A Bicyclic Diterpenoid with a New 15,16-Dinorlabdane Carbon Skeleton from Leonurus japonicus and Its Coagulant Bioactivity
Abstract
:1. Introduction
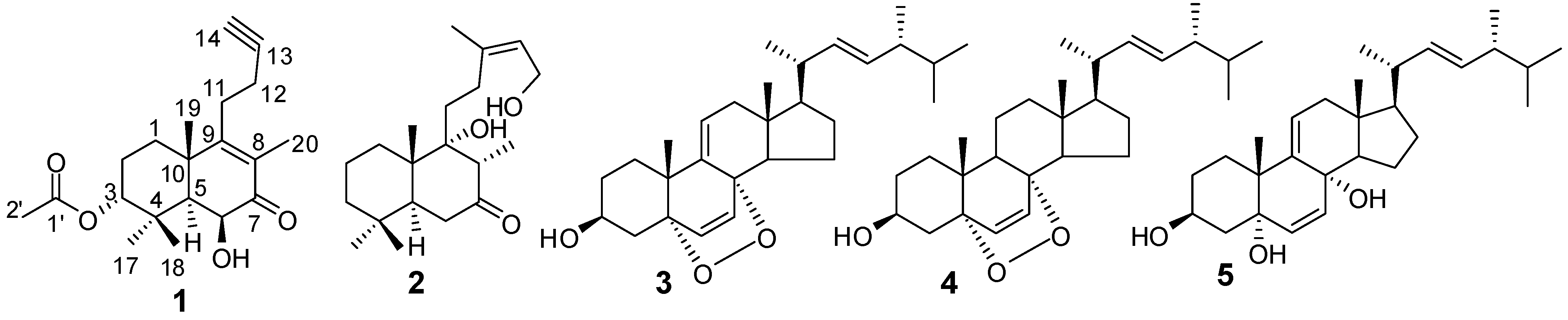
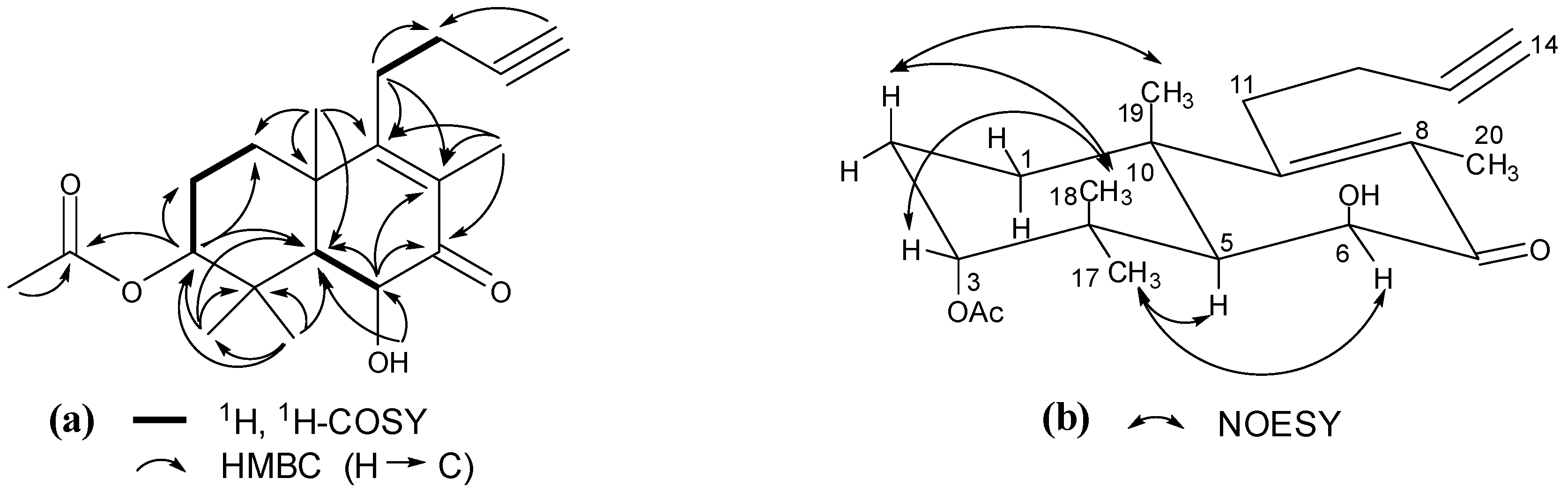
2. Results and Discussion
| No. | δH | δC | No. | δH | δC |
|---|---|---|---|---|---|
| 1 | 1.73 m | 31.7 | 12 | 2.37 m | 18.5 |
| 2 | 2.16 m, 1.72 m | 23.5 | 13 | – | 83.9 |
| 3 | 4.60 t (3.0) | 78.5 | 14 | 2.47 t (2.4) | 70.6 |
| 4 | – | 38.0 | 17 | 1.00 s | 27.5 |
| 5 | 1.97 d (3.0) | 48.5 | 18 | 1.42 s | 24.4 |
| 6 | 4.19 dd (4.2, 3.0) | 71.4 | 19 | 1.48 s | 22.1 |
| 7 | – | 198.1 | 20 | 1.81 s | 11.9 |
| 8 | – | 129.6 | 1ʹ | – | 170.4 |
| 9 | – | 166.5 | 2ʹ | 1.99 s | 21.0 |
| 10 | – | 41.5 | OH-6 | 4.53 d (4.2) | – |
| 11 | 2.58 t (7.8) | 29.5 |
| Sample | Dose | APTT (s) | PT (s) | TT (s) | FIB (g/L) |
|---|---|---|---|---|---|
| Control | Normal saline | 31.22 ± 6.20 | 9.98 ± 0.54 | 53.24 ± 5.91 | 1.59 ± 0.08 |
| Compound 1 | 1 mg/mL | 28.88 ± 5.57 ** | 9.87 ± 0.47* | 49.94 ± 3.46 * | 1.64 ± 0.03 * |
| 0.1 mg/mL | 29.66 ± 4.90 * | 9.94 ± 0.44 | 50.77 ± 4.43 * | 1.66 ± 0.03 * | |
| 0.01 mg/mL | 31.38 ± 4.87 | 10.00 ± 0.49 | 53.28 ± 3.98 | 1.65 ± 0.06 * |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
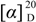 = −17.3 (c = 0.10, MeOH); IR νmax: 3456, 3291, 2941, 2874, 2120, 1728, 1659, 1607, 1461, 1375, 1248, 1128, 1047, 930 cm−1; ESI-MS m/z 355.2 [M+Na]+; HRESI-MS: m/z 355.1883 [M+Na]+ (calcd. for C20H28O4Na, 355.1885); 1H- and 13C-NMR data see Table 1.
= −17.3 (c = 0.10, MeOH); IR νmax: 3456, 3291, 2941, 2874, 2120, 1728, 1659, 1607, 1461, 1375, 1248, 1128, 1047, 930 cm−1; ESI-MS m/z 355.2 [M+Na]+; HRESI-MS: m/z 355.1883 [M+Na]+ (calcd. for C20H28O4Na, 355.1885); 1H- and 13C-NMR data see Table 1.3.4. Blood Coagulation Assay [10]
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 272–273.
- Xiong, L.; Zhou, Q.M.; Peng, C.; Xie, X.F.; Guo, L.; Li, X.H.; Liu, J.; Liu, Z.H.; Dai, O. Sesquiterpenoids from the herb of Leonurus japonicus. Molecules 2013, 18, 5051–5058. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Peng, C.; Li, X.H.; Xiong, L.; He, C.J.; Guo, L.; Cao, Z.X.; Liu, Z.H. Sesquiterpenoids from the herb of Leonurus japonicus. Biochem. Syst. Ecol. 2013, 51, 101–103. [Google Scholar] [CrossRef]
- Hung, T.M.; Luan, T.C.; Vinh, B.T.; Cuong, T.D.; Min, B.S. Labdane-type diterpenoids from Leonurus heterophyllus and their cholinesterase inhibitory activity. Phytother. Res. 2011, 25, 611–614. [Google Scholar] [CrossRef]
- Cateni, F.; Doljak, B.; Zacchigna, M.; Anderluh, M.; Piltaver, A.; Scialino, G.; Banfi, E. New biologically active epidioxysterols from Stereum hirsutum. Bioorg. Med. Chem. Lett. 2007, 17, 6330–6334. [Google Scholar] [CrossRef]
- Ponce, M.A.; Ramirez, J.A.; Galagovsky, L.R.; Gros, E.G.; Erra-Balsells, R. A new look into the reaction between ergosterol and singlet oxygen in vitro. Photochem. Photobiol. Sci. 2002, 1, 749–756. [Google Scholar] [CrossRef]
- Wu, H.; Fronczek, F.R.; Ferreira, D.; Burandt, C.L., Jr.; Zjawiony, J.K. Labdane diterpenoids from Leonurus sibiricus. J. Nat. Prod. 2011, 74, 831–836. [Google Scholar] [CrossRef]
- Moon, H.T.; Jin, Q.; Shin, J.E.; Choi, E.J.; Han, H.K.; Kim, Y.S.; Woo, E.R. Bis-spirolabdane-type diterpenoids from Leonurus sibiricus. J. Nat. Prod. 2010, 73, 123–126. [Google Scholar] [CrossRef]
- Seo, H.K.; Kim, J.S.; Kang, S.S. Labdane diterpenes and flavonoids from Leonurus japonicus. Helv. Chim. Acta 2010, 93, 2045–2051. [Google Scholar] [CrossRef]
- Zhou, J.J.; Xing, N.; Chen, J.; Shi, J.W.; Su, G.L. Effect of artificial colloids on blood coagulation during shock stage of severe burn injury. Chin. Med. J. 2013, 126, 3334–3339. [Google Scholar]
- Hon, P.M.; Lee, C.M.; Shang, H.S.; Cui, Y.X.; Wong, H.N.C.; Chang, H.M. Prehispanolone, a labdane diterpene from Leonurus heterophyllus. Phytochemistry 1991, 30, 354–356. [Google Scholar] [CrossRef]
- Hon, P.M.; Wang, E.S.; Lam, S.K.M.; Choy, Y.M.; Lee, C.M.; Wong, H.N.C. Preleoheterin and leoheterin, two labdane diterpenes from Leonurus heterophyllus. Phytochemistry 1993, 33, 639–641. [Google Scholar] [CrossRef]
- Giang, P.M.; Son, P.T.; Matsunami, K.; Otsuka, H. New labdane-type diterpenoids from Leonurus heterophyllus SW. Chem. Pharm. Bull. 2005, 53, 938–941. [Google Scholar] [CrossRef]
- Giang, P.M.; Son, P.T.; Matsunami, K.; Otsuka, H. New bis-spirolabdane-type diterpenoids from Leonurus heterophyllus SW. Chem. Pharm. Bull. 2005, 53, 1475–1479. [Google Scholar] [CrossRef]
- Romero-González, R.R.; Ávila-Núñez, J.L.; Aubert, L.; Alonso-Amelot, M.E. Labdane diterpenes from Leonurus japonicus leaves. Phytochemistry 2006, 67, 965–970. [Google Scholar] [CrossRef]
- Moon, H.I. Three diterpenes from Leonurus japonicus Houtt protect primary cultured rat cortical cells from glutamate-induced toxicity. Phytother. Res. 2010, 24, 1256–1259. [Google Scholar]
- Gong, H.Q.; Wang, R.; Shi, Y.P. New labdane-type diterpenoids from Leonurus heterophyllus. Helv. Chim. Acta 2012, 95, 618–625. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, F.; Xiong, L.; Zhao, X.-M. A Bicyclic Diterpenoid with a New 15,16-Dinorlabdane Carbon Skeleton from Leonurus japonicus and Its Coagulant Bioactivity. Molecules 2013, 18, 13904-13909. https://doi.org/10.3390/molecules181113904
Peng F, Xiong L, Zhao X-M. A Bicyclic Diterpenoid with a New 15,16-Dinorlabdane Carbon Skeleton from Leonurus japonicus and Its Coagulant Bioactivity. Molecules. 2013; 18(11):13904-13909. https://doi.org/10.3390/molecules181113904
Chicago/Turabian StylePeng, Fu, Liang Xiong, and Xiao-Mei Zhao. 2013. "A Bicyclic Diterpenoid with a New 15,16-Dinorlabdane Carbon Skeleton from Leonurus japonicus and Its Coagulant Bioactivity" Molecules 18, no. 11: 13904-13909. https://doi.org/10.3390/molecules181113904
APA StylePeng, F., Xiong, L., & Zhao, X.-M. (2013). A Bicyclic Diterpenoid with a New 15,16-Dinorlabdane Carbon Skeleton from Leonurus japonicus and Its Coagulant Bioactivity. Molecules, 18(11), 13904-13909. https://doi.org/10.3390/molecules181113904




