Following the Mechanisms of Bacteriostatic versus Bactericidal Action Using Raman Spectroscopy
Abstract
:1. Introduction
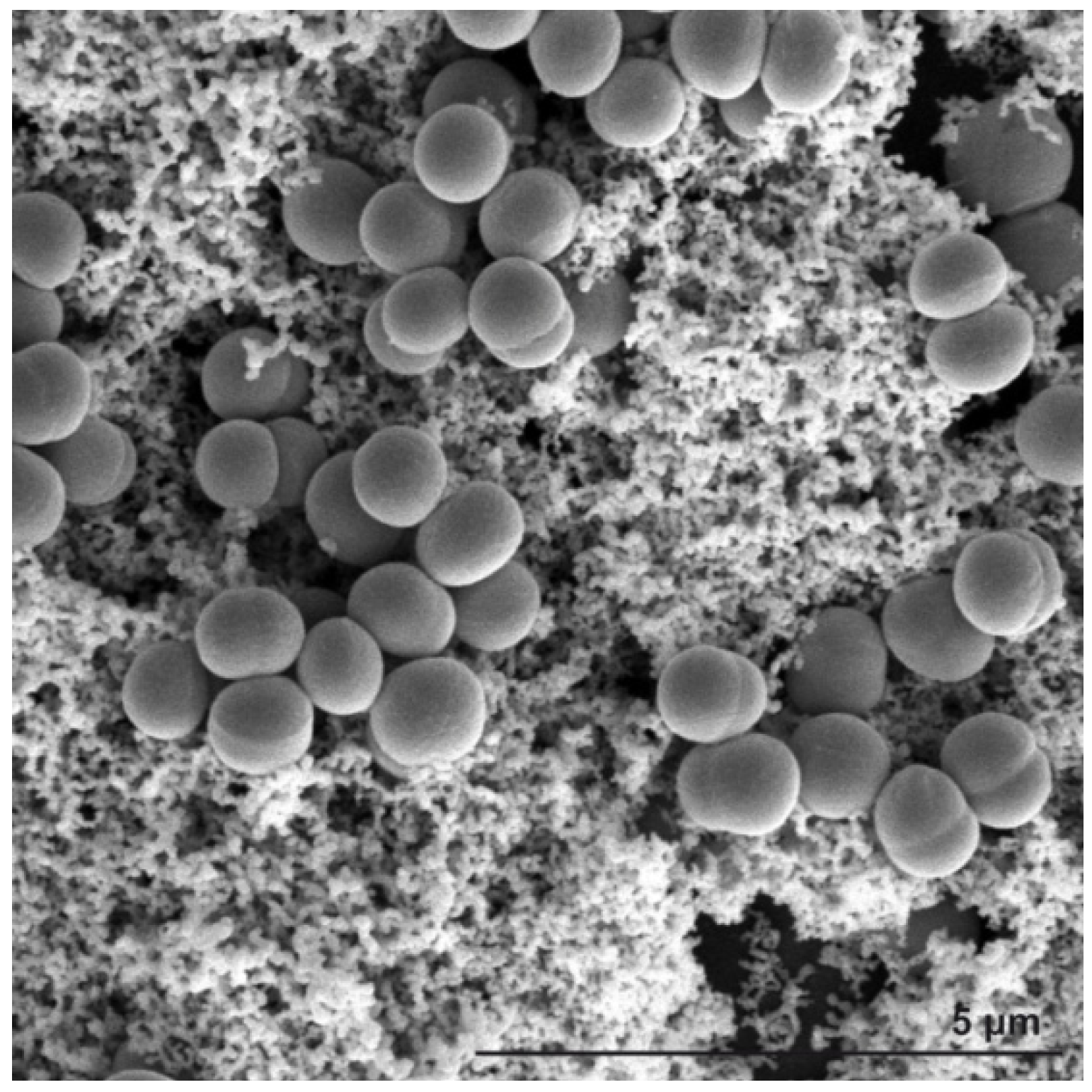
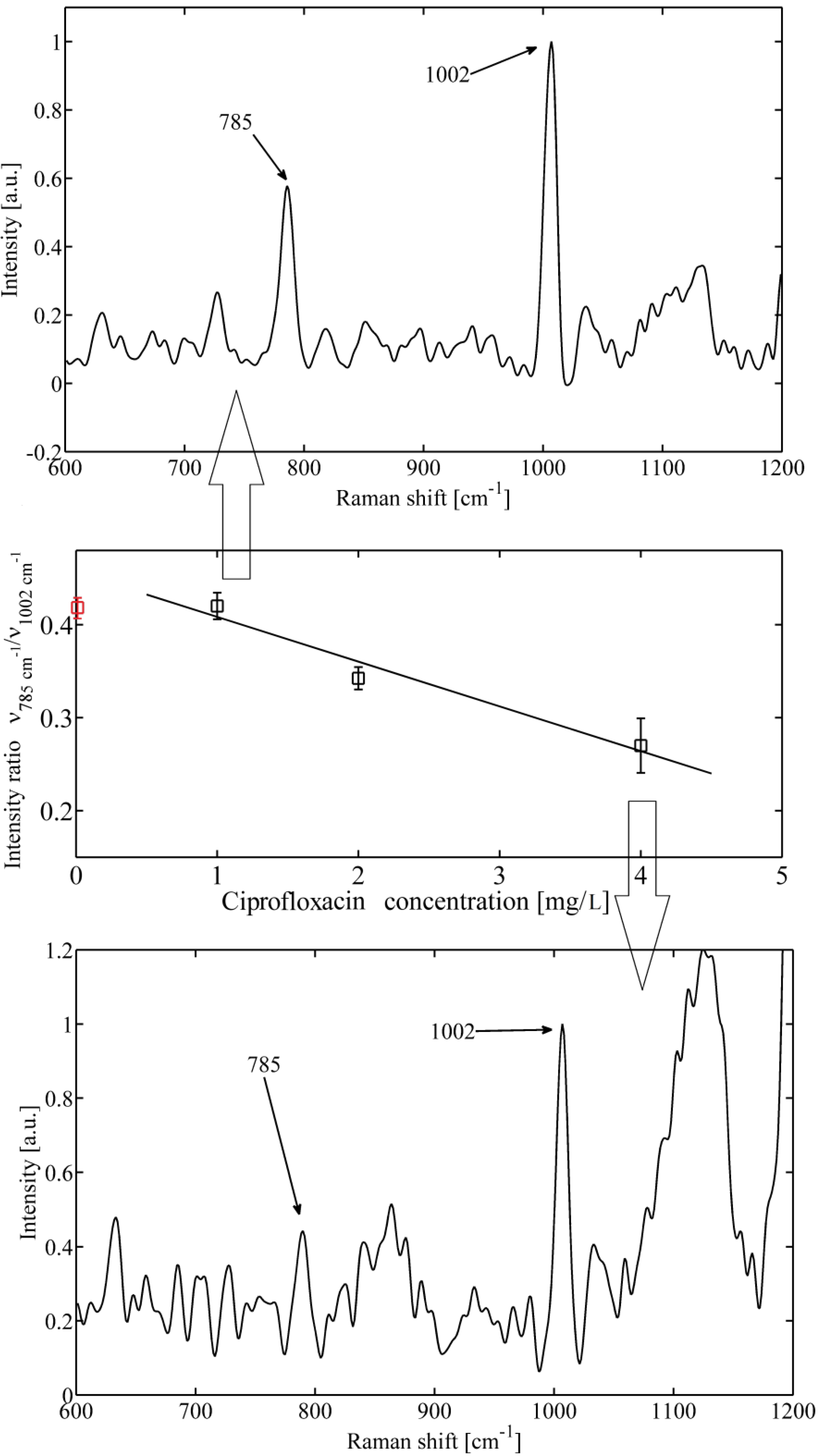
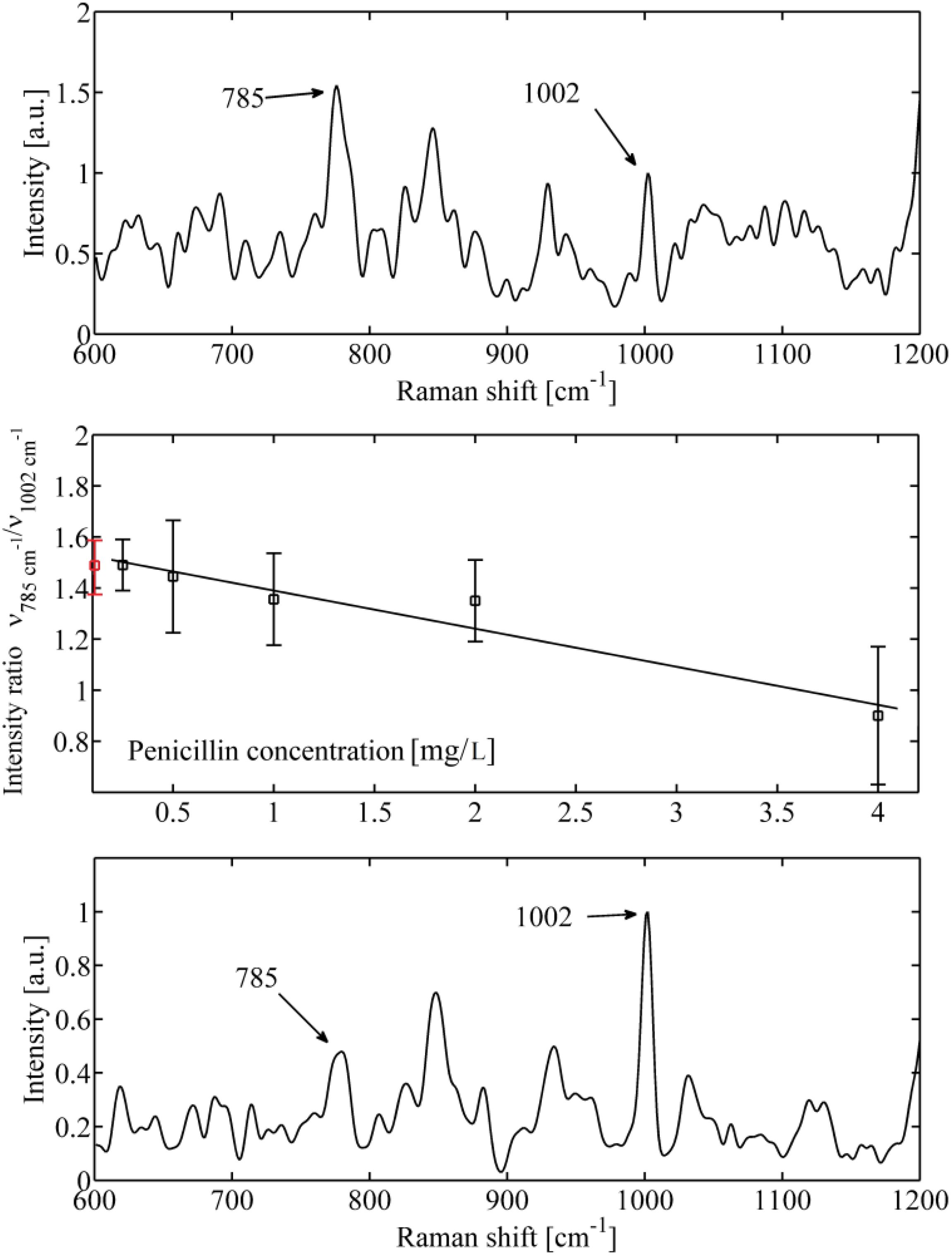
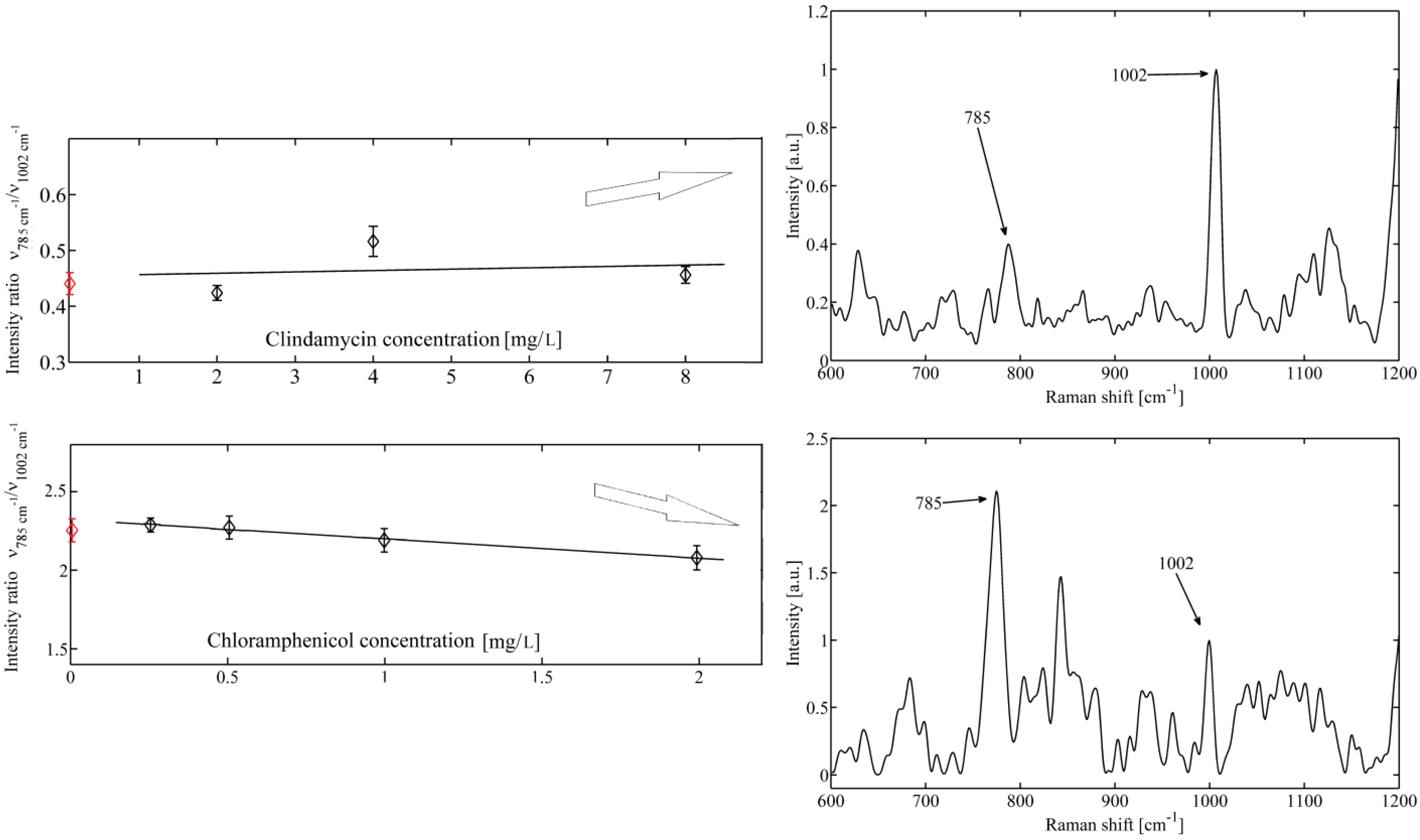
2. Results and Discussion
3. Experimental
3.1. Experimental Setup and Procedures
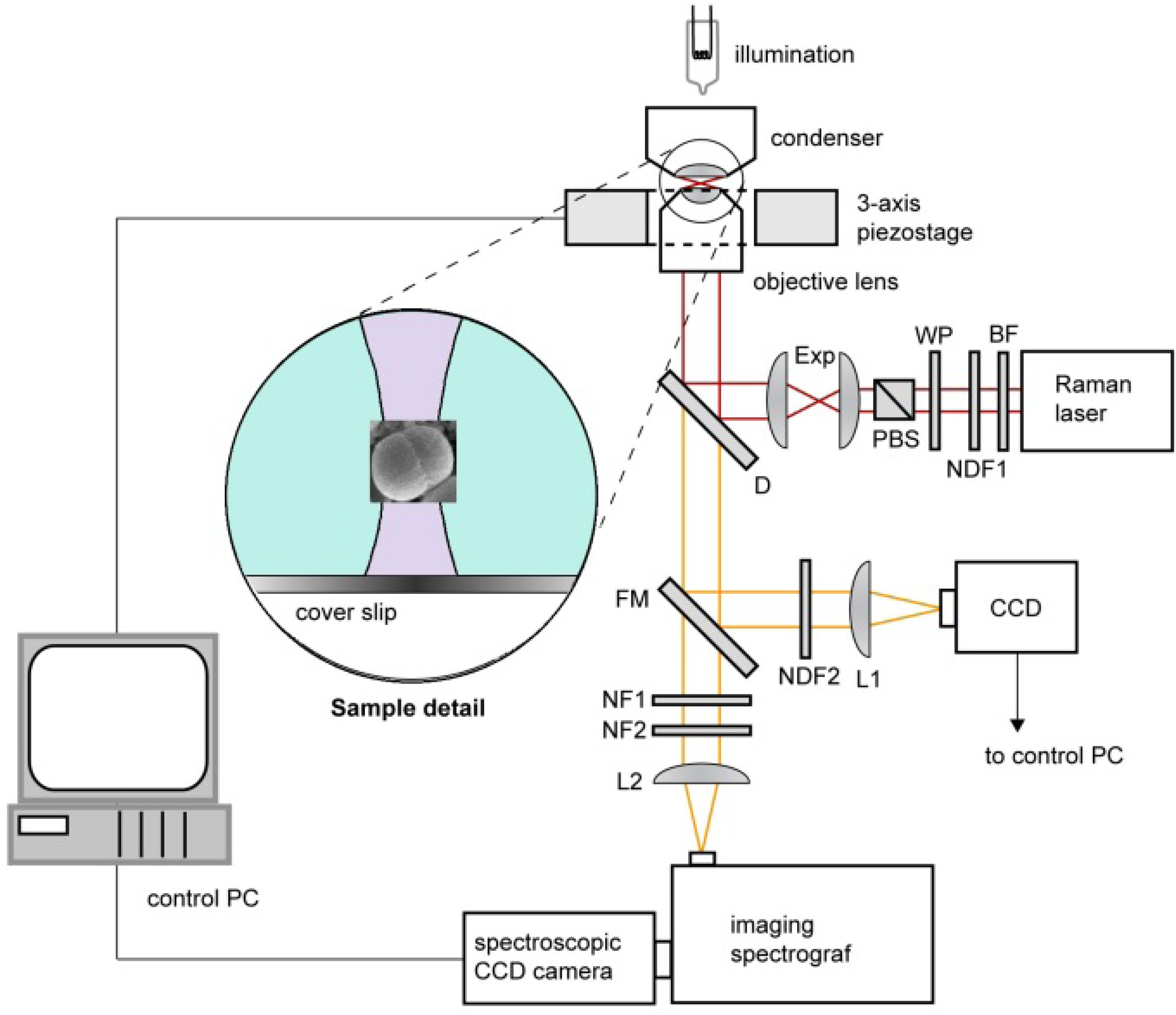
3.2. Spectrum Processing and Analysis
3.3. Sample Preparation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Samek, O.; Al-Marashi, J.F.M.; Telle, H.H. The potential of Raman spectroscopy for the identification of biofilm formation by Staphylococcus epidermidis. Laser Phys. Lett. 2010, 7, 378–383. [Google Scholar] [CrossRef]
- Ruzicka, F.; Hola, V.; Votava, M.; Tejkalova, R.; Horvat, R.; Heroldova, M.; Woznicova, V. Biofilm Detection and the Clinical Significance of Staphylococcus epidermidis Isolates. Folia Microbiol. 2004, 49, 596–600. [Google Scholar]
- Kim, J.; Park, H.-D.; Chung, S. Microfluidic Approaches to Bacterial Biofilm Formation. Molecules 2012, 17, 9818–9834. [Google Scholar]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.-P.; van den Braak, N.; Endtz, H.P.; Naumann, D.; Puppels, G.J. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Method. 2002, 51, 255–271. [Google Scholar] [CrossRef]
- Nagels, N.; Hauchecorne, D.; Herrebout, W.A. Exploring the C-X…π Halogen Bonding Motif: An Infrared and Raman Study of the Complexes of CF3X (X = Cl, Br and I) with the Aromatic Model Compounds Benzene and Toluene. Molecules 2013, 18, 6829–6851. [Google Scholar] [CrossRef]
- Huo, M.-M.; Liu, W.-L.; Zheng, Z.-R.; Zhang, W.; Li, A.-H.; Xu, D.-P. Effect of End Groups on the Raman Spectra of Lycopene and β-Carotene under High Pressure. Molecules 2011, 16, 1973–1980. [Google Scholar] [CrossRef]
- De Gelder, J.; Scheldeman, P.; Leus, K.; Heyndrickx, M.; Vandenabeele, P.; Moens, L.; De Vos, P. Raman spectroscopic study of bacterial endospores. Anal. Bioanal. Chem. 2007, 389, 2143–2151. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Vancanneyt, M.; De Vos, P.; Moens, L. Methods for extracting biochemical information from bacterial Raman spectra: Focus on a group of structurally similar biomolecules—Fatty acids. Anal. Chim. Acta 2007, 603, 167–175. [Google Scholar] [CrossRef]
- Samek, O.; Telle, H.H.; Harris, L.G.; Bloomfield, M.; Mack, D. Raman spectroscopy for rapid discrimination of Staphylococcus epidermidis clones related to medical device-associated infections. Laser Phys. Lett. 2008, 5, 465–470. [Google Scholar] [CrossRef]
- Tuma, R. Raman spectroscopy of proteins: From peptides to large assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Notingher, I.; Hench, L.L. Raman microspectroscopy: A noninvasive tool for studies of individual living cells in vitro. Expert Rev. Med. Devices 2006, 3, 215–234. [Google Scholar] [CrossRef]
- Notingher, I. Raman Spectroscopy cell-based Biosensors. Sensors 2007, 7, 1343–1358. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar]
- Mansour, H.M.; Hickey, A.J. Raman characterization and chemical imaging of biocolloidal self- assemblies, drug delivery systems, and pulmonary inhalation aerosols: A review. AAPS PharmSciTech 2007, 8, 99–109. [Google Scholar] [CrossRef]
- Harz, A.; Rösch, P.; Popp, J. Vibrational spectroscopy—A powerful tool for the rapid identification of microbial cells at the single-cell level. Cytometry Part A 2009, 75A, 104–113. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41, S113–S119. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Paul, M.; Kariv, G.; Goldberg, E.; Raskin, M.; Shaked, H.; Hazzan, R.; Samra, Z.; Paghis, D.; Bishara, J.; Leibovici, L. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob Chemother. 2010, 65, 2658–2665. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Smaill, F. Antibiotic susceptibility and resistance testing: An overview. Can. J. Gastroenterol. 2000, 14, 871–875. [Google Scholar]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbial. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Ashkin, A.; Driedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef]
- Petrov, D.V. Raman spectroscopy of optically trapped particles. J. Opt. A 2007, 9, S139–S156. [Google Scholar] [CrossRef]
- Jonas, A.; Zemanek, P. Light at work: The use of optical forces for particle manipulation, sorting, and analysis. Electrophoresis 2008, 29, 4813–4851. [Google Scholar] [CrossRef]
- Vermeulen, K.C.; Wuite, G.J.L.; Stienen, G.J.M.; Schmidt, C.F. Optical trap stiffness in the presence and absence of spherical aberrations. Appl. Opt. 2006, 45, 1812–1819. [Google Scholar] [CrossRef]
- Brandt, N.N.; Brovko, O.O.; Chikishev, A.Y.; Paraschuk, O.D. Optimization of the rolling-circle filter for Raman background subtraction. Appl. Spectrosc. 2006, 60, 288–293. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; CLSI document M07-A9. Clinical and Laboratory Standards Institut: Wayne, PA, USA, 2012. [Google Scholar]
- Sample Availability: Contact the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bernatová, S.; Samek, O.; Pilát, Z.; Šerý, M.; Ježek, J.; Jákl, P.; Šiler, M.; Krzyžánek, V.; Zemánek, P.; Holá, V.; et al. Following the Mechanisms of Bacteriostatic versus Bactericidal Action Using Raman Spectroscopy. Molecules 2013, 18, 13188-13199. https://doi.org/10.3390/molecules181113188
Bernatová S, Samek O, Pilát Z, Šerý M, Ježek J, Jákl P, Šiler M, Krzyžánek V, Zemánek P, Holá V, et al. Following the Mechanisms of Bacteriostatic versus Bactericidal Action Using Raman Spectroscopy. Molecules. 2013; 18(11):13188-13199. https://doi.org/10.3390/molecules181113188
Chicago/Turabian StyleBernatová, Silvie, Ota Samek, Zdeněk Pilát, Mojmír Šerý, Jan Ježek, Petr Jákl, Martin Šiler, Vladislav Krzyžánek, Pavel Zemánek, Veronika Holá, and et al. 2013. "Following the Mechanisms of Bacteriostatic versus Bactericidal Action Using Raman Spectroscopy" Molecules 18, no. 11: 13188-13199. https://doi.org/10.3390/molecules181113188
APA StyleBernatová, S., Samek, O., Pilát, Z., Šerý, M., Ježek, J., Jákl, P., Šiler, M., Krzyžánek, V., Zemánek, P., Holá, V., Dvořáčková, M., & Růžička, F. (2013). Following the Mechanisms of Bacteriostatic versus Bactericidal Action Using Raman Spectroscopy. Molecules, 18(11), 13188-13199. https://doi.org/10.3390/molecules181113188




