectral deconvolution, 161 individual compounds were identified and labeled as potentially characteristic analytes found in both low and high boiling fractions issued from distillation of mature ylang-ylang flowers. Compared to the most recent full GC-MS characterization, this represents 75 new compounds, essentially consisting of terpenes, terpenoid esters, and alcohols.
1. Introduction
Ylang-ylang essential oil, together with jasmine, rose and neroli, is one of the few essential oils extracted from flowers that are exploited at a large scale. Ylang-ylang essential oil is distilled from the mature fresh flowers of the Annonaceae family tropical tree Cananga odorata [Lam.] Hook f. and Thomson forma genuina. The plant originates from the Indonesian archipelago, but is currently exploited in the Western Indian Ocean islands, mainly in Comoros Islands, Mayotte and Madagascar [,]. Ylang-ylang essential oil is mainly used by the cosmetic industry in applications ranging from high grade perfume conception to soap manufacture, but also, to a lesser extent, in aromatherapy or even as a food ingredient [,,,]. In addition to a great cultural and tourism value of the plant, the production of ylang-ylang essential oil plays an important economic role as the oil represents the second most important export product for the Comoros Islands, after clovers [,].
Ylang-ylang essential oil production has the particularity of relying on a fractionation based on distillation times, resulting in four to five grades of oil that have different commercial applications. Commercial grades strongly differ in their chemical composition, the first fraction being richer in very volatile compounds like esters, aldehydes or alcohols, while the last fraction is richer in less volatile compounds like sesquiterpenes []. In the past, the chemical composition of ylang-ylang essential oil fractions has been studied by gas chromatography coupled to mass spectrometry (GC-MS), especially quadrupole analyzers, revealing its complexity [,]. This permitted researchers to highlight the major components of the oil and use them for quality and/or origin control. In the aroma and flavor industries, the standard published by the French standardization system [AFNOR, ISO 3063:2004(E)] is considered to be the reference. This ISO standard is based on the measurement and comparison of 15 major compounds, but only allows distinguishing between two groups of essential oils, based on geographic origin: Mayotte/Comoros Islands and Madagascar [].
Despite its economic and social importance, little is known about the chemical variability of ylang-ylang essential oil and factors potentially causing it. In a previous work based on the monitoring of the 15 “AFNOR” compounds, we highlighted significant variations in oil compositions between islands, but also within plantations on a same island. Moreover, the genetic differentiation pattern was shown to be different from the chemical differentiation pattern, which indicated a possible important environmental effect (climate, edaphic conditions, soil composition, solar exposition and agronomic practices) []. To refine those findings, we recently carried out a deeper and more exhaustive GC-MS investigation of the chemical characterization of four fractions of ylang-ylang essential oil distillated in controlled conditions in four different locations (Grand Comore, Mayotte, Nosi Bé and Ambanja). As a result, a total of 119 potentially characteristic compounds were reported, among which 32 compounds had never been reported before. Quantification performed by GC coupled to flame ionization detection (FID) further allowed us to build regression trees that permitted to differentiate the four geographic origins for the four fractions []. The chemical polymorphism that was highlighted in that study represents an interesting tool for perfumers and flavorists in search of new specific raw material for their compositions.
Comprehensive two-dimensional gas chromatography (GC×GC) is an already well-established technique used to perform separations of highly complex mixtures of GC-amenable compounds [,,]. It has successfully been applied for the analysis of specific essential oils [,,]. As compared to one-dimensional gas chromatography (1DGC), the use of two separation mechanisms results in a significant increase of peak capacity, whereas modulation can provide an improvement in sensitivity []. Coupling this technique to time-of-flight mass spectrometer (TOFMS), capable of acquiring up to 500 full-range spectra per second [], offers the possibility of mass deconvolution—an additional tool to resolve coelutions in the mass spectral domain.
Based on the complexity of the 1DGC chromatogram generated in our previous study [], we investigated the use of GC×GC-TOFMS for a more exhaustive analysis of selected ylang-ylang essential oil fractions with the aim of improving the differentiation approach. For this conceptual study, we focused our efforts on the first and the last fractions issued from the distillation of an ylang-ylang essential oil produced from fresh flowers collected in Mayotte.
2. Results and Discussion
Figure 1 represents the total ion current (TIC) surface plot of the signal recorded for the most volatile fraction of the ylang-ylang essential oil from Mayotte (fraction 1). The background contour line represents the reconstructed 1D trace, issued from the summation of chromatographic signals collected along the second dimension retention time axis (2tR), and reflects the separation that could be achieved using classical 1DGC.
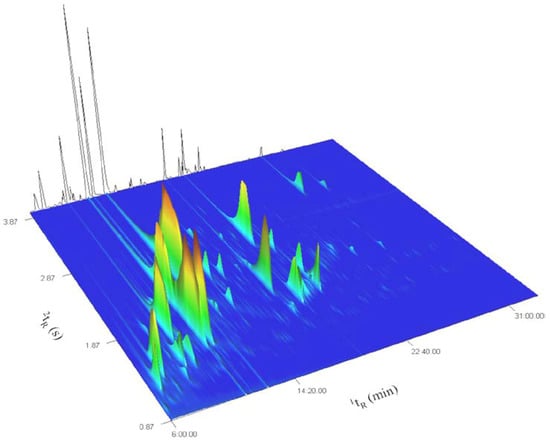
Figure 1.
GC×GC TOFMS TIC surface plot of fraction 1 of ylang-ylang essential oil.
Several 1DGC coelutions are visible and the number of peaks separated in the two-dimensional chromatographic space clearly surpasses the number of peaks separated in the reconstructed 1DGC trace. This includes situations where peaks are present at levels that differ of several orders of magnitude, for which the identification of the low level coeluters would have been particularly difficult, even with the help of mass spectral deconvolution. The use of the combination of a low polar 5% phenyl phase for the first dimension (1D) with a medium polar 50% phenyl polysilphenylene phase for the second dimension (2D) allowed an efficient use of the available chromatographic space. Peak widths at half height in 2D ranged from 100 ms to 150 ms and were distributed over 3 s of the 4 s modulation period (PM). Tailing was observed for some of the most abundant compounds in 2D. This resulted in a slight wrap-around of the tails but did not practically affect the separation efficiency as it did not create coelution issues.
Several hundred peaks were detected and their deconvoluted mass spectral signatures were processed against mass spectrometry libraries. In several cases, it appeared that, despite the use of two chromatographic separation axes, some peaks were still coeluting and were separated by mass spectral deconvolution. The TOFMS acquisition rate of 100 spectra s−1 permitted to differentiate between peaks with the same first dimension retention times (1tR) (identical linear retention indices (IT)) but exhibiting slight differences in 2tR values. Figure 2 illustrates such a case (1tR(A) = 1tR(B), 2tR(A) = 2.32 s and 2tR(B) = 2.36 s) where the peak apexes of compounds A and B were only 40 ms apart of each other.
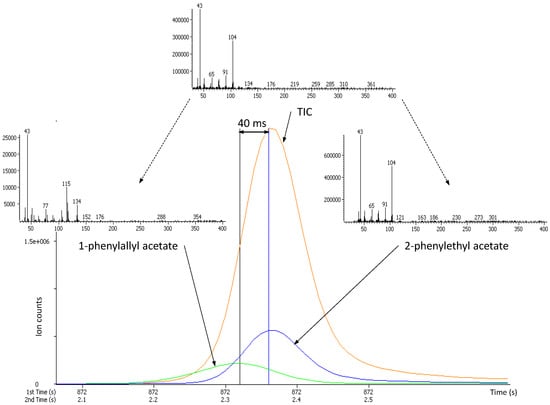
Figure 2.
Deconvoluted ion current (DIC) traces for two coeluting esters. DICs were reconstructed based on unique masses of m/z 115 for 1-phenylallyl acetate and m/z 104 for 2-phenylethyl acetate; the signal for 1-phenylallyl acetate has been magnified 20 times for clarity. The mass spectrum on the top represents the raw data. The mass spectra on the left and on the right represent the deconvoluted signals of 1-phenylallyl acetate and 2-phenylethyl acetate, respectively.
In such a critical scenario (only four full mass spectra acquired in between peak apexes), the deconvolution software was able to separate two MS signals and further successively identified them by mass library searching (forward-reverse similarities of 826–897 and forward-reverse similarities of 935–952, respectively for compound A and B). The lower score of library matching for compound A (1-phenylallyl acetate), compared to compound B (2-phenylethyl acetate) is, most probably, to be related to the much lower concentration, resulting in lowering the intensity of the MS signal.
After first data processing, peak tables for fractions 1 and 4 accounted for 681 and 839 hits, respectively. During the second processing, artifacts and column bleeding were removed, leaving 370 and 446 hits for fractions 1 and 4, respectively. From those, 161 individual compounds were identified and labeled as potentially characteristic analytes found in either fraction 1 or 4. This selection was based on manual review of the large peak tables, focusing on signals that exhibited signal to noise ratio (S/N) values greater than 200, and forward and reverse library match similarity factor over 750 and 800, respectively. Selected compounds, along with their calculated IT, 1tR, 2tR, molecular formula, and a relative abundance are listed in Table 1. As a comparison, the previous GC-MS study [] reported 96 compounds present in either fraction 1 or 4. Amongst those 96 compounds, 79 were also found in the list of 161 compounds generated from GC×GC-TOFMS analysis.

Table 1.
Selected compounds from fractions 1 and 4 of ylang-ylang essential oil.
| No. | Name | IT | 1tR (min:s), 2tR (s) | Formula | Relative abundance % | |
|---|---|---|---|---|---|---|
| Fraction 1 | Fraction 4 | |||||
| 1 | 3-hexen-1-ol # | 867 | 6:16, 1.28 | C6H12O | tr | - |
| 2 | 3-methyl-3-buten-1-ol acetate | 885 | 6:36, 1.26 | C7H12O2 | 2.16 | 0.22 |
| 3 | heptanal | 907 | 7:00, 1.29 | C7H14O | 0.05 † | - |
| 4 | 3-methyl-2-butenyl acetate | 923 | 7:16, 1.38 | C7H12O2 | 4.24 | 0.32 |
| 5 | α-pinene | 941 | 7:36, 1.17 | C10H16 | 0.1 | 0.04 |
| 6 | benzaldehyde | 967 | 8:04, 1.87 | C7H6O | 0.21 | 0.05 |
| 7 | sabinene # | 978 | 8:16, 1.27 | C10H16 | tr | - |
| 8 | 6-methyl-5-hepten-2-one | 986 | 8:24, 1.52 | C8H14O | 0.07 | 0.03 † |
| 9 | β-myrcene | 993 | 8:32, 1.27 | C10H16 | 0.29 | tr |
| 10 | decane * | 1000 | 8:40, 1.13 | C10H22 | tr | 0.03 |
| 11 | ( 3Z)-3-hexenyl acetate | 1003 | 8:44, 1.47 | C8H14O2 | 0.5 | 0.03 |
| 12 | n-hexyl acetate | 1009 | 8:52, 1.42 | C8H16O2 | 0.97 | 0.07 |
| 13 | α-phellandrene * | 1012 | 8:56, 1.33 | C10H16 | tr | tr |
| 14 | p-cresyl methyl ether | 1025 | 9:12, 1.88 | C8H10O | 9.7 | 1.63 |
| 15 | β-limonene* | 1034 | 9:24, 1.36 | C10H16 | 0.13 | 0.15 |
| 16 | 1,8-cineole | 1037 | 9:28, 1.45 | C10H18O | 1.11 | 0.22 |
| 17 | benzyl alcohol | 1037 | 9:28, 2.13 | C7H8O | 0.53 | tr |
| 18 | β-ocimene * | 1046 | 9:40, 1.38 | C10H16 | 0.07 | tr |
| 19 | ester (MW 174) | 1046 | 9:40, 1.71 | C8H14O4 | 0.28 | tr |
| 20 | phenyl acetaldehyde | 1046 | 9:40, 2.16 | C8H8O | 0.17 | tr † |
| 21 | p-cresol | 1070 | 10:12, 2.10 | C7H8O | 0.19 | 0.05 |
| 22 | cis-linalool oxide (furanoid) | 1073 | 10:16, 1.53 | C10H18O2 | 0.02 | - |
| 23 | trans-linalool oxide (furanoid) | 1088 | 10:36, 1.59 | C10H18O2 | tr † | - |
| 24 | 2-methoxyphenol | 1089 | 10:36, 2.18 | C7H8O2 | 0.7 | tr |
| 25 | terpinolene # | 1097 | 10:48, 1.78 | C10H16 | 0.07 | - |
| 26 | methyl benzoate | 1098 | 10:48, 2.25 | C8H8O2 | 6.05 | 0.69 |
| 27 | undecane * | 1100 | 10:36, 1.24 | C11H24 | - | tr |
| 28 | linalool | 1100 | 10:52, 1.76 | C10H18O | 8.95 | 0.34 |
| 29 | levoglucosenone * | 1101 | 10:52, 3.22 | C6H6O3 | tr | 0.07 |
| 30 | monoterpene (MW 136) * | 1112 | 11:08, 1.34 | C10H16 | 0.05 | 0.42 |
| 31 | methyl 3-methylbutanoate * | 1117 | 11:16, 1.58 | C6H12O2 | tr | - |
| 32 | methyl caprylate * | 1123 | 11:24, 1.54 | C9H18O2 | 0.43 | 7.24 |
| 33 | α-pyronene * | 1126 | 11:28, 1.52 | C10H16 | 0.04 | - |
| 34 | plinol A * | 1132 | 11:36, 1.67 | C10H18O | 0.14 | - |
| 35 | monoterpene (MW 136) * | 1134 | 11:40, 1.39 | C10H16 | 0.03 | 0.17 |
| 36 | phenylacetonitrile | 1138 | 11:44, 2.79 | C8H7N | 0.02 | tr |
| 37 | veratrole | 1141 | 11:48, 2.39 | C8H10O2 | 0.07 | tr † |
| 38 | plinol D * | 1149 | 12:00, 1.73 | C10H18O | 0.05 | - |
| 39 | 1-phenyl-2-propen-1-ol * | 1152 | 12:04, 2.28 | C9H10O | tr | tr |
| 40 | benzyl acetate | 1167 | 12:24, 2.57 | C9H10O2 | 27.48 | 0.07 |
| 41 | ethyl benzoate | 1175 | 12:36, 2.15 | C9H10O2 | 0.42 | - |
| 42 | 2-methoxy-4-methylphenol | 1192 | 13:00, 2.29 | C8H10O2 | 0.03 | tr |
| 43 | methyl salicylate | 1198 | 13:08, 2.30 | C8H8O3 | 0.32 | 0.15 |
| 44 | dodecane * | 1200 | 13:12, 1.27 | C12H26 | tr | tr |
| 45 | α-terpineol | 1200 | 13:12, 1.88 | C10H18O | 0.26 | tr |
| 46 | methyl chavicol | 1201 | 13:12, 2.10 | C10H12O | 0.06 | - |
| 47 | 1-methoxy-4-propylbenzene * | 1209 | 13:24, 1.96 | C10H14O | tr | tr |
| 48 | nerol # | 1225 | 13:48, 1.82 | C10H18O | tr | - |
| 49 | linalyl acetate # | 1249 | 14:24, 1.63 | C12H20O2 | 0.03 | - |
| 50 | geraniol | 1249 | 14:24, 1.92 | C10H18O | 0.36 | tr |
| 51 | 1-phenylallyl acetate * | 1255 | 14:32, 2.32 | C11H12O2 | tr | tr |
| 52 | 2-phenylethyl acetate | 1255 | 14:32, 2.36 | C10H12O2 | 0.57 | tr |
| 53 | 4-methoxy benzaldehyde * | 1261 | 14:40, 2.90 | C8H8O2 | tr | - |
| 54 | geranial | 1268 | 14:52, 2.04 | C10H16O | 0.03 | tr † |
| 55 | diethyl 1,5-pentanedioate * | 1274 | 15:00, 2.14 | C9H16O4 | tr | tr |
| 56 | trans-anethol | 1290 | 15:24, 2.31 | C10H12O | 0.36 | tr |
| 57 | 1 H-indole * | 1299 | 15:36, 3.28 | C8H7N | tr | tr |
| 58 | 2-phenylnitroethane | 1301 | 15:40, 2.96 | C8H9NO2 | 0.25 | 0.03 |
| 59 | vinyl butyrate * | 1309 | 15:52, 2.07 | C6H10O2 | tr | tr |
| 60 | cinnamyl alcohol | 1309 | 15:52, 2.81 | C9H10O | tr | tr † |
| 61 | p-vinylguaiacol | 1314 | 16:00, 2.56 | C9H10O2 | tr † | tr |
| 62 | sesquiterpene (MW 204) * | 1318 | 15:48, 2.11 | C15H24 | - | tr |
| 63 | diethyl ( 2E)-3-methyl-2-pentanedioate * | 1325 | 16:16, 2.17 | C10H16O4 | tr | 0.02 |
| 64 | 2,5-dimethyl-3-methylene-1,5-heptadiene * | 1330 | 16:24, 1.54 | C10H16 | tr | 0.04 |
| 65 | methyl 2-methoxybenzoate # | 1334 | 16:28, 2.97 | C9H10O3 | tr | tr |
| 66 | bicycloelemene | 1338 | 16:36, 1.58 | C15H24 | 0.03 | 0.29 |
| 67 | 5-indanol | 1339 | 16:36, 2.66 | C9H10O | tr | tr |
| 68 | ester (MW 190) * | 1341 | 16:40, 2.14 | C12H14O2 | tr | tr |
| 69 | benzyl butyrate | 1347 | 16:48, 2.32 | C11H14O2 | 0.06 | tr † |
| 70 | methyl 2-aminobenzoate * | 1347 | 16:48, 2.99 | C8H9NO2 | tr | tr |
| 71 | α-cubebene* | 1354 | 17:00, 1.53 | C15H24 | tr | 0.09 |
| 72 | eugenol | 1355 | 17:00, 2.47 | C10H12O2 | tr | - |
| 73 | benzenepropanol, acetate * | 1371 | 17:24, 2.39 | C11H14O2 | 0.03 | tr |
| 74 | neryl acetate | 1373 | 17:28, 1.95 | C12H20O2 | 2.74 | 0.21 |
| 75 | geranyl acetate | 1376 | 17:32, 2.00 | C12H20O2 | 2 | - |
| 76 | methyl 4-methoxybenzoate | 1377 | 17:32, 2.79 | C9H10O3 | 0.08 | tr |
| 77 | α-ylangene | 1378 | 17:16, 1.67 | C15H24 | - | 0.06 |
| 78 | butyl benzoate | 1376 | 17:32, 2.31 | C11H14O2 | 0.04 | tr † |
| 79 | α-copaene | 1384 | 17:44, 1.59 | C15H24 | 0.11 | 0.76 |
| 80 | sesquiterpene (MW 204) * | 1389 | 17:32, 1.72 | C15H24 | - | 0.07 |
| 81 | β-bourbonene | 1392 | 17:56, 1.64 | C15H24 | tr † | tr |
| 82 | β-cubebene | 1395 | 18:00, 1.64 | C15H24 | 0.13 | 0.56 |
| 83 | vanillin | 1399 | 18:04, 3.30 | C8H8O3 | tr | 0.05 |
| 84 | tetradecane * | 1400 | 18:08, 1.35 | C14H30 | tr | tr |
| 85 | sesquiterpene (MW 204) * | 1403 | 17:52, 1.71 | C15H24 | - | tr |
| 86 | 2-methoxy-4-(1-propenyl)-phenol * | 1407 | 18:16, 2.59 | C10H12O2 | tr | tr |
| 87 | p-anisyl acetate | 1418 | 18:32, 2.77 | C10H12O3 | 0.04 | tr |
| 88 | β-ylangene * | 1429 | 18:48, 1.79 | C15H24 | 1.71 | 0.73 |
| 89 | β-copaene | 1440 | 19:04, 1.78 | C15H24 | 0.72 | 0.12 |
| 90 | sesquiterpene (MW 204) * | 1442 | 18:48, 1.88 | C15H24 | - | 7.48 |
| 91 | cinnamyl acetate | 1447 | 19:12, 2.78 | C11H12O2 | 0.9 | 1.59 |
| 92 | 3-methyl-3-butenyl benzoate * | 1449 | 19:16, 2.37 | C12H14O2 | 0.03 | tr |
| 93 | isoeugenol | 1452 | 19:20, 2.65 | C10H12O2 | 0.63 | 0.38 |
| 94 | β-caryophyllene | 1455 | 19:24, 1.79 | C15H24 | 0.37 | 0.3 |
| 95 | aromandendrene* | 1460 | 19:32, 1.79 | C15H24 | 0.06 | 1.53 |
| 96 | α-humulene | 1467 | 19:24, 1.96 | C15H24 | - | 6.2 |
| 97 | isogermacrene-D | 1472 | 19:48, 1.77 | C15H24 | 0.03 | 1.83 |
| 98 | α-ionene* | 1483 | 20:04, 1.78 | C13H18 | tr † | tr |
| 99 | germacrene-D | 1495 | 20:20, 1.80 | C11H22O | tr | 2.76 |
| 100 | 3-methyl-2-butenyl benzoate | 1492 | 20:16, 2.47 | C12H14O2 | 0.39 | 0.21 |
| 101 | pentadecane* | 1500 | 20:28, 1.41 | C15H32 | tr | tr |
| 102 | ( Z,E)-α-farnesene* | 1503 | 20:16, 1.87 | C15H24 | - | 0.19 |
| 103 | α-muurolene | 1503 | 20:16, 2.03 | C15H24 | - | 0.31 |
| 104 | ( E,E)-α-farnesene | 1506 | 20:36, 1.79 | C15H24 | 1.62 | 10.1 |
| 105 | β-curcumenene | 1512 | 20:44, 1.90 | C15H24 | 0.39 | 2.73 |
| 106 | γ-cadinene | 1521 | 20:56, 1.88 | C15H24 | tr | 2.14 |
| 107 | δ-cadinene | 1527 | 21:04, 1.92 | C15H24 | 0.28 | 0.61 |
| 108 | guaiacyl acetone * | 1528 | 21:04, 3.26 | C10H12O3 | tr | tr |
| 109 | zonarene | 1547 | 21:32, 1.87 | C15H24 | tr † | tr |
| 110 | sesquiterpene (MW 202) * | 1540 | 21:04, 2.05 | C15H22 | - | tr |
| 111 | benzyl 4-methylpentanoate * | 1548 | 21:32, 2.33 | C13H18O2 | tr | tr |
| 112 | elemol | 1556 | 21:44, 1.99 | C15H26O | tr | 0.02 |
| 113 | cis-3-hexenyl benzoate | 1577 | 22:12, 2.37 | C13H16O2 | tr | tr |
| 114 | germacren D-4-ol * | 1589 | 22:28, 2.02 | C15H26O | 0.06 | tr |
| 115 | caryophyllene oxide | 1595 | 22:36, 2.17 | C15H24O | 0.06 | tr |
| 116 | hexadecane * | 1600 | 22:24, 1.51 | C16H34 | tr | tr |
| 117 | guaiol | 1607 | 22:52, 2.05 | C15H26O | tr | 0.46 |
| 118 | isoeugenol acetate | 1607 | 22:52, 2.89 | C12H14O3 | tr | tr |
| 119 | oxygenated sesquiterpene (MW 220) * | 1625 | 22:56, 2.22 | C15H24O | - | 0.06 |
| 120 | sesquiterpene (MW 206) * | 1625 | 22:56, 2.70 | C15H26 | - | tr |
| 121 | copaborneol | 1637 | 23:32, 2.11 | C15H26O | tr | 0.03 |
| 122 | sesquiterpene (MW 202) * | 1643 | 23:20, 2.33 | C15H22 | - | tr |
| 123 | τ-muurolol | 1652 | 23:52, 2.17 | C15H26O | 0.06 | 4.43 |
| 124 | α-cadinol | 1664 | 24:08, 2.23 | C15H26O | 0.07 | 1.52 |
| 125 | oxygenated sesquiterpene (MW 222) * | 1671 | 23:56, 2.58 | C15H26O | - | tr |
| 126 | bulnesol * | 1673 | 24:00, 2.34 | C15H26O | tr | 0.05 |
| 127 | sesquiterpene (MW 200) * | 1674 | 24:00, 2.63 | C15H20 | - | tr |
| 128 | farnesene* | 1679 | 24:08, 2.35 | C15H24 | - | 0.11 |
| 129 | sesquiterpene (MW 204) * | 1682 | 24:12, 2.01 | C15H24 | - | 0.02 |
| 130 | ( 2Z,6E)-farnesol * | 1686 | 24:16, 2.37 | C15H26O | - | 0.02 |
| 131 | sesquiterpene (MW 204)* | 1686 | 24:16, 2.76 | C15H24 | - | 0.02 |
| 132 | cetene* | 1694 | 24:48, 1.52 | C16H32 | tr | 0.04 |
| 133 | oxygenated sesquiterpene (MW 222)* | 1695 | 24:28, 2.49 | C15H26O | - | tr |
| 134 | ester (MW 196)* | 1704 | 24:40, 1.99 | C12H20O2 | - | tr |
| 135 | globulol* | 1708 | 24:44, 2.53 | C15H26O | - | tr |
| 136 | ( 2Z,6Z)-farnesol* | 1717 | 25:16, 2.10 | C15H26O | 0.09 | 1.43 |
| 137 | sesquiterpene (MW 206)* | 1724 | 25:04, 2.57 | C15H26 | - | tr |
| 138 | ledane* | 1732 | 25:36, 1.70 | C15H26 | tr | 0.15 |
| 139 | ( 2E,6E)-farnesal* | 1739 | 25:44, 2.20 | C15H24O | tr | tr |
| 140 | (2 E,6E)-farnesol | 1741 | 25:24, 2.49 | C15H26O | - | 0.03 |
| 141 | benzyl benzoate | 1776 | 26:28, 3.22 | C14H12O2 | 0.97 | 1.24 |
| 142 | cis-2-methyl-7-octadecene* | 1794 | 26:52, 1.54 | C19H38 | tr | 0.05 |
| 143 | octadecane* | 1800 | 27:00, 1.49 | C18H38 | tr | tr |
| 144 | octadecanal* | 1818 | 27:20, 1.83 | C18H36O | tr | 0.02 |
| 145 | ( 2E,6E)-farnesyl acetate | 1832 | 27:36, 2.07 | C17H28O2 | 0.05 | 2.05 |
| 146 | cis-Z-α-bisabolene epoxide* | 1875 | 28:04, 2.95 | C15H24O | - | tr |
| 147 | benzyl salicylate | 1881 | 28:32, 3.18 | C14H12O3 | 0.21 | 4.18 |
| 148 | nonadecane * | 1900 | 28:56, 1.51 | C19H40 | tr | tr |
| 149 | hexadecanoic acid * | 1958 | 29:40, 2.10 | C16H32O2 | - | 0.27 |
| 150 | geranyl benzoate | 1965 | 29:48, 2.72 | C17H22O2 | - | tr |
| 151 | eicosane # | 2000 | 30:48, 1.54 | C20H42 | tr | tr |
| 152 | heneicosane * | 2100 | 32:36, 1.57 | C21H44 | tr | tr |
| 153 | benzyl cinnamate | 2102 | 32:16, 3.80 | C16H14O2 | - | tr |
| 154 | docosane * | 2200 | 34:20, 1.59 | C22H46 | tr | tr |
| 155 | tricosane | 2300 | 35:56, 1.62 | C23H48 | tr | tr |
| 156 | tetracosane | 2400 | 37:32, 1.66 | C24H50 | tr | tr |
| 157 | pentacosane * | 2500 | 39:04, 1.70 | C25H52 | tr | tr |
| 158 | hexacosane * | 2600 | 40:32, 1.74 | C26H54 | tr | tr |
| 159 | heptacosane * | 2700 | 41:56, 1.79 | C27H56 | tr | tr |
| 160 | octacosane * | 2800 | 43:16, 1.86 | C28H58 | tr | - |
| 161 | nonacosane * | 2900 | 44:36, 2.02 | C29H60 | tr | - |
# compound not detected by 1DGC analysis in neither of fractions 1 nor 4, but found by GC×GC analysis; * compound not detected before in ylang-ylang essential oil; † compound not detected by 1DGC in a specified fraction but found by GC×GC analysis; tr (trace): relative content < 0.02%; all identifications are based on retention indices, mass spectra and zone of elution.
A first observation is that 82 extra potentially characteristic analytes were thus reported. It is interesting to note that, among this group of 82 analytes, seven were already reported in either fraction 2 or 3 in the classical GC-MS study []. Such crossed presence of analytes in various fractions could potentially challenge the discrimination between fractions. So it is for the fact that, as 79 identical compounds were found in either fraction 1 or 4 from both GC and GC×GC studies, 17 compounds reported in the GC-MS study were not identified in the GC×GC study. Among these 17 compounds reported in the GC-MS study, three were confirmed by standard injections (3-methyl-2-buten-1-ol, 3-methyl butyl acetate, and p-cresyl acetate). None of the identities of the other missing compounds had been strictly verified, so that they could have been identified as other analytes in the GC×GC study. Moreover, 50% of them were reported at trace level (relative abundance below 0.02%), making proper identification quite difficult. Other factors such as chromatographic coelutions, the lack of deconvolution, the presence of interferences in MS data, and the use of slightly different mass spectral libraries could also be involved in this difference.
From the 75 (82−7) compounds that were not reported in the earlier study, the majority belonged to terpenes, terpenoid esters or alcohols, while 14 of them were alkanes that are not responsible for the aroma of essential oil. Some of the analytes e.g., 3-hexen-1-ol, sabinene or terpinolene were not found by 1DGC in neither of fractions 1 nor 4, but were detected in the present study; others like β-bourborene, p-vinylguaiacol or zonarene were missing in fraction 1, while 6-methyl-2-buten-1-yl acetate, phenylacetaldehyde or geranial, among others, were absent in fraction 4, but identified in the GC×GC analysis (Table 1).
Figure 3 shows the differences between the two distillate fractions collected after 25 min (a): fraction 1 and 8 h (b): fraction 4 of distillation of mature ylang-ylang flowers harvested in Mayotte. As already mentioned, fraction 4 is richer in high boiling compounds while fraction 1 is richer in more volatile ones.
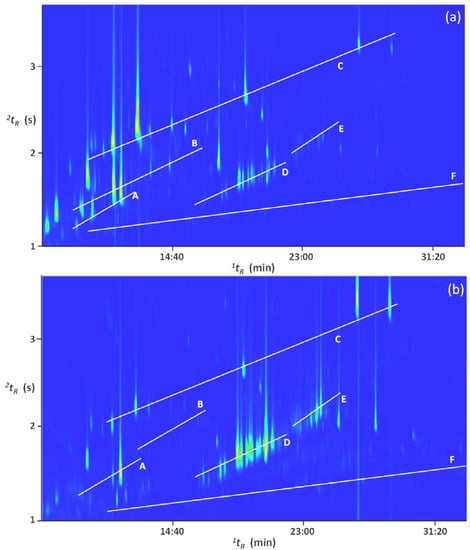
Figure 3.
GC×GC total ion chromatogram (TIC) contour plots of two distillate fractions (a) collected after 25 min (fraction 1) and (b) after 8 h (fraction 4) of distillation of mature ylang-ylang flowers harvested in Mayotte. Letters A, B, C, D, E, and F correspond to monoterpenes, oxygenated monoterpenes, esters, sesquiterpenes, oxygenated sesquiterpenes, and alkanes, respectively.
Bubble plots presented in Figure 4 further illustrate the differences between the two fractions in terms of relative abundances of the major families of compounds present in the oil. GC×GC analysis allowed much better separation and led to easier identification as compounds with similar chemical properties eluted forming groups in the retention space. Several sesquiterpenes, present especially in fraction 4, could not be identified due to lack of standards and their mass spectra similarity.
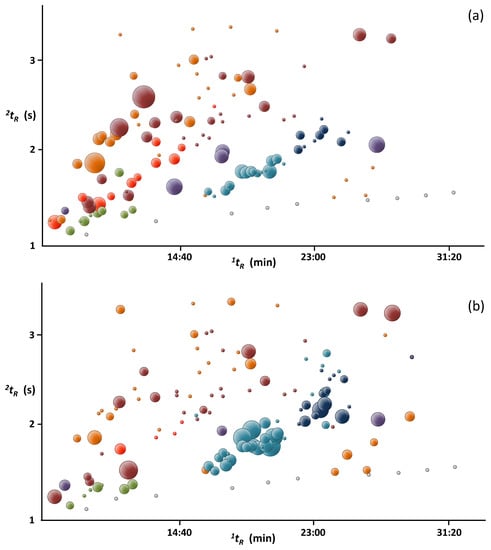
Figure 4.
GC×GC bubble plots of two distillate fractions (a) collected after 25 min (fraction 1) and (b) after 8 h (fraction 4) of distillation of mature ylang-ylang flowers harvested in Mayotte. Chemical classes: alkanes—grey; monoterpenes—green; oxygenated monoterpenes—red; terpenoid esters—purple; sesquiterpenes—light blue; oxygenated sesquiterpenes—dark blue; esters—dark red; others—orange. The size of the bubbles reflects the relative abundances found in Table 1. Alkanes C22–C29are not shown.
Thus, further investigation is necessary to fully characterize the ylang-ylang essential oil. One field of efforts is the writing of specific scripts [,,] that could allow reprocessing of data to highlight chromatographic zones specific to certain families of compounds and enhance the detectability of low abundance analytes.
3. Experimental
3.1. Plant Material and Essential Oil Distillation
Fresh, mature flowers of C. odorata were collected between 7 am and 8 am in July 2009 in Mavigoni, Mayotte. Seven trees were randomly selected in the plantation and the flowers were pooled to make one composite sample for the distillation, which was performed directly on the field with portable equipment, within an hour of the harvest (350 g of mature flowers from seven trees; 50 g of flowers collected from each tree). The flowers were subjected to hydrodistillation for 8 h using a Clevenger-type apparatus (equipped with a 2 L reactor and a 235 mm vertical column). The flowers were added to the water at 70 °C and then brought to the boil. Four separate fractions of the distillate were collected after 25 min (fraction 1), 1 h (fraction 2), 3 h (fraction 3), and 8 h (fraction 4). The essential oils were dried over anhydrous sodium sulfate (0.20 g of anhydrous sodium sulfate for 1.5 mL of essential oil) and kept in amber vials at 4 °C until use. The essential oils were diluted in diethyl ether (4 µL essential oil in 174 µL diethyl ether) containing 2 µL methyl octanoate standard (Sigma-Aldrich, St. Louis, MO, USA) previously diluted in diethyl ether (25 µL standard in 75 µL diethyl ether) Further details on solvents and consumables are available in a previous report []. The present study was carried out on fraction 1 and fraction 4.
3.2. GC×GC-TOFMS Parameters
The GC×GC TOFMS system consisted of an Agilent 7890 (Agilent Technologies, Palo Alto, CA, USA) gas chromatograph and a Pegasus 4D TOFMS (LECO, St. Joseph, MI, USA) equipped with a liquid nitrogen quadruple jet thermal modulator and a secondary oven. The first dimension column was a low-polarity crossbond® silarylene phase exhibiting similar selectivity to 5% phenyl/95% dimethyl polysiloxane phases (Rxi®-5Sil MS; 30 m × 0.25 mm i.d. × 0.25 μm film thickness; Restek Corp., Bellefonte, PA, USA) connected by means of a deactivated universal Press-Tight® connector (Restek Corp.) to the second dimension. The 2D GC column consisted in a medium polarity 50% phenyl polysilphenylene-siloxane phase (BPX50; 1.2 m × 0.10 mm i.d. × 0.10 μm film thickness; SGE International, Victoria, Australia). The 2D column was installed in the separate oven located inside the main GC oven, providing more flexible temperature control. The carrier gas was helium at a constant flow rate of 1 mL min−1 and the injector split ratio of the split/splitless injector was set to 1:20. The main oven temperature was ramped from 45 °C to 85 °C at 20 °C min−1 and then to 285 °C at 5 °C min−1, with a final isothermal period of 10 min at 285 °C. The secondary oven was programmed with a 5 °C offset above the primary oven. The modulation period was 4 s and a modulator temperature offset of 15 °C above the main oven was applied. The hot pulse duration was set at 600 ms. Mass spectra were acquired in the range m/z 30–400 at an acquisition rate of 100 spectra s−1. The ion source temperature was set at 230 °C and the transfer line temperature was set at 250 °C. The detector voltage was 1,500 V and the ionization electron energy (EI source) was set at 70 eV. Samples were acquired using LECO ChromaTOF® software version 4.32. A solvent acquisition delay of 6 min was used to protect the MS analyzer from excessive solvent exposure.
3.3. Data Processing
Data were processed using LECO ChromaTOF® software version 4.33. Automatic peak finding with mass deconvolution were used to create a raw peak table, based on minimum signal to noise ratio of 100 and library matches as requirements for a peak to be included in peak tables. The signal to noise ratio was based on the so-called “unique mass”, the most specific mass extracted for an analyte after deconvolution of the MS signal. Further classification processes were applied to remove the chromatographic noise (column bleed) and potential peak tailing issues. Library searching was carried out using NIST/EPA/NIH Mass Spectral Library (NIST 11) and Wiley Registry of Mass Spectral Data (9th Edition). Library similarity factors were reported on a scale of 1,000 unit, the higher the match factor, the better the match, for both forward and reverse searches. Linear retention indices in the first dimension (IT) were calculated within the ChromaTOF® software using retention times observed for alkane mixture C8–C20 (Fluka, Belgium) analyzed under the same chromatographic conditions as for samples. The relative deviation of all IT obtained in the experiments is lower than 3% according to the 5% phenyl methyl column indices previously reported for these compounds. The first and the second dimension retention times, IT and mass spectrometry data were used for compound identifications. Relative abundances (% values) calculations of the compounds were based on the ratio between the peak area of each compound and the sum of areas of all selected compounds.
4. Conclusions
Although our previous report on the GC-MS analysis of distillate fractions collected at different times of distillation of mature ylang-ylang flowers freshly harvested permitted the update of the chemical composition of ylang-ylang essential oils, the present study demonstrates the possibility to extract more information when GC×GC-TOFMS is used to separate the hundreds of components of the oil. The gain in information on additional individual compounds has a potential interest in enhancing the statistical treatment of the data by highlighting subtle differences between samples, further improving the chemical distinction between extracts of essential oils originating from different geographical locations. On a more fundamental basis, enlarging the list of specific compounds and gaining a better description of chemical polymorphisms could ultimately contribute to detect adulterations or to study the role of the different components of “terroir” effect.
Acknowledgments
This research was partially supported by the Fonds National de la Recherche Scientifique FRFC project 2.4583.09.C. Céline Benini has received a PhD fellowship from Fund for Research Training in Industry and Agriculture. The authors thank the Centre de cooperation Internationale en Recherche Agronomique pour le Développement of Mayotte. Restek Corporation is acknowledged for supporting chromatographic consumables and columns.
- Sample Availability: Samples of the essential oil fraction are available from the authors.
References
- Benini, C.; Danflous, J.P.; Wathelet, J.P.; du Jardin, P.; Fauconnier, M.L. Ylang-ylang [Cananga odorata (Lam.) Hook. f. & Thomson]: An unknown essential oil plant in an endangered sector. Biotechnologie Agronomie Société et Environnement 2010, 14, 693–705. [Google Scholar]
- Benini, C.; Ringuet, M.; Wathelet, J.-P.; Lognay, G.; du Jardin, P.; Fauconnier, M.-L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012, 27, 356–366. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, L.G. Safety assessment of ylang-ylang oil as a food ingredient. Food Chem. Toxicol. 2008, 46, 433–445. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Randriamiharisoa, R.; Bianchini, J.P. Composition of the essential oil of Ylang-Ylang (Cananga odorata Hook Fil. & Thomson forma genuina) from Madagascar. J. Agric. Food Chem. 1986, 34, 481–487. [Google Scholar]
- Craig Elevitch. Cananga odorata (ylang-ylang). Species profiles for Pacific island agroforestry. Available online: http://www.traditionaltree.org/ (accessed on 3 April 2012).
- FAOSTAT. Exports: Commodities by countries. Available online: http://faostat.fao.org/site/342/defaut.aspx/ (accessed on 13 January 2013).
- Stashenko, E.E.; Torres, W.; Morales, J.R.M. A study of the compositional variation of the essential oil of ylang-ylang (Cananga odorata Hook Fil. & Thomson, forma genuina) during flower development. J. High Resolut. Chromatogr. 1995, 18, 101–104. [Google Scholar] [CrossRef]
- AFNOR. French Standard. In Oil of Ylang-ylang [Cananga odorata (Lam.) Hook. f. & Thomson forma genuina]; AFNOR: Paris, France, 2005.
- Benini, C.; Mahy, G.; Bizoux, J.-P.; Wathelet, J.-P.; du Jardin, P.; Brostaux, Y.; Fauconnier, M.-L. Comparative chemical and molecular variability of Cananga odorata (Lam.) Hook. f. & Thomson forma genuina (Ylang-Ylang) in the western indian ocean islands: Implication for valorization. Chem. Biodivers. 2012, 9, 1389–1402. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensive two-dimensional gas chromatography (GCxGC) IV. Further applications, conclusions and perspectives. Trends Anal. Chem. 2006, 25, 821–840. [Google Scholar] [CrossRef]
- Górecki, T.; Harynuk, J.; Panić, O. The evolution of comprehensive two-dimensional gas chromatography (GCxGC). J. Sep. Sci. 2004, 27, 359–379. [Google Scholar] [CrossRef]
- Cordero, C.; Rubiolo, P.; Sgorbini, B.; Galli, M.; Bicchi, C. Comprehensive two-dimensional gas chromatography in the analysis of volatile samples of natural origin: A multidisciplinary approach to evaluate the influence of second dimension column coated with mixed stationary phases on system orthogonality. J. Chromatogr. A 2006, 1132, 268–279. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P. Opportunities for ultra-high resolution analysis of essential oils using comprehensive two-dimensional gas chromatography: A review. Flavour Fragr. J. 2003, 18, 179–191. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Shellie, R.A.; Purcaro, G.; Conte, L.S.; Dugo, P.; Dugo, G.; Mondello, L. Analysis of fresh and aged tea tree essential oils by using GC×GC-qMS. J. Chromatogr. Sci. 2010, 48, 262–266. [Google Scholar]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef]
- Phillips, J.B.; Beens, J. Comprehensive two-dimensional gas chromatography: A hyphenated method with strong coupling between the two dimensions. J. Chromatogr. A 1999, 856, 331–347. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensivetwo-dimensional gas chromatography (GCxGC) II. Modulation and detection. Trends Anal. Chem. 2006, 25, 540–553. [Google Scholar] [CrossRef]
- Hilton, D.C.; Jones, R.S.; Sjödin, A. A method for rapid, non-targeted screening for environmental contaminants in household dust. J. Chromatogr. A 2010, 1217, 6851–6856. [Google Scholar] [CrossRef]
- Brokl, M.; Bishop, L.; Wright, C.G.; Liu, C.; McAdam, K.; Focant, J.-F. Analysis of mainstream tobacco smoke particulate phase using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Sep. Sci. 2013. [Google Scholar] [CrossRef]
- Brasseur, C.; Dekeirsschieter, J.; Schotsmans, E.M.J.; de Koning, S.; Wilson, A.S.; Haubruge, E.; Focant, J.-F. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J. Chromatogr. A 2012, 1255, 163–170. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).