Anti-Inflammatory Effect of Neoechinulin A from the Marine Fungus Eurotium sp. SF-5989 through the Suppression of NF-кB and p38 MAPK Pathways in Lipopolysaccharide-Stimulated RAW264.7 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Neoechinulins A (1) and B (2)
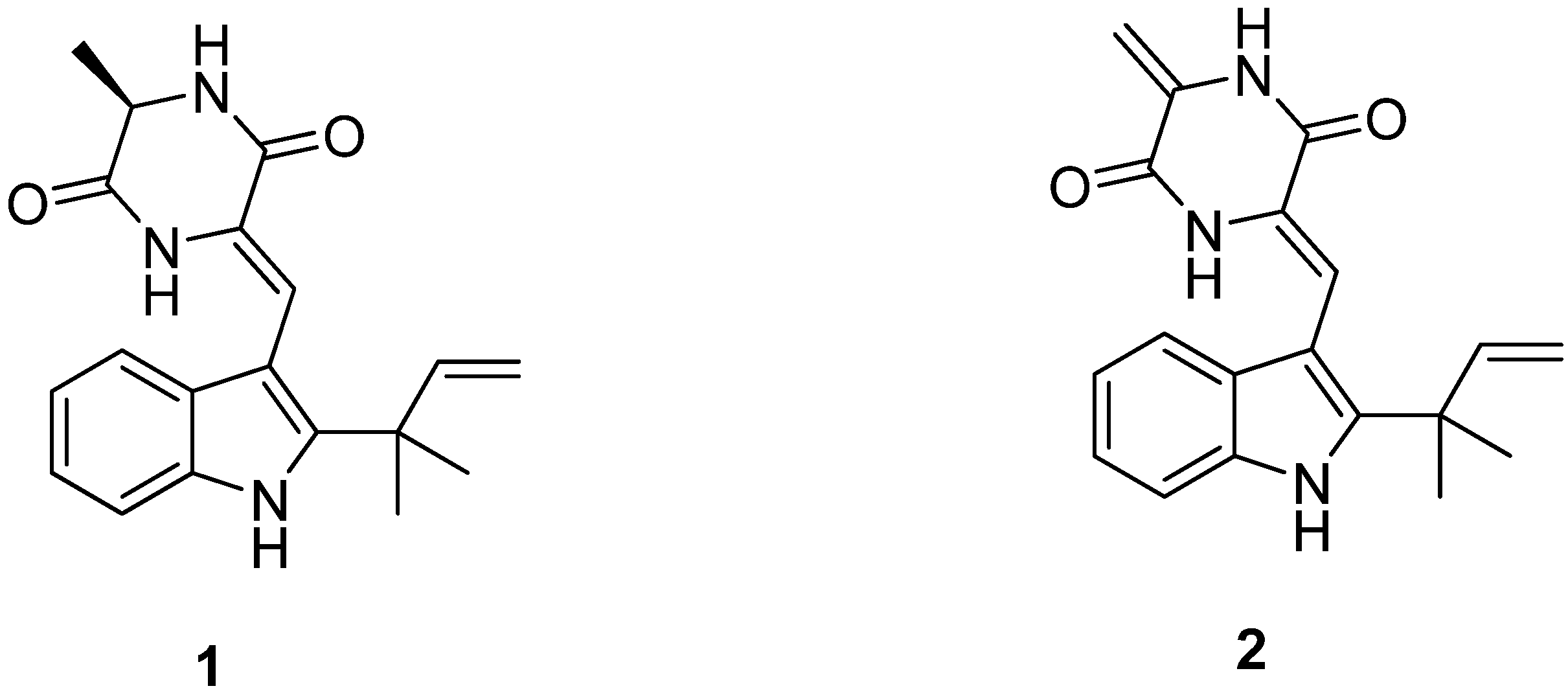
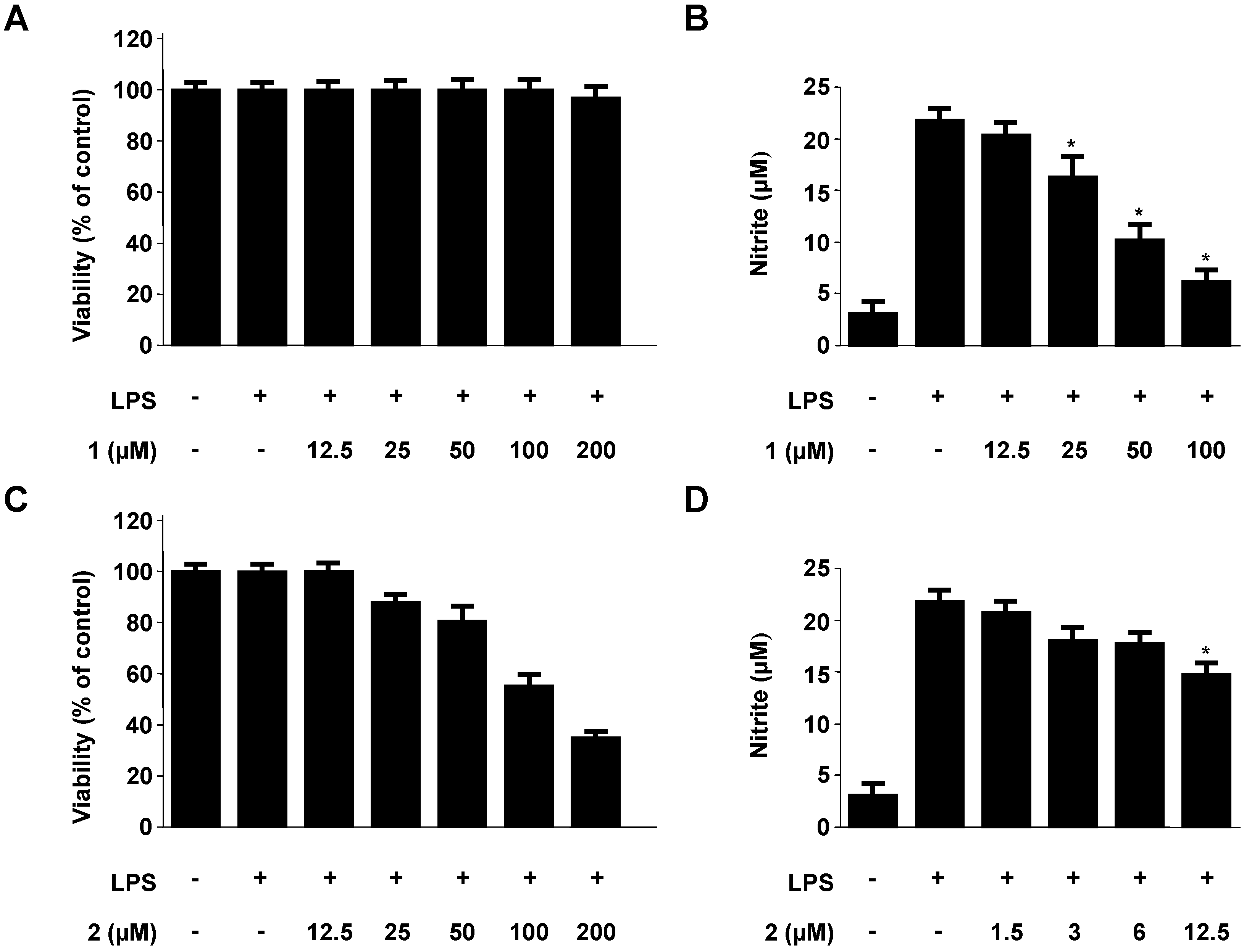
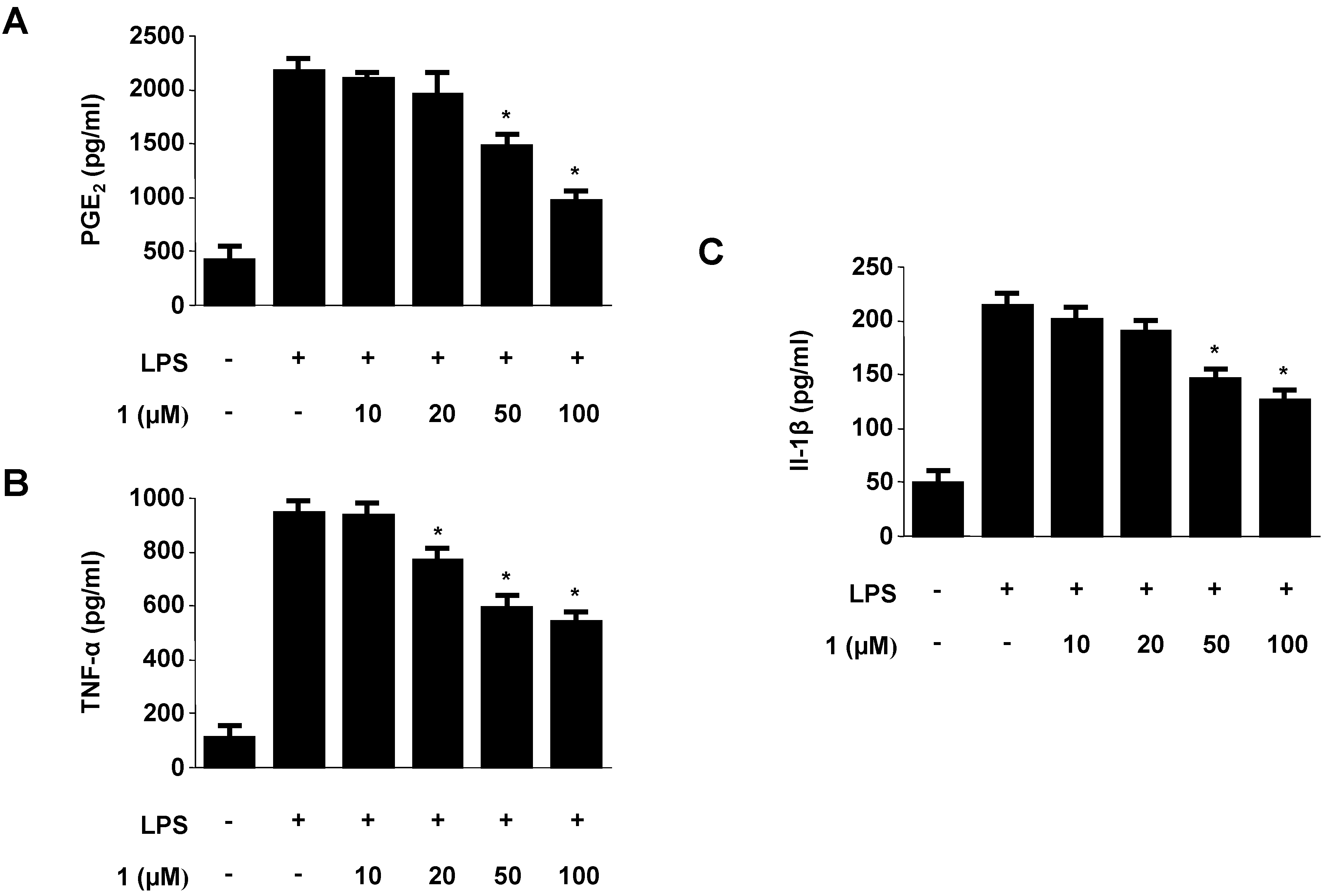
2.2. Effects of Neoechinulins A (1) and B (2) on Cell Viability and Pro-Inflammatory Mediators/Cytokines Production
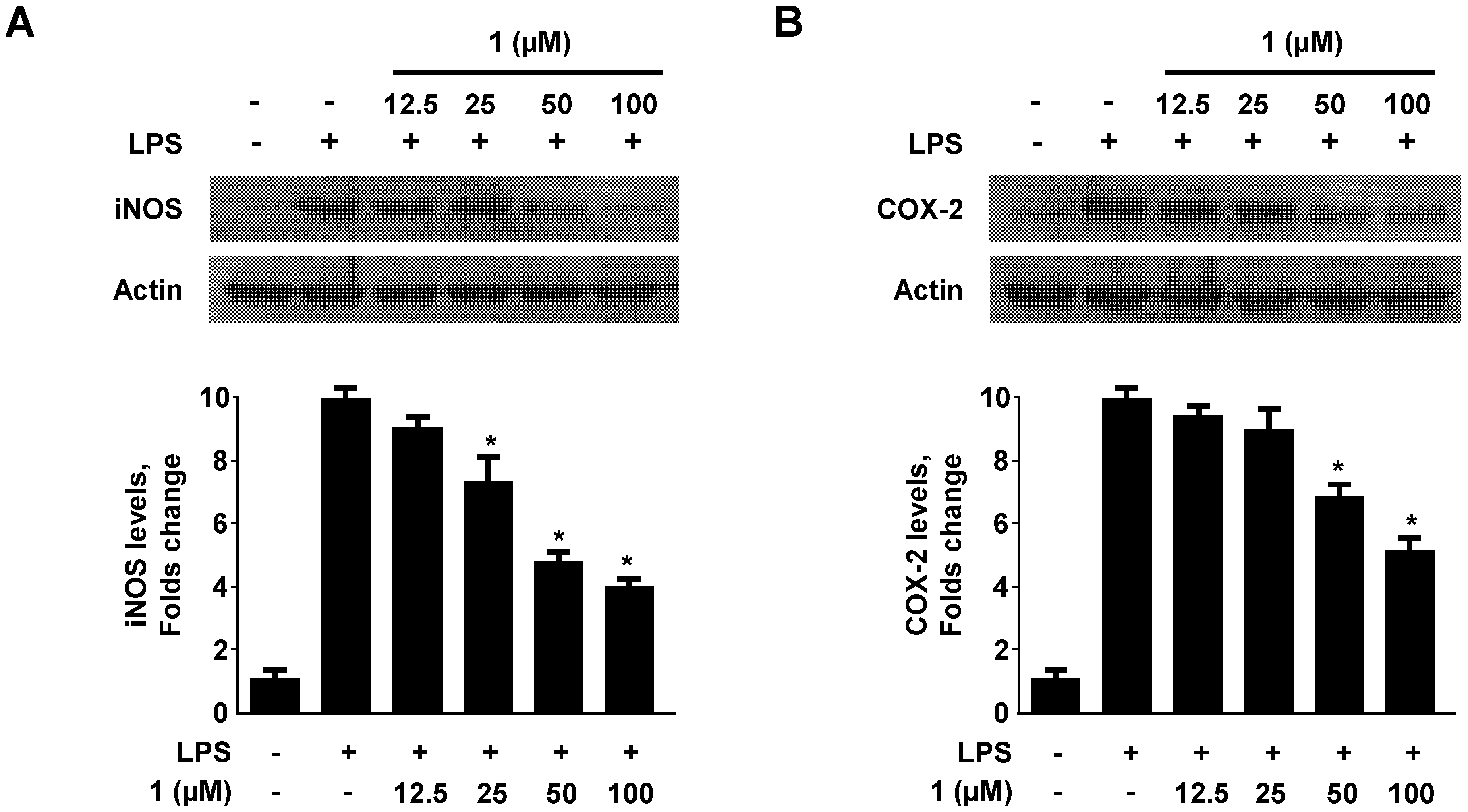
2.3. Effect of Neoechinulin A (1) on iNOS and COX-2 Expression
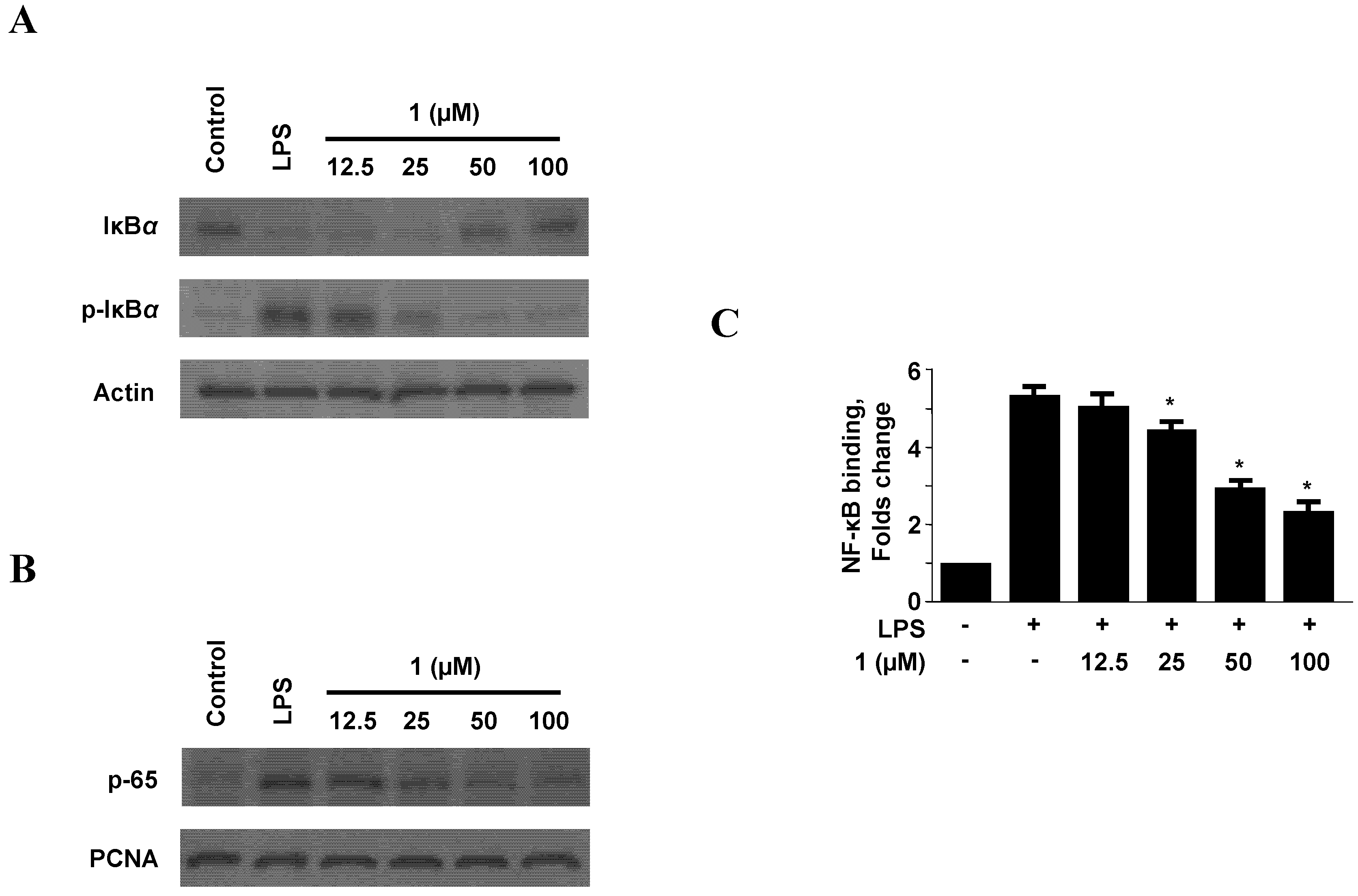
2.4. Effect of Neoechinulin A (1) on NF-κB Activation
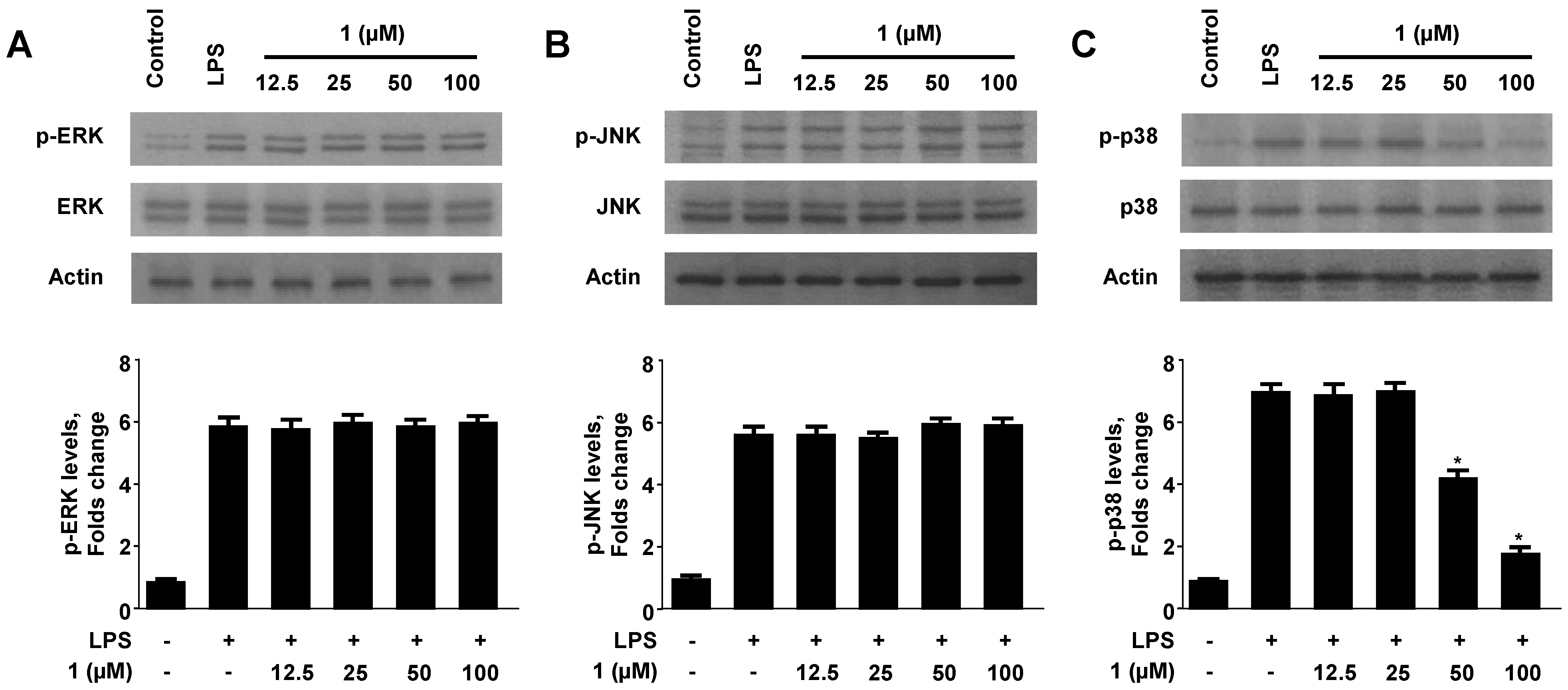
2.5. Effect of Neoechinulin A (1) on the Phosphorylation of MAPKs
3. Experimental
3.1. General
3.2. Specimen Collection and Identification of the Marine-Derived Fungus Eurotium sp. SF-5989
3.3. Fermentation, Extraction and Isolation of Neoechinulins A (1) and B (2) from Eurotium sp. SF-5989
3.4. Cell Culture and Viability Assay
3.5. Determination of Nitrite Production and PGE2, TNF-α, and IL-1β Assays
3.6. Preparation of Cytosolic and Nuclear Fractions
3.7. Western Blot Analysis
3.8. DNA Binding Activity of NF-κB
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Anderson, N.; Borlak, J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008, 60, 311–357. [Google Scholar]
- Chung, C.P.; Avalos, I.; Raggi, P.; Stein, C.M. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 1228–1233. [Google Scholar]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef]
- Mayeux, P.R. Pathobiology of lipopolysaccharide. J. Toxicol. Environ. Health. 1997, 51, 415–435. [Google Scholar]
- Boje, K.M. Nitric oxide neurotoxicity in neurodegenerative diseases. Front. Biosci. 2004, 9, 763–776. [Google Scholar] [CrossRef]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef]
- Nathan, C.F.; Hibbs, J.B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar]
- Yoon, W.J.; Ham, Y.M.; Yoo, B.S.; Moon, J.Y.; Koh, J.; Hyun, C.G. Oenothera. laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. J. Biosci. Bioeng. 2009, 107, 429–438. [Google Scholar]
- Zhang, G.; Ghosh, S. Toll-like receptor-mediated NF-kappaB activation: A phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 2001, 107, 13–19. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases--regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar]
- Rao, K.M. Map kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar]
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology 2008, 123, 348–357. [Google Scholar]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as antiinfective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar]
- Martins, M.B.; Carvalho, I. Diketopiperazines: biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar]
- Kuramochi, K.; Aoki, T.; Nakazaki, A.; Kamisuki, S.; Takeno, M.; Ohnishia, K.; Kimoto, K.; Watanabe, N.; Kamakura, T.; Arai, T.; et al. Synthesis of neoechinulin A and derivatives. Synthesis 2008, 23, 3810–3818. [Google Scholar]
- Marchelli, R.; Dossena, A.; Pochini, A.; Dradi, E. The structures of five new didehydropeptides related to neoechinulin, isolated from Aspergillus. amstelodami. J. Chem. Soc. Perkin Trans. 1 1977, 17, 713–717. [Google Scholar]
- Koleva, Y.K.; Madden, J.C.; Cronin, M.T. Formation of categories from structure-activity relationships to allow read-across for risk assessment: Toxicity of alpha, beta-unsaturated carbonyl compounds. Chem. Res. Toxicol. 2008, 21, 2300–2312. [Google Scholar]
- Sawa, T.; Ohshima, H. Nitrative DNA damage in inflammation and its possible role in carcinogenesis. Nitric Oxide 2006, 14, 91–100. [Google Scholar]
- Cheng, P.Y.; Lee, Y.M.; Wu, Y.S.; Chang, T.W.; Jin, J.S.; Yen, M.H. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem. Pharmacol. 2007, 73, 793–804. [Google Scholar]
- Kimura, H.; Hokari, R.; Miura, S.; Shigematsu, T.; Hirokawa, M.; Akiba, Y.; Kurose, I.; Higuchi, H.; Fujimori, H.; Tsuzuki, Y.; et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut 1998, 42, 180–187. [Google Scholar]
- Szabo, C.; Thiemermann, C.; Wu, C.C.; Perretti, M.; Vane, J.R. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc. Nat. Acad. Sci. USA. 1994, 91, 271–275. [Google Scholar]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A therapeutic target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar]
- Choi, J.H.; Park, Y.N.; Li, Y.; Jin, M.H.; Lee, J.; Lee, Y.; Son, J.K.; Chang, H.W.; Lee, E. Flowers of Inula. japonica attenuate inflammatory responses. Immune Netw. 2010, 10, 145–152. [Google Scholar]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar]
- Bonizzi, G. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar]
- Choi, M.S.; Lee, S.H.; Cho, H.S.; Kim, Y.; Yun, Y.P.; Jung, H.Y. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur. J. Pharmacol. 2007, 556, 181–189. [Google Scholar]
- Costa, G.; Francisco, V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Intracellular signaling pathways modulated by phenolic compounds: Application for new anti-inflammatory drugs discovery. Curr. Med. Chem. 2012, 19, 2876–2900. [Google Scholar]
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 26.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar]
- Korb, A.; Tohidast-Akrad, M.; Cetin, E.; Axmann, R.; Smolen, J.; Schett, G. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2745–2756. [Google Scholar]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar]
- Titheradge, M.A. The enzymatic measurement of nitrate and nitrite. Meth. Mol. Biol. 1998, 100, 83–91. [Google Scholar]
- Li, D.L.; Li, X.M.; Wang, B.G. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium. rubrum: Structural elucidation and DPPH radical scavenging activity. J. Microbiol. Biotechnology 2009, 19, 675–680. [Google Scholar]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Wang, B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium. rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Proksch, P.; Wang, B.G. Benzaldehyde derivatives from Eurotium. rubrum, an endophytic fungus derived from the mangrove plant Hibiscus tiliaceus. Chem. Pharm. Bull. Tokyo 2008, 56, 1282–1285. [Google Scholar]
- Dossena, A.; Marchelli, R.; Pochini, A. New metabolites of Aspergillus. amstelodami related to the biogenesis of neoechinulin. J. Chem. Soc. Chem. Commun. 1974, 771–772. [Google Scholar]
- Slack, G.J.; Puniani, E.; Frisvad, J.C.; Samson, R.A.; Miller, J.D. Secondary metabolites from Eurotium. species, Aspergillus. calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009, 113, 480–490. [Google Scholar]
- Pettit, G.R.; Hogan, F.; Xu, J.P.; Tan, R.; Nogawa, T.; Cichacz, Z.; Pettit, R.K.; Du, J.; Ye, Q.H.; Cragg, G.M.; et al. Antineoplastic agents. 563. New sources of naturally occurring cancer cell growth inhibitors from marine organisms, terrestrial plants, and microorganisms (1a,). J. Nat. Prod. 2008, 71, 438–444. [Google Scholar]
- Kimoto, K.; Aoki, T.; Shibata, Y.; Kamisuki, S.; Sugawara, F.; Kuramochi, K.; Nakazaki, A.; Kobayashi, S.; Kuroiwa, K.; Watanabe, N.; Arai, T. Structure-activity relationships of neoechinulin A analogues with cytoprotection against peroxynitrite-induced PC12 cell death. J. Antibiot. 2007, 60, 614–621. [Google Scholar]
- Maruyama, K.; Ohuchi, T.; Yoshida, K.; Shibata, Y.; Sugawara, F.; Arai, T. Protective properties of neoechinulin A against SIN-1-induced neuronal cell death. J. Biochem. 2004, 136, 81–87. [Google Scholar]
- Yagi, R.; Doi, M. Isolation of an Antioxidative Substance Produced by Aspergillus. repens. Biosci. Biotechnol. Biochem. 1999, 63, 932–933. [Google Scholar]
- Li, Y.; Li, X.; Kim, S.K.; Kang, J.S.; Choi, H.D.; Rho, J.R.; Son, B.W. Golmaenone, a new diketopiperazine alkaloid from the marine-derived fungus Aspergillus. sp. Chem. Pharm. Bull. Tokyo. 2004, 52, 375–376. [Google Scholar]
- Miller, J.D.; Sun, M.; Gilyan, A.; Roy, J.; Rand, T.G. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem-Biol. Inter. 2010, 183, 113–124. [Google Scholar]
- Dewapriya, P.; Li, Y.X.; Himaya, S.W.; Pangestuti, R.; Kim, S.K. Neoechinulin A suppresses amyloid-β oligomer-induced microglia activation and thereby protects PC-12 cells from inflammation-mediated toxicity. Neurotoxicology 2013, 35, 30–40. [Google Scholar] [CrossRef]
- Rand, T.G.; Dipenta, J.; Robbins, C.; Miller, J.D. Effects of low molecular weight fungal compounds on inflammatory gene transcription and expression in mouse alveolar macrophages. Chem-Biol. Inter. 2011, 190, 139–147. [Google Scholar]
- Sample Availability: Samples of the neoechinulins A and B are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, K.-S.; Cui, X.; Lee, D.-S.; Sohn, J.H.; Yim, J.H.; Kim, Y.-C.; Oh, H. Anti-Inflammatory Effect of Neoechinulin A from the Marine Fungus Eurotium sp. SF-5989 through the Suppression of NF-кB and p38 MAPK Pathways in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Molecules 2013, 18, 13245-13259. https://doi.org/10.3390/molecules181113245
Kim K-S, Cui X, Lee D-S, Sohn JH, Yim JH, Kim Y-C, Oh H. Anti-Inflammatory Effect of Neoechinulin A from the Marine Fungus Eurotium sp. SF-5989 through the Suppression of NF-кB and p38 MAPK Pathways in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Molecules. 2013; 18(11):13245-13259. https://doi.org/10.3390/molecules181113245
Chicago/Turabian StyleKim, Kyoung-Su, Xiang Cui, Dong-Sung Lee, Jae Hak Sohn, Joung Han Yim, Youn-Chul Kim, and Hyuncheol Oh. 2013. "Anti-Inflammatory Effect of Neoechinulin A from the Marine Fungus Eurotium sp. SF-5989 through the Suppression of NF-кB and p38 MAPK Pathways in Lipopolysaccharide-Stimulated RAW264.7 Macrophages" Molecules 18, no. 11: 13245-13259. https://doi.org/10.3390/molecules181113245
APA StyleKim, K.-S., Cui, X., Lee, D.-S., Sohn, J. H., Yim, J. H., Kim, Y.-C., & Oh, H. (2013). Anti-Inflammatory Effect of Neoechinulin A from the Marine Fungus Eurotium sp. SF-5989 through the Suppression of NF-кB and p38 MAPK Pathways in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Molecules, 18(11), 13245-13259. https://doi.org/10.3390/molecules181113245



