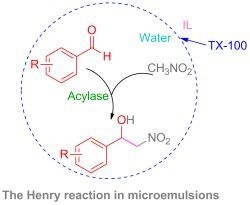
The Henry Reaction in [Bmim][PF6]-based Microemulsions Promoted by Acylase
Abstract
:1. Introduction
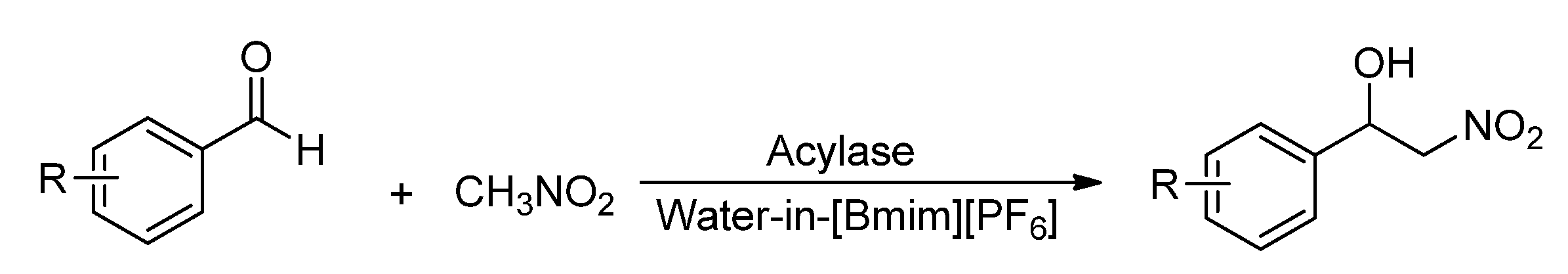
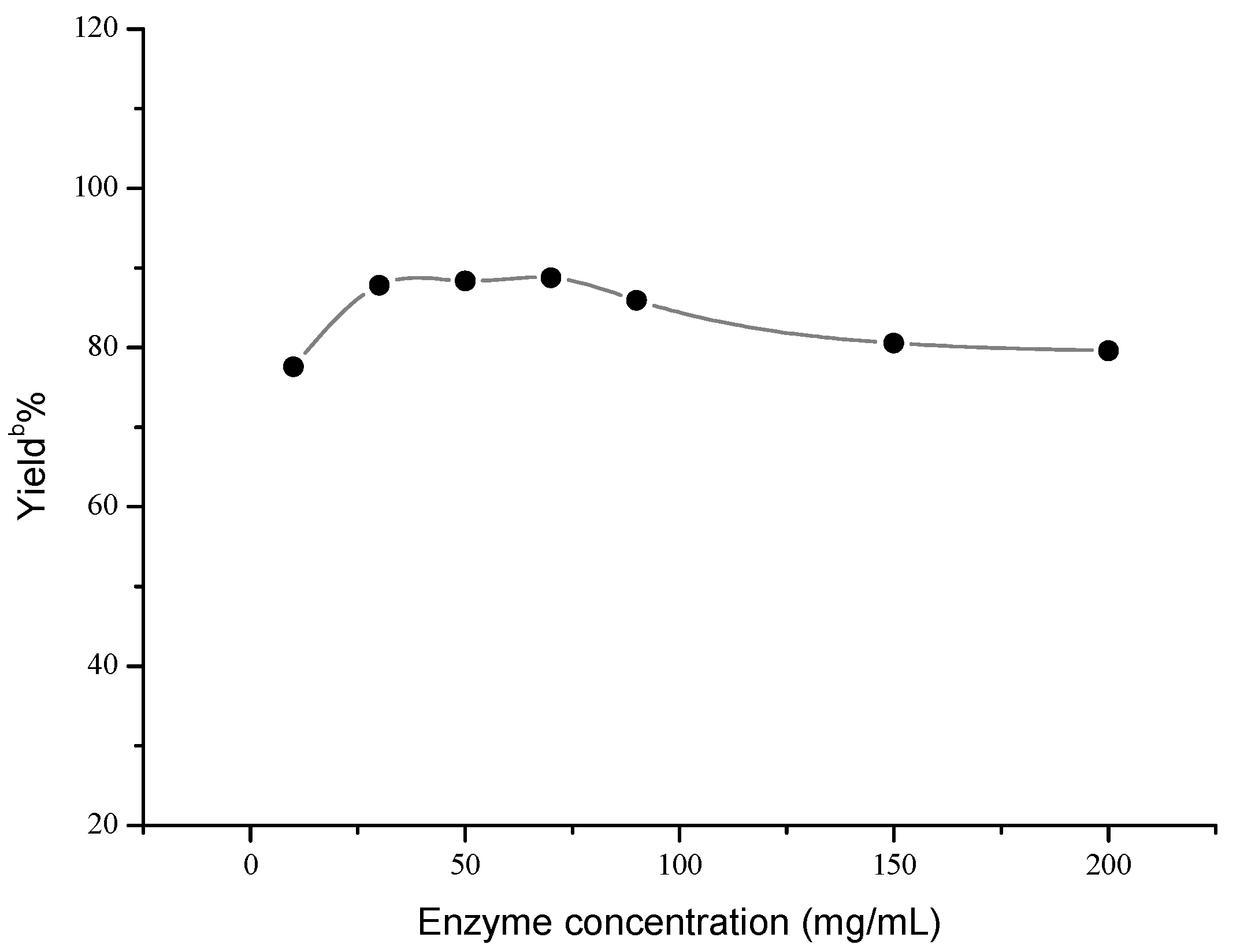
2. Results and Discussion
 | ||
|---|---|---|
| Entry | Enzyme | Yield (%) b |
| 1 | Amano acylase from Aspergillus oryzae | 88 |
| 2 | Lipase from Rhizopus niveus | 72 |
| 3 | Amano lipase PS from Burkholderia cepacia | 71 |
| 4 | Amano lipase from Pseudomonas fluorescens | 70 |
| 5 | Lipase from Candida rugosa | 68 |
| 6 | Lipase from bovine pancreas | 68 |
| 7 | Amano lipase M from Mucor javanicus | 67 |
| 8 | Amano lipase A from Aspergillus niger | 66 |
| 9 | Acylase I from Aspergillus melleus | 65 |
| 10 | Bovine serum albumin | 64 |
| 11 | Control test c | 62 |
| 12 | Control test d | 24 |
| 13 | Control test e | 1 |
| Entry | ω0 | Water (%) | TX-100 (%) | [Bmim]PF6 (%) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | 4 | 6.7 | 60 | 33.3 | 84 |
| 2 | 8 | 13.3 | 60 | 26.7 | 85 |
| 3 | 10 | 16.7 | 60 | 23.3 | 88 |
| 4 | 12 | 20.0 | 60 | 20.0 | 82 |
| 5 | 14 | 23.4 | 60 | 16.6 | 80 |
| 6 | 16 | 26.7 | 60 | 13.3 | 75 |
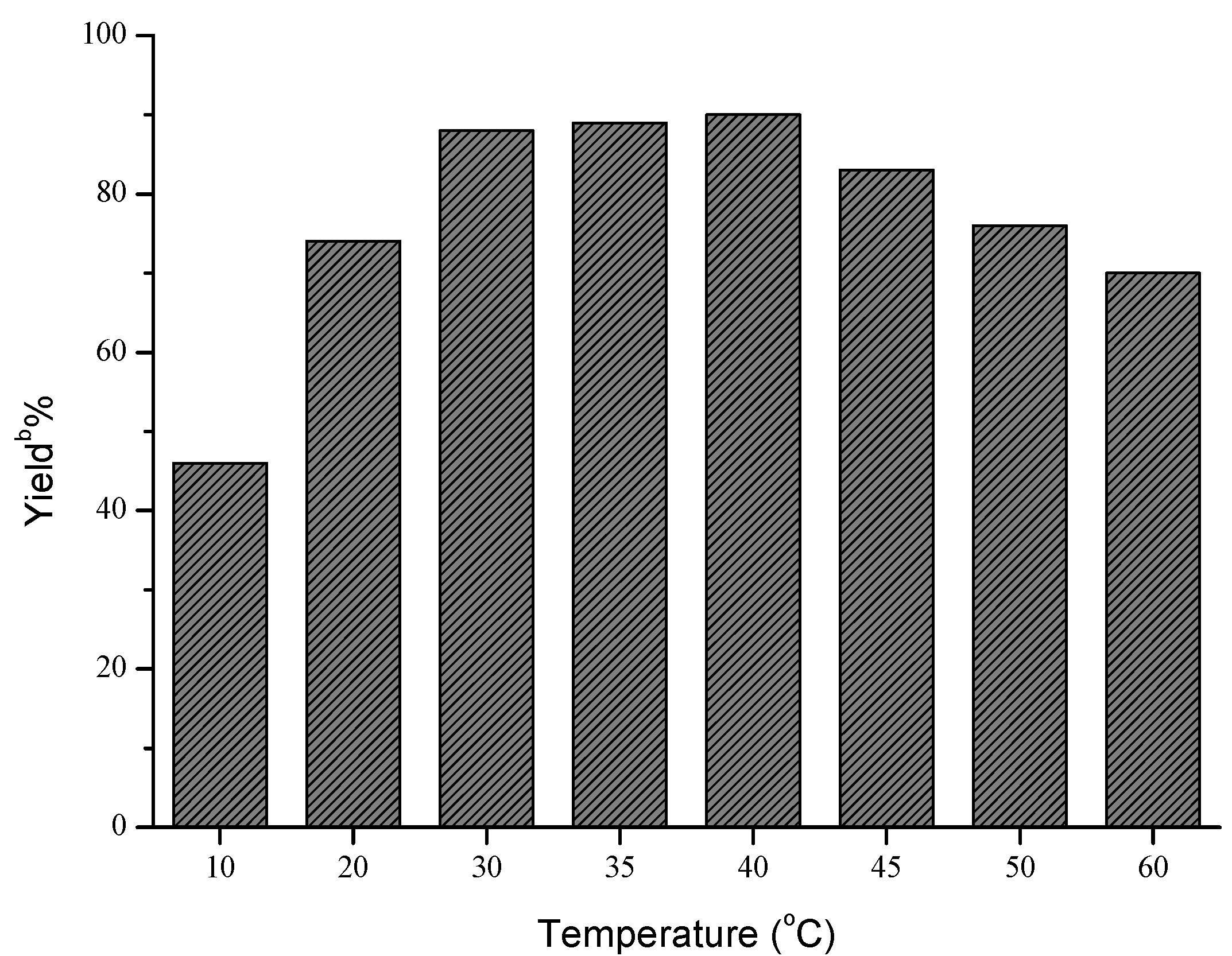
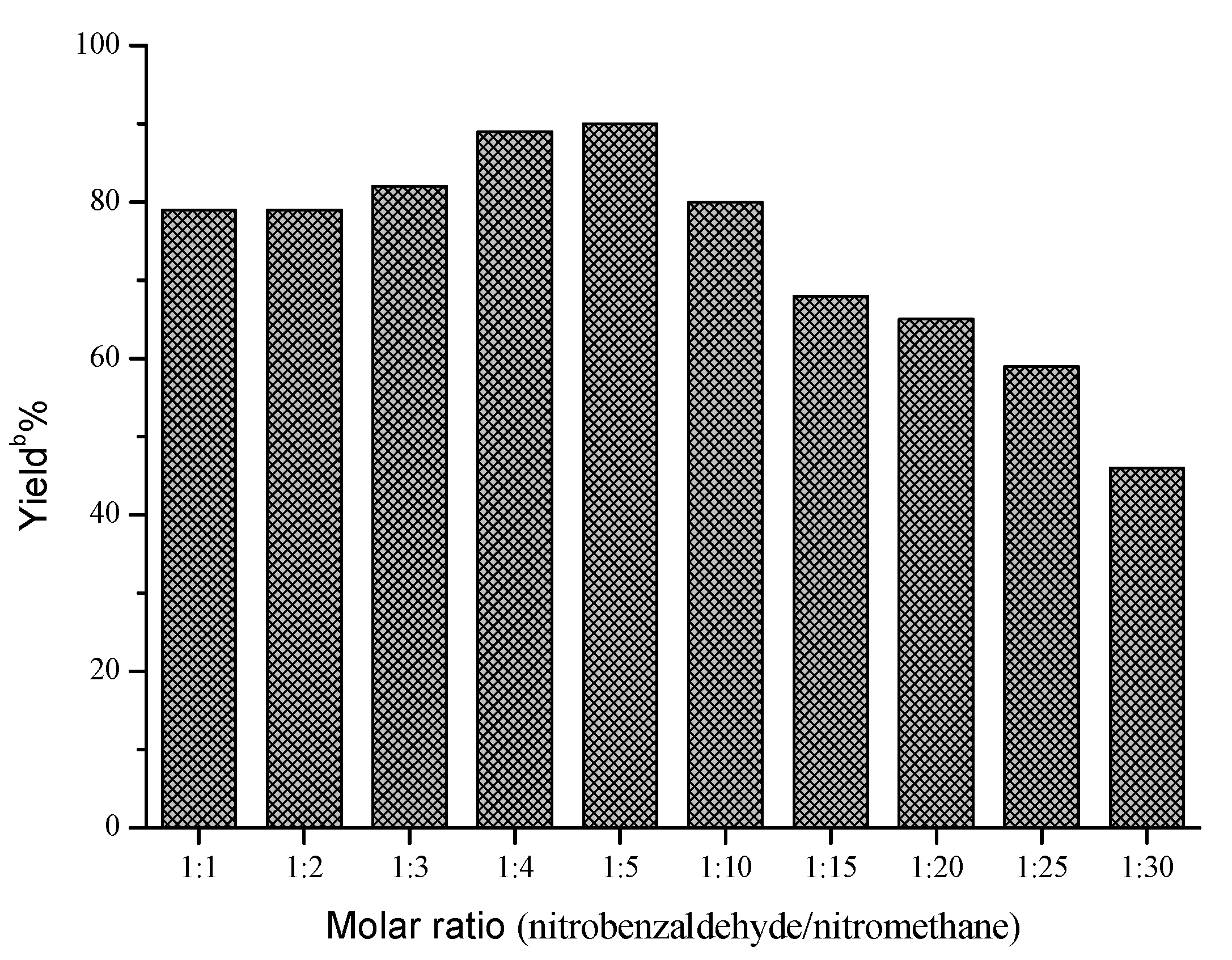
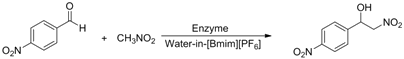
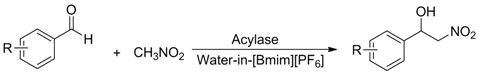 | |||
|---|---|---|---|
| Entry | R | Products | Yield b(%) |
| 1 | H | a | 44 |
| 2 | 3-CH3 | b | 28 |
| 3 | 4-CH3 | c | 30 |
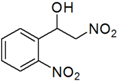
| 4 | 2-NO2 | d | 87 |
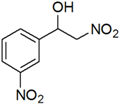
| 5 | 3-NO2 | e | 79 |
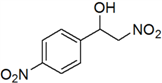
| 6 | 4-NO2 | f | 90 |
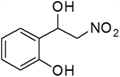
| 7 | 2-OH | g | 22 |
| 8 | 3-OH | h | 48 |
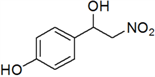
| 9 | 4-OH | i | 55 |
| 10 | 4-OCH3 | j | 40 |
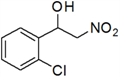
| 11 | 2-Cl | k | 77 |
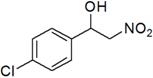
| 12 | 4-Cl | l | 66 |
3. Experimental
3.1. Materials and Analytical Methods
3.2. General Procedure for Henry reaction
3.3. Physical and 1H-NMR Data of Some Representative Henry Products

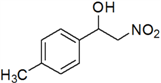
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Milner, S.E.; Moody, T.S.; Maguire, A.R. Biocatalytic Approaches to the Henry (Nitroaldol) Reaction. Eur. J. Org. Chem. 2012, 2012, 3059–3067. [Google Scholar] [CrossRef]
- Kisanga, P.B.; Verkade, J.G. P(RNCH2CH2)3N: An efficient promoter for the nitroaldol (Henry) reaction. J. Org. Chem. 1999, 64, 4298–4303. [Google Scholar] [CrossRef]
- Heffner, R.J.; Jiang, J.; Joullie, M.M. Total synthesis of (−)-nummularine F. J. Am. Chem. Soc. 1992, 114, 10181–10189. [Google Scholar] [CrossRef]
- Saraswat, A.; Sharma, L.; Singh, S.; Siddiqui, I.; Singh, R. Chemoselective Henry reaction catalyzed by electro-generated base. Res. Chem. Intermed. 2013, 1–7. [Google Scholar]
- Jiang, T.; Gao, H.; Han, B.; Zhao, G.; Chang, Y.; Wu, W.; Gao, L.; Yang, G. Ionic liquid catalyzed Henry reactions. Tetrahedron Lett. 2004, 45, 2699–2701. [Google Scholar] [CrossRef]
- Rokhum, L.; Bez, G. Ethyl acrylate conjugated polystyryl-diphenylphosphine—An extremely efficient catalyst for Henry reaction under solvent-free conditions (SolFC). Can. J. Chem. 2012, 91, 300–306. [Google Scholar] [CrossRef]
- Khan, F.A.; Dash, J.; Satapathy, R.; Upadhyay, S.K. Hydrotalcite catalysis in ionic liquid medium: A recyclable reaction system for heterogeneous Knoevenagel and nitroaldol condensation. Tetrahedron Lett. 2004, 45, 3055–3058. [Google Scholar] [CrossRef]
- Simoni, D.; Rondanin, R.; Morini, M.; Baruchello, R.; Invidiata, F.P. 1,5,7-Triazabicyclo[4.4.0]dec-1-ene (TBD), 7-methyl-TBD (MTBD) and the polymer-supported TBD (P-TBD): Three efficient catalysts for the nitroaldol (Henry) reaction and for the addition of dialkyl phosphites to unsaturated systems. Tetrahedron Lett. 2000, 41, 1607–1610. [Google Scholar] [CrossRef]
- Bora, P.P.; Bez, G. Henry reaction in aqueous media at neutral pH. Eur. J. Org. Chem. 2013, 2013, 2922–2929. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, X.; Xie, H.-Y.; Liu, B.-K.; Lin, X.-F. Hydrolase-catalyzed fast Henry reaction of nitroalkanes and aldehydes in organic media. J. Biotechnol. 2010, 145, 240–243. [Google Scholar] [CrossRef]
- Majhi, A.; Kadam, S.T.; Kim, S.S. TMEDA catalyzed Henry (nitroaldol) reaction under metal and solvent-free conditions. Bull. Korean Chem. Soc. 2009, 30, 1767–1770. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Parrini, M. A one pot, solvent-free synthesis of acyclic α-nitro ketones through the nitroaldol reaction. Tetrahedron Lett. 1998, 39, 7963–7964. [Google Scholar] [CrossRef]
- Changsheng, R.M.-A.H.R.; Gan, X.C.; Lai, G.; Wang, Z. Rapid microwave-assisted henry reaction in solvent-free processes. Synlett 2006, 2006, 387–390. [Google Scholar]
- Tanaka, K.; Hachiken, S. Enantioselective Henry reaction catalyzed by trianglamine–Cu (OAc)2 complex under solvent-free conditions. Tetrahedron Lett. 2008, 49, 2533–2536. [Google Scholar] [CrossRef]
- Reddy, K.R.; Rajasekhar, C.V.; Krishna, G.G. Zinc-proline complex: An efficient, reusable catalyst for direct nitroaldol reaction in aqueous media. Synth. Commun. 2007, 37, 1971–1976. [Google Scholar] [CrossRef]
- Ren, Y.; Cai, C. Iodine catalysis in aqueous medium: An improved reaction system for Knoevenagel and Nitroaldol condensation. Catalysis Lett. 2007, 118, 134–138. [Google Scholar] [CrossRef]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Protein-mediated nitroaldol addition in aqueous media. Catalytic promiscuity or unspecific catalysis? Org. Process Res. Dev. 2010, 15, 236–240. [Google Scholar]
- Purkarthofer, T.; Gruber, K.; Gruber-Khadjawi, M.; Waich, K.; Skranc, W.; Mink, D.; Griengl, H. A biocatalytic Henry reaction—The hydroxynitrile lyase from Hevea brasiliensis also catalyzes nitroaldol reactions. Angew. Chem. Int. Ed. 2006, 45, 3454–3456. [Google Scholar] [CrossRef]
- Tang, R.-C.; Guan, Z.; He, Y.-H.; Zhu, W. Enzyme-catalyzed Henry (nitroaldol) reaction. J. Mol. Catal. B: Enzym. 2010, 63, 62–67. [Google Scholar] [CrossRef]
- Gruber-Khadjawi, M.; Purkarthofer, T.; Skranc, W.; Griengl, H. Hydroxynitrile lyase-catalyzed enzymatic nitroaldol (Henry) reaction. Adv. Synth. Catal. 2007, 349, 1445–1450. [Google Scholar] [CrossRef]
- Humble, M.S.; Berglund, P. Biocatalytic promiscuity. Eur. J. Org. Chem. 2011, 2011, 3391–3401. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Le, Z.-G.; Guo, L.-T.; Jiang, G.-F.; Yang, X.-B.; Liu, H.-Q. Henry reaction catalyzed by Lipase A from Aspergillus niger. Green Chem. Lett. Rev. 2013, 6, 277–281. [Google Scholar] [CrossRef]
- Xue, L.; Qiu, H.; Li, Y.; Lu, L.; Huang, X.; Qu, Y. A novel water-in-ionic liquid microemulsion and its interfacial effect on the activity of laccase. Colloids Surf. B 2011, 82, 432–437. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocatalysis in water-in-ionic liquid microemulsions: A case study with horseradish peroxidase. Langmuir 2008, 25, 977–982. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Gournis, D.; Papadopoulos, G.K.; Stamatis, H. Lipases in water-in-ionic liquid microemulsions: Structural and activity studies. J. Mol. Catal. B: Enzym. 2009, 60, 50–56. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Zou, F.; Lu, L.; Zhao, Y.; Huang, X.; Qu, Y. The catalytic efficiency of lipase in a novel water-in-[Bmim][PF6] microemulsion stabilized by both AOT and Triton X-100. Colloids Surf. B 2012, 92, 360–366. [Google Scholar] [CrossRef]
- Gao, Y.; Han, S.; Han, B.; Li, G.; Shen, D.; Li, Z.; Du, J.; Hou, W.; Zhang, G. TX-100/water/1-butyl-3-methylimidazolium hexafluorophosphate microemulsions. Langmuir 2005, 21, 5681–5684. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xia, W.-J.; Xie, Z.-B.; Jiang, G.-F.; Le, Z.-G. The Henry Reaction in [Bmim][PF6]-based Microemulsions Promoted by Acylase. Molecules 2013, 18, 13910-13919. https://doi.org/10.3390/molecules181113910
Xia W-J, Xie Z-B, Jiang G-F, Le Z-G. The Henry Reaction in [Bmim][PF6]-based Microemulsions Promoted by Acylase. Molecules. 2013; 18(11):13910-13919. https://doi.org/10.3390/molecules181113910
Chicago/Turabian StyleXia, Wen-Jian, Zong-Bo Xie, Guo-Fang Jiang, and Zhang-Gao Le. 2013. "The Henry Reaction in [Bmim][PF6]-based Microemulsions Promoted by Acylase" Molecules 18, no. 11: 13910-13919. https://doi.org/10.3390/molecules181113910
APA StyleXia, W.-J., Xie, Z.-B., Jiang, G.-F., & Le, Z.-G. (2013). The Henry Reaction in [Bmim][PF6]-based Microemulsions Promoted by Acylase. Molecules, 18(11), 13910-13919. https://doi.org/10.3390/molecules181113910