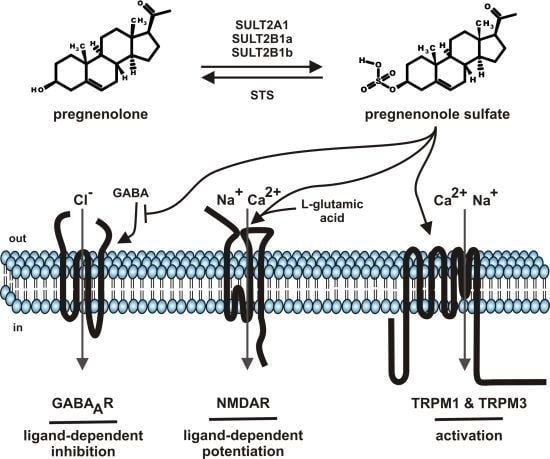
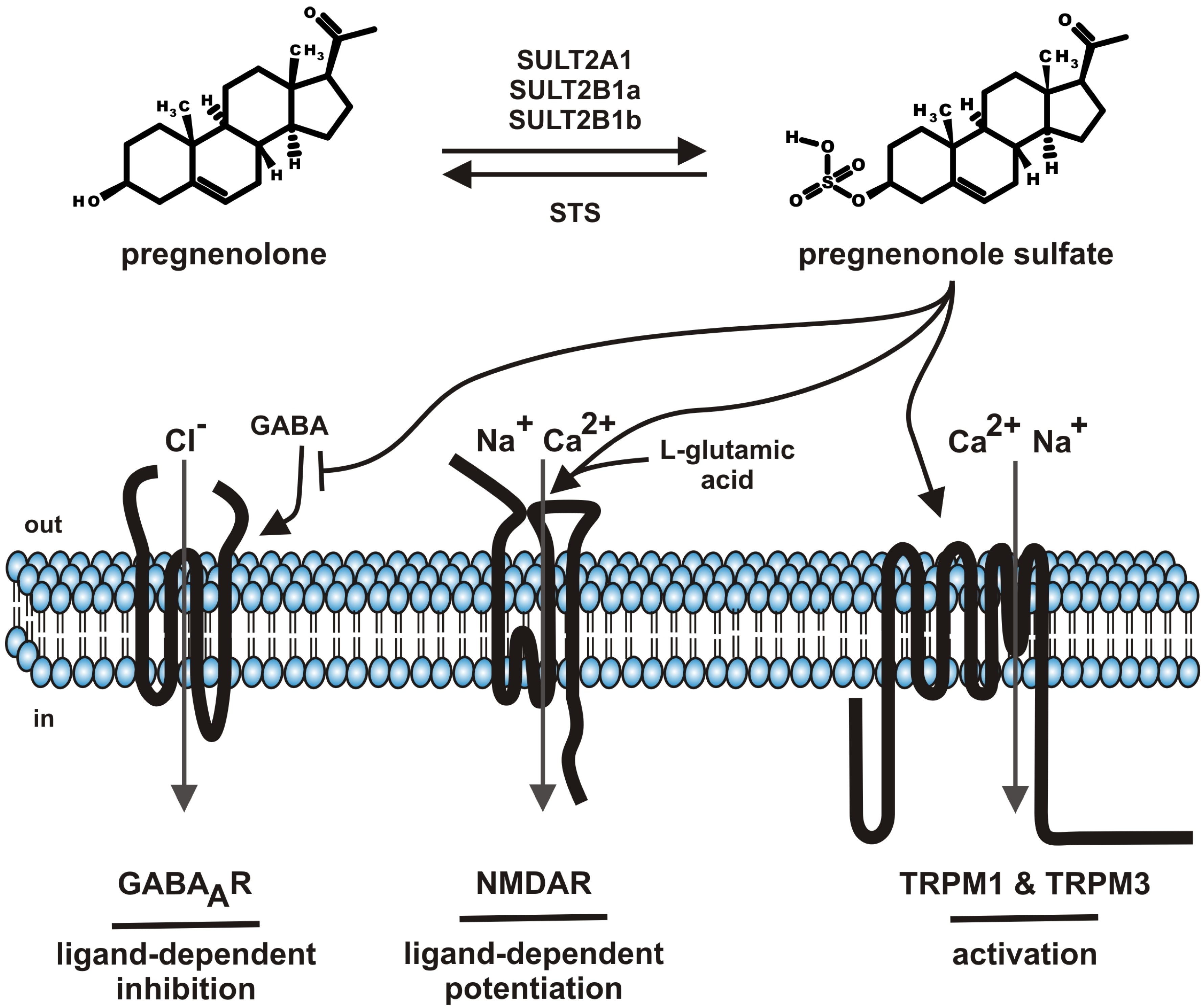
Pregnenolone Sulfate: From Steroid Metabolite to TRP Channel Ligand
Abstract
:1. Introduction
2. Pregnenolone Sulfate—Synthesis and Metabolism
3. Pregnenolone Sulfate Levels in Serum and Tissues
4. Transporter—Cellular Uptake of Pregnenolone Sulfate
5. Molecular Targets of Pregnenolone Sulfate
| Molecular target | Mode of action | EC50/IC50 values | Physiological impact |
|---|---|---|---|
| GABAA channel | inhibition | IC50 7.2 µM | long term potentiation, memory & learning, anxiolysis, general anesthesia epilepsy, musclar cramps |
| (GABA 3 µM) [63] | |||
| NMDA receptor | potentiation | EC50 33 µM | neuronal development, synapse formation |
| (NMDA 5 µM) [64] | |||
| TRPM1 | activation | melanocyte function, melanin synthesis, phototransduction | |
| TRPM3 | activation | EC50 23 µM (– 80 mV) [6] | pain modulation, insulin secretion, neuronal development |
| EC50 12 µM (+ 80 mV) [6] | |||
| [Pregnenolone EC50 15 µM | |||
| (–80 mV)] [6] | |||
| [Pregnenolone EC50 14 µM | |||
| (+80 mV)] [6] |
5.1. GABAA Channels
5.2. NMDA Receptors
5.3. TRP Channels (TRPM1, TRPM3)
6. Conclusions
Conflicts of Interest
References
- Wehling, M.; Losel, R. Non-genomic steroid hormone effects: membrane or intracellular receptors? J. Steroid Biochem. Mol. Biol. 2006, 102, 180–183. [Google Scholar] [CrossRef]
- Sooksawate, T.; Simmonds, M.A. Influence of membrane cholesterol on modulation of the GABAA receptor by neuroactive steroids and other potentiators. Br. J. Pharmacol. 2001, 134, 1303–1311. [Google Scholar] [CrossRef]
- Dubrovsky, B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol. Biochem. Behav. 2006, 84, 644–655. [Google Scholar] [CrossRef]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef]
- Zheng, P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog. Neurobiol. 2009, 89, 134–152. [Google Scholar] [CrossRef]
- Wagner, T.F.; Loch, S.; Lambert, S.; Straub, I.; Mannebach, S.; Mathar, I.; Düfer, M.; Lis, A.; Flockerzi, V.; Philipp, S.E.; et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 2008, 10, 1421–1430. [Google Scholar] [CrossRef]
- Falany, C.N. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997, 11, 206–216. [Google Scholar]
- Strott, C.A. Sulfonation and molecular action. Endocr. Rev. 2002, 23, 703–732. [Google Scholar] [CrossRef]
- Burel, D.; Li, J.H.; Do-Rego, J.L.; Wang, A.F.; Luu-The, V.; Pelletier, G.; Tillet, Y.; Taragnat, C.; Kwon, H.B.; Seong, J.Y.; Vaudry, H. Gonadotropin-releasing hormone stimulates the biosynthesis of pregnenolone sulfate and dehydroepiandrosterone sulfate in the hypothalamus. Endocrinology 2013, 154, 2114–2128. [Google Scholar] [CrossRef]
- Her, C.; Wood, T.C.; Eichler, E.E.; Mohrenweiser, H.W.; Ramagli, L.S.; Siciliano, M.J.; Weinshilboum, R.M. Human hydroxysteroid sulfotransferase SULT2B1: two enzymes encoded by a single chromosome 19 gene. Genomics 1998, 53, 284–295. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Maeda, N.; Okumura, K.; Tanaka, E.; Suzuki, T.; Miyasho, T.; Haeno, S.; Ueda, H.; Hoshi, N.; Yokota, H. Downregulation of cytochrome P450scc as an initial adverse effect of adult exposure to diethylstilbestrol on testicular steroidogenesis. Environ. Toxicol. 2013, in press. [Google Scholar]
- Parte, P.; Balasinor, N.; Gill-Sharma, M.K.; Maitra, A.; Juneja, H.S. Temporal effect of tamoxifen on cytochrome P450 side chain cleavage gene expression and steroid concentration in adult male rats. J. Steroid Biochem. Mol. Biol. 2002, 82, 349–358. [Google Scholar] [CrossRef]
- Wu, L.; Yan, J.; Qu, S.C.; Feng, Y.Q.; Jiang, X.L. Abnormal regulation for progesterone production in placenta with prenatal cocaine exposure in rats. Placenta 2012, 33, 977–981. [Google Scholar] [CrossRef]
- Kohjitani, A.; Fuda, H.; Hanyu, O.; Strott, C.A. Regulation of SULT2B1a (pregnenolone sulfotransferase) expression in rat C6 glioma cells: relevance of AMPA receptor-mediated NO signaling. Neurosci. Lett. 2008, 430, 75–80. [Google Scholar] [CrossRef]
- Kauffman, F.C. Sulfonation in pharmacology and toxicology. Drug Metab. Rev. 2004, 36, 823–843. [Google Scholar] [CrossRef]
- Geyer, J.; Wilke, T.; Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 372, 413–431. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Meier, P.J. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch. 2004, 447, 653–665. [Google Scholar] [CrossRef]
- Ghosh, D. Human sulfatases: a structural perspective to catalysis. Cell. Mol. Life Sci. 2007, 64, 2013–2022. [Google Scholar] [CrossRef]
- Kriz, L.; Bicikova, M.; Hampl, R. Roles of steroid sulfatase in brain and other tissues. Physiol. Res. 2008, 57, 657–668. [Google Scholar]
- Reed, M.J.; Purohit, A.; Woo, L.W.; Newman, S.P.; Potter, B.V. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr. Rev. 2005, 26, 171–202. [Google Scholar]
- Hernandez-Martin, A.; Gonzalez-Sarmiento, R.; De Unamuno, P. X-linked ichthyosis: an update. Br. J. Dermatol. 1999, 141, 617–627. [Google Scholar] [CrossRef]
- Chibbar, R.; Mitchell, B.F. Steroid sulfohydrolase in human chorion and decidua: studies using pregnenolone sulfate and dehydroepiandrosterone sulfate as substrate. J. Clin. Endocrinol. Metab. 1990, 70, 1693–1701. [Google Scholar] [CrossRef]
- Kauffman, F.C.; Whittaker, M.; Anundi, I.; Thurman, R.G. Futile cycling of a sulfate conjugate by isolated hepatocytes. Mol. Pharmacol. 1991, 39, 414–420. [Google Scholar]
- Payne, A.H.; Jaffe, R.B. Androgen formation from pregnenolone sulfate by the human fetal ovary. J. Clin. Endocrinol. Metab. 1974, 39, 300–304. [Google Scholar] [CrossRef]
- Stone, B.A.; Seamark, R.F.; Kelly, R.W.; Deam, S. Production of steroids and release of prostaglandins by spherical pig blastocysts in vitro. Aust. J. Biol. Sci. 1986, 39, 283–293. [Google Scholar]
- de Peretti, E.; Mappus, E. Pattern of plasma pregnenolone sulfate levels in humans from birth to adulthood. J. Clin. Endocrinol. Metab. 1983, 57, 550–556. [Google Scholar] [CrossRef]
- Bicikova, M.; Klak, J.; Hill, M.; Zizka, Z.; Hampl, R.; Calda, P. Two neuroactive steroids in midpregnancy as measured in maternal and fetal sera and in amniotic fluid. Steroids 2002, 67, 399–402. [Google Scholar] [CrossRef]
- Chang, R.J.; Buster, J.E.; Blakely, J.L.; Okada, D.M.; Hobel, C.J.; Abraham, G.E.; Marshall, J.R. Simultaneous comparison of Δ5-3β-hydroxysteroid levels in the fetoplacental circulation of normal pregnancy in labor and not in labor. J. Clin. Endocrinol. Metab. 1976, 42, 744–751. [Google Scholar] [CrossRef]
- Conrad, S.H.; Pion, R.J.; Kitchin, J.D., 3rd. Pregnenolone sulfate in human pregnancy plasma. J. Clin. Endocrinol. Metab. 1967, 27, 114–119. [Google Scholar] [CrossRef]
- Hill, M.; Parizek, A.; Klak, J.; Hampl, R.; Sulcova, J.; Havlikova, H.; Lapcik, O.; Bicikova, M.; Fait, T.; Kancheva, R.; et al. Neuroactive steroids, their precursors and polar conjugates during parturition and postpartum in maternal and umbilical blood: 3.3β-hydroxy-5-ene steroids. J. Steroid Biochem. Mol. Biol. 2002, 82, 241–250. [Google Scholar] [CrossRef]
- Klak, J.; Hill, M.; Parizek, A.; Havlikova, H.; Bicikova, M.; Hampl, R.; Fait, T.; Sulcova, J.; Pouzar, V.; Kancheva, R.; Starka, L. Pregnanolone isomers, pregnenolone and their polar conjugates around parturition. Physiol. Res. 2003, 52, 211–221. [Google Scholar]
- Mathur, R.S.; Landgrebe, S.; Moody, L.O.; Powell, S.; Williamson, H.O. Plasma steroid concentrations in maternal and umbilical circulation after spontaneous onset of labor. J. Clin. Endocrinol. Metab. 1980, 51, 1235–1238. [Google Scholar] [CrossRef]
- Norman, R.J.; Deppe, W.M.; Joubert, S.M.; Marivate, M. Umbilical artery concentrations of androstenedione increased in early labour in the leading twin fetus. Br. J. Obstet. Gynaecol. 1984, 91, 776–780. [Google Scholar] [CrossRef]
- Scommegna, A.; Burd, L.; Bieniarz, J. Progesterone and pregnenolone sulfate in pregnancy plasma. Am. J. Obstet. Gynecol. 1972, 113, 60–65. [Google Scholar]
- Nathanielsz, P.W.; Elsner, C.; Magyar, D.; Fridshal, D.; Freeman, A.; Buster, J.E. Time trend analysis of plasma unconjugated and sulfoconjugated estrone and 3β-Δ5-steroids in fetal and maternal sheep plasma in relation to spontaneous parturition at term. Endocrinology 1982, 110, 1402–1407. [Google Scholar] [CrossRef]
- McKay, S.A.; Jenkin, G.; Thorburn, G.D. Peripheral plasma concentrations of pregnenolone sulphate, pregnenolone, progesterone and 20α-hydroxy-4-pregnen-3-one in ewes throughout the oestrous cycle. J. Endocrinol. 1987, 113, 231–237. [Google Scholar] [CrossRef]
- Wang, M.; Seippel, L.; Purdy, R.H.; Backstrom, T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J. Clin. Endocrinol. Metab. 1996, 81, 1076–1082. [Google Scholar] [CrossRef]
- de la Torre, B.; Hedman, M.; Noren, S.; Sjoberg, B. Blood and tissue steroid levels and their interrelationship in men with pathological conditions of the reproductive organs. Int. J. Androl. 1986, 9, 241–249. [Google Scholar] [CrossRef]
- Leinonen, P.; Ruokonen, A.; Kontturi, M.; Vihko, R. Effects of estrogen treatment on human testicular unconjugated steroid and steroid sulfate production in vivo. J. Clin. Endocrinol. Metab. 1981, 53, 569–573. [Google Scholar] [CrossRef]
- Ruokonen, A.; Lukkarinen, O.; Vihko, R. Secretion of steroid sulfates from human testis and their response to a single intramuscular injection of 5000 IU hCG. J. Steroid Biochem. 1981, 14, 1357–1360. [Google Scholar] [CrossRef]
- de Peretti, E.; Forest, M.G.; Loras, B.; Morel, Y.; David, M.; Francois, R.; Bertrand, J. Usefulness of plasma pregnenolone sulfate in testing pituitary-adrenal function in children. Acta Endocrinol. Suppl. (Copenh) 1986, 279, 259–263. [Google Scholar]
- Bicikova, M.; Szamel, I.; Hill, M.; Tallova, J.; Starka, L. Allopregnanolone, pregnenolone sulfate, and epitestosterone in breast cyst fluid. Steroids 2001, 66, 55–57. [Google Scholar] [CrossRef]
- Hedman, M.; Nilsson, E.; de la Torre, B. Low sulpho-conjugated steroid hormone levels in systemic lupus erythematosus (SLE). Clin. Exp. Rheumatol. 1989, 7, 583–588. [Google Scholar]
- Hedman, M.; Nilsson, E.; de la Torre, B. Low blood and synovial fluid levels of sulpho-conjugated steroids in rheumatoid arthritis. Clin. Exp. Rheumatol. 1992, 10, 25–30. [Google Scholar]
- Tagawa, N.; Tamanaka, J.; Fujinami, A.; Kobayashi, Y.; Takano, T.; Fukata, S.; Kuma, K.; Tada, H.; Amino, N. Serum dehydroepiandrosterone, dehydroepiandrosterone sulfate, and pregnenolone sulfate concentrations in patients with hyperthyroidism and hypothyroidism. Clin. Chem. 2000, 46, 523–528. [Google Scholar]
- Tallova, J.; Tomandl, J.; Bicikova, M.; Simickova, M. Homocysteine in breast cyst fluid. Eur. J. Clin. Invest. 2001, 31, 623–627. [Google Scholar] [CrossRef]
- Schumacher, M.; Liere, P.; Akwa, Y.; Rajkowski, K.; Griffiths, W.; Bodin, K.; Sjovall, J.; Baulieu, E.E. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem. Int. 2008, 52, 522–540. [Google Scholar] [CrossRef]
- Weill-Engerer, S.; David, J.P.; Sazdovitch, V.; Liere, P.; Eychenne, B.; Pianos, A.; Schumacher, M.; Delacourte, A.; Baulieu, E.E.; Akwa, Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J. Clin. Endocrinol. Metab. 2002, 87, 5138–5143. [Google Scholar] [CrossRef]
- Corpechot, C.; Synguelakis, M.; Talha, S.; Axelson, M.; Sjovall, J.; Vihko, R.; Baulieu, E.E.; Robel, P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983, 270, 119–125. [Google Scholar] [CrossRef]
- Jo, D.H.; Abdallah, M.A.; Young, J.; Baulieu, E.E.; Robel, P. Pregnenolone, dehydroepiandrosterone, and their sulfate and fatty acid esters in the rat brain. Steroids 1989, 54, 287–297. [Google Scholar] [CrossRef]
- Caldeira, J.C.; Wu, Y.; Mameli, M.; Purdy, R.H.; Li, P.K.; Akwa, Y.; Savage, D.D.; Engen, J.R.; Valenzuela, C.F. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J. Neurochem. 2004, 90, 1530–1539. [Google Scholar] [CrossRef]
- Xu, B.; Yang, R.; Chang, F.; Chen, L.; Xie, G.; Sokabe, M.; Chen, L. Neurosteroid PREGS protects neurite growth and survival of newborn neurons in the hippocampal dentate gyrus of APPswe/PS1dE9 mice. Curr. Alzheimer Res. 2012, 9, 361–372. [Google Scholar] [CrossRef]
- St-Pierre, M.V.; Hagenbuch, B.; Ugele, B.; Meier, P.J.; Stallmach, T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J. Clin. Endocrinol. Metab. 2002, 87, 1856–1863. [Google Scholar] [CrossRef]
- Grube, M.; Kock, K.; Karner, S.; Reuther, S.; Ritter, C.A.; Jedlitschky, G.; Kroemer, H.K. Modification of OATP2B1-mediated transport by steroid hormones. Mol. Pharmacol. 2006, 70, 1735–1741. [Google Scholar] [CrossRef]
- Geyer, J.; Doring, B.; Meerkamp, K.; Ugele, B.; Bakhiya, N.; Fernandes, C.F.; Godoy, J.R.; Glatt, H.; Petzinger, E. Cloning and functional characterization of human sodium-dependent organic anion transporter (SLC10A6). J. Biol. Chem. 2007, 282, 19728–19741. [Google Scholar] [CrossRef]
- Grosser, G.; Fietz, D.; Gunther, S.; Bakhaus, K.; Schweigmann, H.; Ugele, B.; Brehm, R.; Petzinger, E.; Bergmann, M.; Geyer, J. Cloning and functional characterization of the mouse sodium-dependent organic anion transporter Soat (Slc10a6). J. Steroid Biochem. Mol. Biol. 2013, 138C, 90–99. [Google Scholar]
- Fang, F.; Christian, W.V.; Gorman, S.G.; Cui, M.; Huang, J.; Tieu, K.; Ballatori, N. Neurosteroid transport by the organic solute transporter OSTα-OSTβ. J. Neurochem. 2010, 115, 220–233. [Google Scholar] [CrossRef]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Pregnenolone sulfate potentiates the inwardly rectifying K channel Kir2.3. PLoS One 2009, 4, e6311. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Wardwell-Swanson, J. Modulation of cloned human neuronal voltage-gated potassium channels (hKv1.1 and hKv2.1) by neurosteroids. Pflügers Arch. 1998, 437, 49–55. [Google Scholar] [CrossRef]
- Kudo, K.; Tachikawa, E.; Kashimoto, T. Inhibition by pregnenolone sulfate of nicotinic acetylcholine response in adrenal chromaffin cells. Eur. J. Pharmacol. 2002, 456, 19–27. [Google Scholar] [CrossRef]
- Horishita, T.; Ueno, S.; Yanagihara, N.; Sudo, Y.; Uezono, Y.; Okura, D.; Sata, T. Inhibition by pregnenolone sulphate, a metabolite of the neurosteroid pregnenolone, of voltage-gated sodium channels expressed in Xenopus oocytes. J. Pharmacol. Sci. 2012, 120, 54–58. [Google Scholar] [CrossRef]
- Wu, F.S.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 1991, 40, 333–336. [Google Scholar]
- Irwin, R.P.; Maragakis, N.J.; Rogawski, M.A.; Purdy, R.H.; Farb, D.H.; Paul, S.M. Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons. Neurosci. Lett. 1992, 141, 30–34. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bluet-Pajot, M.T.; Robel, P.; Baulieu, E.E. Pregnenolone sulfate antagonizes barbiturate-induced hypnosis. Pharmacol. Biochem. Behav. 1989, 33, 701–703. [Google Scholar] [CrossRef]
- Majewska, M.D.; Demirgoren, S.; London, E.D. Binding of pregnenolone sulfate to rat brain membranes suggests multiple sites of steroid action at the GABAA receptor. Eur. J. Pharmacol. 1990, 189, 307–315. [Google Scholar] [CrossRef]
- Majewska, M.D.; Mienville, J.M.; Vicini, S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci. Lett. 1988, 90, 279–284. [Google Scholar] [CrossRef]
- Zaman, S.H.; Shingai, R.; Harvey, R.J.; Darlison, M.G.; Barnard, E.A. Effects of subunit types of the recombinant GABAA receptor on the response to a neurosteroid. Eur. J. Pharmacol. 1992, 225, 321–330. [Google Scholar] [CrossRef]
- Akk, G.; Li, P.; Manion, B.D.; Evers, A.S.; Steinbach, J.H. Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the α1β2γ2L GABAA receptor. Mol. Pharmacol. 2007, 71, 461–472. [Google Scholar]
- Twede, V.; Tartaglia, A.L.; Covey, D.F.; Bamber, B.A. The neurosteroids dehydroepiandrosterone sulfate and pregnenolone sulfate inhibit the UNC-49 GABA receptor through a common set of residues. Mol. Pharmacol. 2007, 72, 1322–1329. [Google Scholar] [CrossRef]
- Baker, C.; Sturt, B.L.; Bamber, B.A. Multiple roles for the first transmembrane domain of GABAA receptor subunits in neurosteroid modulation and spontaneous channel activity. Neurosci. Lett. 2010, 473, 242–247. [Google Scholar] [CrossRef]
- Elfverson, M.; Linde, A.M.; Le Greves, P.; Zhou, Q.; Nyberg, F.; Johansson, T. Neurosteroids allosterically modulate the ion pore of the NMDA receptor consisting of NR1/NR2B but not NR1/NR2A. Biochem. Biophys. Res. Commun. 2008, 372, 305–308. [Google Scholar] [CrossRef]
- Johansson, T.; Frandberg, P.A.; Nyberg, F.; Le Greves, P. Molecular mechanisms for nanomolar concentrations of neurosteroids at NR1/NR2B receptors. J. Pharmacol. Exp. Ther. 2008, 324, 759–768. [Google Scholar]
- Alexander, S.P.; Mathie, A.; Peters, J.A. Guide to Receptors and Channels (GRAC), 5th edition. Br. J. Pharmacol. 2011, 164 (Suppl. 1), S1–324. [Google Scholar] [CrossRef]
- Ceccon, M.; Rumbaugh, G.; Vicini, S. Distinct effect of pregnenolone sulfate on NMDA receptor subtypes. Neuropharmacology 2001, 40, 491–500. [Google Scholar] [CrossRef]
- Horak, M.; Vlcek, K.; Chodounska, H.; Vyklicky, L., Jr. Subtype-dependence of N-methyl-D-aspartate receptor modulation by pregnenolone sulfate. Neuroscience 2006, 137, 93–102. [Google Scholar] [CrossRef]
- Malayev, A.; Gibbs, T.T.; Farb, D.H. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br. J. Pharmacol. 2002, 135, 901–909. [Google Scholar] [CrossRef]
- Petrovic, M.; Sedlacek, M.; Cais, O.; Horak, M.; Chodounska, H.; Vyklicky, L., Jr. Pregnenolone sulfate modulation of N-methyl-D-aspartate receptors is phosphorylation dependent. Neuroscience 2009, 160, 616–628. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Fukuchi, J.; Chao, J.; Igarashi, K.; Williams, K. An aspartate residue in the extracellular loop of the N-methyl-D-aspartate receptor controls sensitivity to spermine and protons. Mol. Pharmacol. 1996, 49, 1131–1141. [Google Scholar]
- Low, C.M.; Lyuboslavsky, P.; French, A.; Le, P.; Wyatte, K.; Thiel, W.H.; Marchan, E.M.; Igarashi, K.; Kashiwagi, K.; Gernert, K.; Williams, K.; Traynelis, S.F.; Zheng, F. Molecular determinants of proton-sensitive N-methyl-D-aspartate receptor gating. Mol. Pharmacol. 2003, 63, 1212–1222. [Google Scholar] [CrossRef]
- Jang, M.K.; Mierke, D.F.; Russek, S.J.; Farb, D.H. A steroid modulatory domain on NR2B controls N-methyl-D-aspartate receptor proton sensitivity. Proc. Natl. Acad. Sci. USA 2004, 101, 8198–8203. [Google Scholar] [CrossRef]
- Kostakis, E.; Smith, C.; Jang, M.K.; Martin, S.C.; Richards, K.G.; Russek, S.J.; Gibbs, T.T.; Farb, D.H. The Neuroactive Steroid Pregnenolone Sulfate Stimulates Trafficking of Functional N-Methyl D-Aspartate Receptors to the Cell Surface via a Noncanonical, G Protein, and Ca2+-Dependent Mechanism. Mol. Pharmacol. 2013, 84, 261–274. [Google Scholar] [CrossRef]
- Valenzuela, C.F.; Partridge, L.D.; Mameli, M.; Meyer, D.A. Modulation of glutamatergic transmission by sulfated steroids: role in fetal alcohol spectrum disorder. Brain Res. Rev. 2008, 57, 506–519. [Google Scholar] [CrossRef]
- Chen, L.; Miyamoto, Y.; Furuya, K.; Mori, N.; Sokabe, M. PREGS induces LTP in the hippocampal dentate gyrus of adult rats via the tyrosine phosphorylation of NR2B coupled to ERK/CREB [corrected] signaling. J. Neurophysiol. 2007, 98, 1538–1548. [Google Scholar] [CrossRef]
- Sabeti, J.; Nelson, T.E.; Purdy, R.H.; Gruol, D.L. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: role for L-type calcium channels and sigma-receptors. Hippocampus 2007, 17, 349–369. [Google Scholar] [CrossRef]
- Sliwinski, A.; Monnet, F.P.; Schumacher, M.; Morin-Surun, M.P. Pregnenolone sulfate enhances long-term potentiation in CA1 in rat hippocampus slices through the modulation of N-methyl-D-aspartate receptors. J. Neurosci. Res. 2004, 78, 691–701. [Google Scholar] [CrossRef]
- Whittaker, M.T.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate induces NMDA receptor dependent release of dopamine from synaptic terminals in the striatum. J. Neurochem. 2008, 107, 510–521. [Google Scholar] [CrossRef]
- Johansson, T.; Elfverson, M.; Zhou, Q.; Nyberg, F. Allosteric modulation of the NMDA receptor by neurosteroids in rat brain and the impact of long term morphine administration. Biochem. Biophys. Res. Commun. 2010, 401, 504–508. [Google Scholar] [CrossRef]
- Lambert, S.; Drews, A.; Rizun, O.; Wagner, T.F.; Lis, A.; Mannebach, S.; Plant, S.; Portz, M.; Meissner, M.; Philipp, S.E.; Oberwinkler, J. Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J. Biol. Chem. 2011, 286, 12221–12233. [Google Scholar] [CrossRef]
- Zamudio-Bulcock, P.A.; Valenzuela, C.F. Pregnenolone sulfate increases glutamate release at neonatal climbing fiber-to-Purkinje cell synapses. Neuroscience 2011, 175, 24–36. [Google Scholar] [CrossRef]
- Zamudio-Bulcock, P.A.; Everett, J.; Harteneck, C.; Valenzuela, C.F. Activation of steroid-sensitive TRPM3 channels potentiates glutamatergic transmission at cerebellar Purkinje neurons from developing rats. J. Neurochem. 2011, 119, 474–485. [Google Scholar] [CrossRef]
- Duncan, L.M.; Deeds, J.; Hunter, J.; Shao, J.; Holmgren, L.M.; Woolf, E.A.; Tepper, R.I.; Shyjan, A.W. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998, 58, 1515–1520. [Google Scholar]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef]
- Hellwig, N.; Albrecht, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 2005, 118, 917–928. [Google Scholar] [CrossRef]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Voets, T.; Hoefs, S.; Weidema, F.; Prenen, J.; Nilius, B.; Bindels, R.J. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003, 22, 776–785. [Google Scholar] [CrossRef]
- Harteneck, C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 307–314. [Google Scholar] [CrossRef]
- Harteneck, C.; Frenzel, H.; Kraft, R. N-(p-amylcinnamoyl)anthranilic acid (ACA): A phospholipase A2 inhibitor and TRP channel blocker. Cardiovasc. Drug Rev. 2007, 25, 61–75. [Google Scholar] [CrossRef]
- Harteneck, C.; Klose, C.; Krautwurst, D. Synthetic modulators of TRP channel activity. Adv. Exp. Med. Biol. 2011, 704, 87–106. [Google Scholar] [CrossRef]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar]
- Grimm, C.; Kraft, R.; Schultz, G.; Harteneck, C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol. Pharmacol. 2005, 67, 798–805. [Google Scholar]
- Majeed, Y.; Agarwal, A.K.; Naylor, J.; Seymour, V.A.; Jiang, S.; Muraki, K.; Fishwick, C.W.; Beech, D.J. Cis-isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br. J. Pharmacol. 2010, 161, 430–441. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef]
- Majeed, Y.; Tumova, S.; Green, B.L.; Seymour, V.A.; Woods, D.M.; Agarwal, A.K.; Naylor, J.; Jiang, S.; Picton, H.M.; Porter, K.E.; et al. Pregnenolone sulphate-independent inhibition of TRPM3 channels by progesterone. Cell Calcium 2012, 51, 1–11. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Oberwinkler, J.; Lis, A.; Giehl, K.M.; Flockerzi, V.; Philipp, S.E. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J. Biol. Chem. 2005, 280, 22540–22548. [Google Scholar]
- Wagner, T.F.; Drews, A.; Loch, S.; Mohr, F.; Philipp, S.E.; Lambert, S.; Oberwinkler, J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflügers Arch. 2010, 460, 755–765. [Google Scholar] [CrossRef]
- Naylor, J.; Li, J.; Milligan, C.J.; Zeng, F.; Sukumar, P.; Hou, B.; Sedo, A.; Yuldasheva, N.; Majeed, Y.; Beri, D.; et al. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ. Res. 2010, 106, 1507–1515. [Google Scholar]
- Klose, C.; Straub, I.; Riehle, M.; Ranta, F.; Krautwurst, D.; Ullrich, S.; Meyerhof, W.; Harteneck, C. Fenamates as TRP channel blockers: Mefenamic acid selectively blocks TRPM3. Br. J. Pharmacol. 2011, 162, 1757–1769. [Google Scholar] [CrossRef]
- Hoffmann, A.; Grimm, C.; Kraft, R.; Goldbaum, O.; Wrede, A.; Nolte, C.; Hanisch, U.K.; Richter-Landsberg, C.; Bruck, W.; Kettenmann, H.; et al. TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J. Neurochem. 2010, 114, 654–665. [Google Scholar] [CrossRef]
- Hunter, J.J.; Shao, J.; Smutko, J.S.; Dussault, B.J.; Nagle, D.L.; Woolf, E.A.; Holmgren, L.M.; Moore, K.J.; Shyjan, A.W. Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1). Genomics 1998, 54, 116–123. [Google Scholar] [CrossRef]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2009, 2, ra21. [Google Scholar] [CrossRef]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef]
- Shen, Y.; Heimel, J.A.; Kamermans, M.; Peachey, N.S.; Gregg, R.G.; Nawy, S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J. Neurosci. 2009, 29, 6088–6093. [Google Scholar] [CrossRef]
- Cao, Y.; Pahlberg, J.; Sarria, I.; Kamasawa, N.; Sampath, A.P.; Martemyanov, K.A. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 7905–7910. [Google Scholar]
- Koike, C.; Obara, T.; Uriu, Y.; Numata, T.; Sanuki, R.; Miyata, K.; Koyasu, T.; Ueno, S.; Funabiki, K.; Tani, A.; et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA 2010, 107, 332–337. [Google Scholar] [CrossRef]
- Morgans, C.W.; Zhang, J.; Jeffrey, B.G.; Nelson, S.M.; Burke, N.S.; Duvoisin, R.M.; Brown, R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19174–19178. [Google Scholar]
- Shen, Y.; Rampino, M.A.; Carroll, R.C.; Nawy, S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer. Proc. Natl. Acad. Sci. USA 2012, 109, 8752–8757. [Google Scholar] [CrossRef]
- Shim, H.; Wang, C.T.; Chen, Y.L.; Chau, V.Q.; Fu, K.G.; Yang, J.; McQuiston, A.R.; Fisher, R.A.; Chen, C.K. Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice. J. Bio.l Chem. 2012, 287, 14873–14879. [Google Scholar] [CrossRef]
- Xu, Y.; Dhingra, A.; Fina, M.E.; Koike, C.; Furukawa, T.; Vardi, N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J. Neurophysiol. 2012, 107, 948–957. [Google Scholar] [CrossRef]
- Guarneri, P.; Guarneri, R.; Cascio, C.; Pavasant, P.; Piccoli, F.; Papadopoulos, V. Neurosteroidogenesis in rat retinas. J. Neurochem. 1994, 63, 86–96. [Google Scholar]
- Cascio, C.; Guarneri, R.; Russo, D.; De Leo, G.; Guarneri, M.; Piccoli, F.; Guarneri, P. Pregnenolone sulfate, a naturally occurring excitotoxin involved in delayed retinal cell death. J. Neurochem. 2000, 74, 2380–2391. [Google Scholar]
- Cascio, C.; Guarneri, R.; Russo, D.; De Leo, G.; Guarneri, M.; Piccoli, F.; Guarneri, P. A caspase-3-dependent pathway is predominantly activated by the excitotoxin pregnenolone sulfate and requires early and late cytochrome c release and cell-specific caspase-2 activation in the retinal cell death. J. Neurochem. 2002, 83, 1358–1371. [Google Scholar] [CrossRef]
- Guarneri, P.; Russo, D.; Cascio, C.; De Leo, G.; Piccoli, T.; Sciuto, V.; Piccoli, F.; Guarneri, R. Pregnenolone sulfate modulates NMDA receptors, inducing and potentiating acute excitotoxicity in isolated retina. J. Neurosci. Res. 1998, 54, 787–797. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harteneck, C. Pregnenolone Sulfate: From Steroid Metabolite to TRP Channel Ligand. Molecules 2013, 18, 12012-12028. https://doi.org/10.3390/molecules181012012
Harteneck C. Pregnenolone Sulfate: From Steroid Metabolite to TRP Channel Ligand. Molecules. 2013; 18(10):12012-12028. https://doi.org/10.3390/molecules181012012
Chicago/Turabian StyleHarteneck, Christian. 2013. "Pregnenolone Sulfate: From Steroid Metabolite to TRP Channel Ligand" Molecules 18, no. 10: 12012-12028. https://doi.org/10.3390/molecules181012012
APA StyleHarteneck, C. (2013). Pregnenolone Sulfate: From Steroid Metabolite to TRP Channel Ligand. Molecules, 18(10), 12012-12028. https://doi.org/10.3390/molecules181012012