Protective Effects of the Key Compounds Isolated from Corni fructus against β-Amyloid-Induced Neurotoxicity in PC12 Cells
Abstract
:1. Introduction
2. Results and Discussion
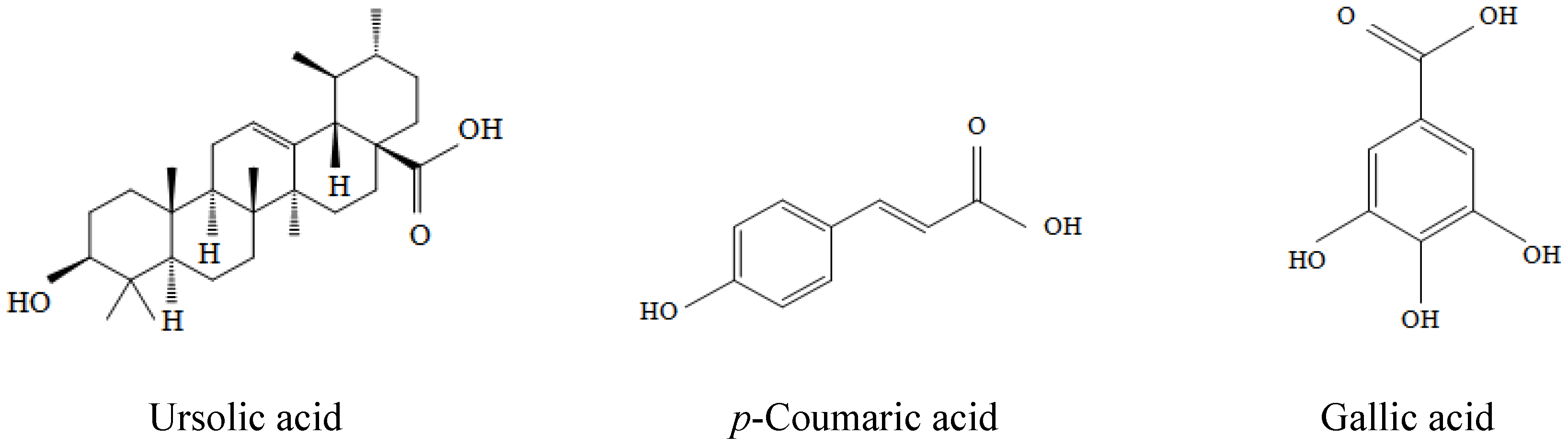
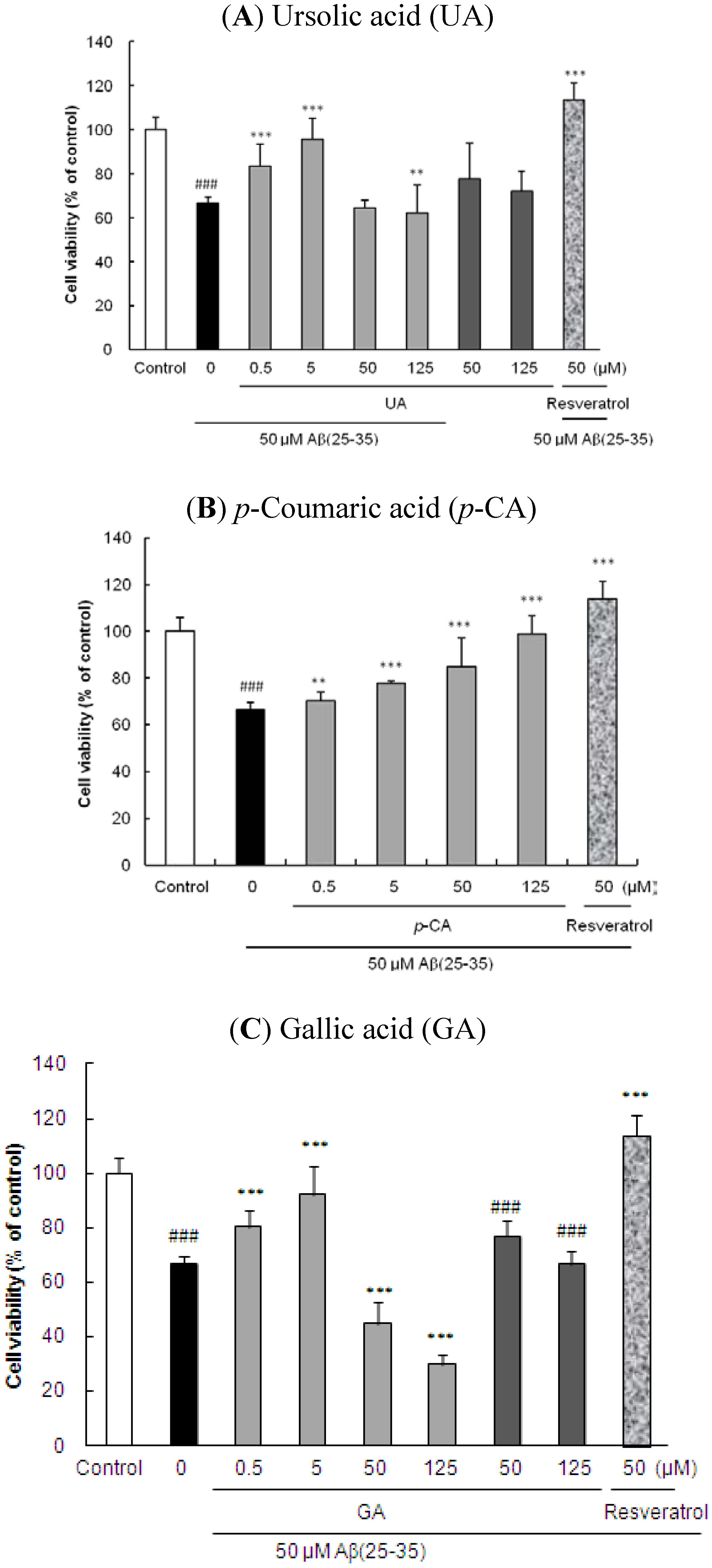
2.1. Ursolic Acid, p-Coumaric Acid and Gallic Acid Protected PC12 Cells against Aβ(25–35)-Induced Cytotoxicity
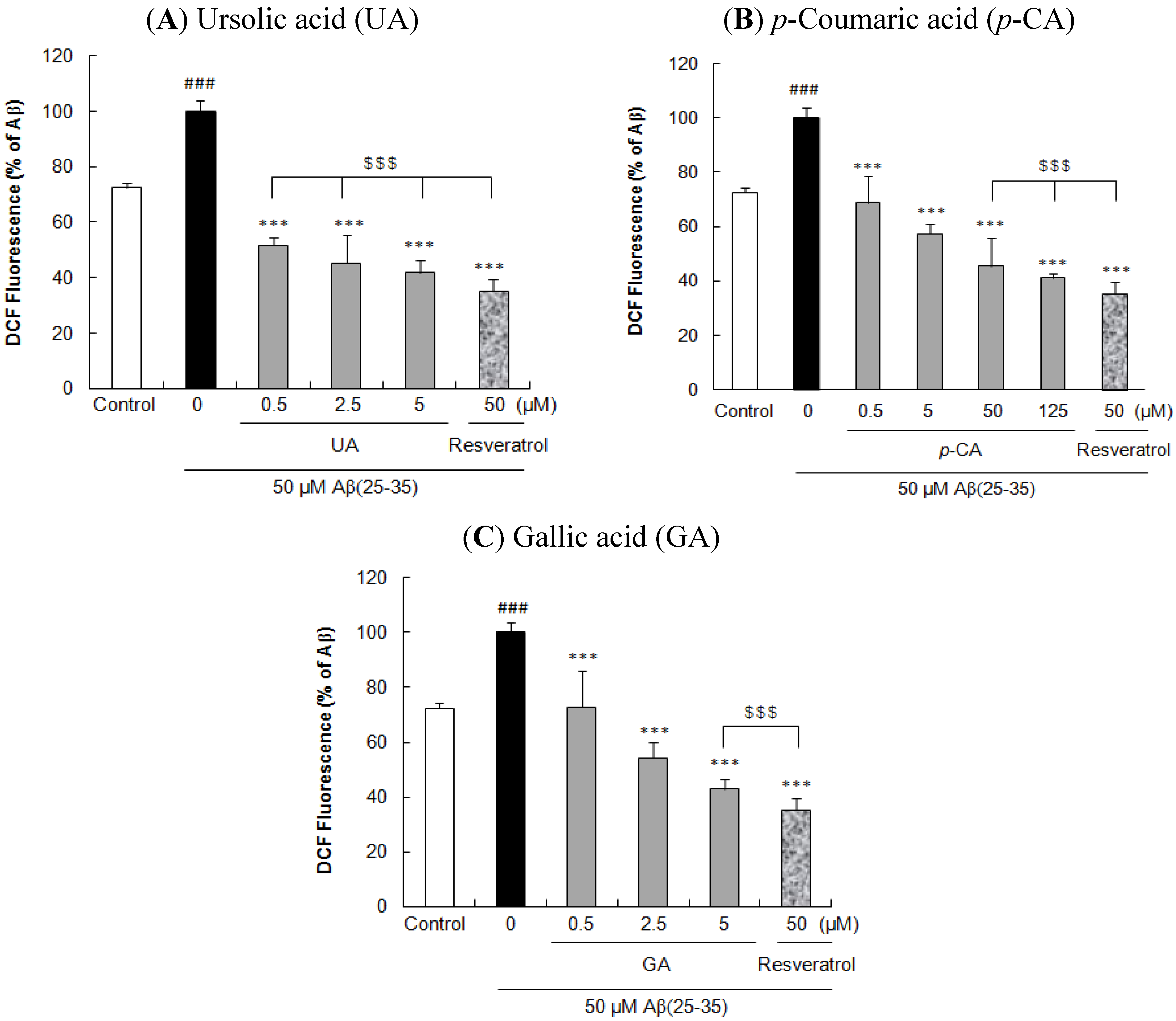
2.2. Ursolic Acid, p-Coumaric Acid and Gallic acid Protected PC12 Cells against Aβ(25–35)-Induced Intracellular ROS Accumulation in PC12 Cells
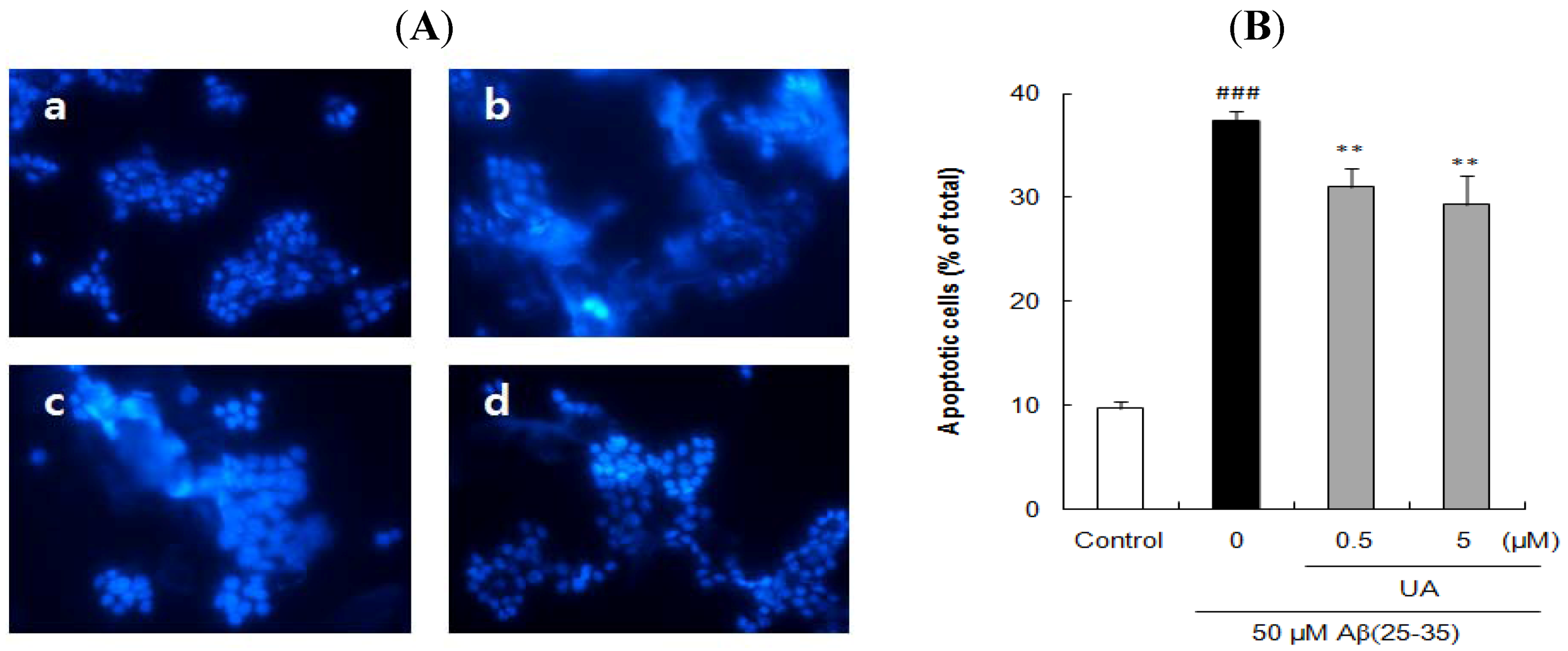
2.3. Ursolic Acid, p-Coumaric Acid and Gallic Acid Protected PC12 Cells against Aβ(25–35)-Induced Apoptosis
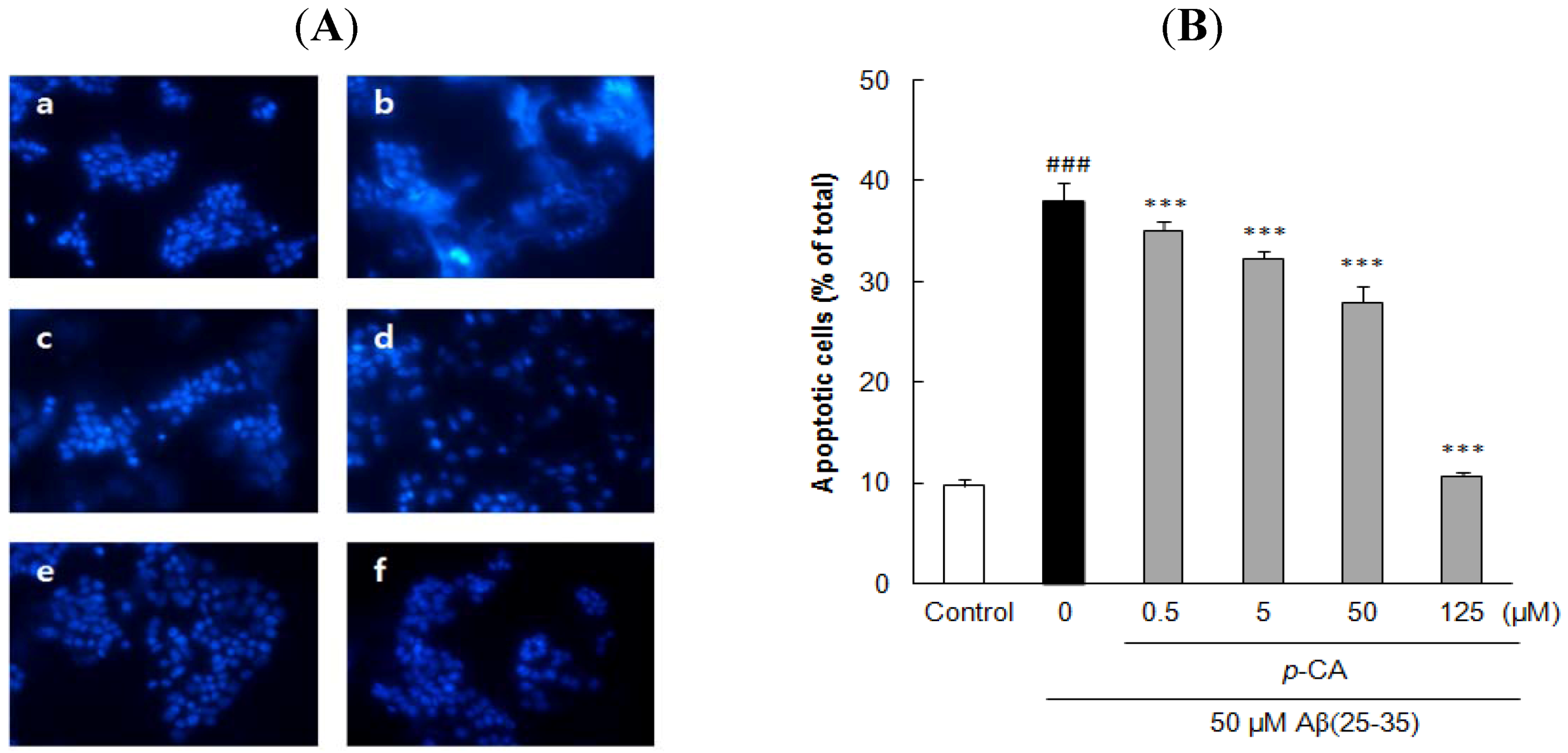
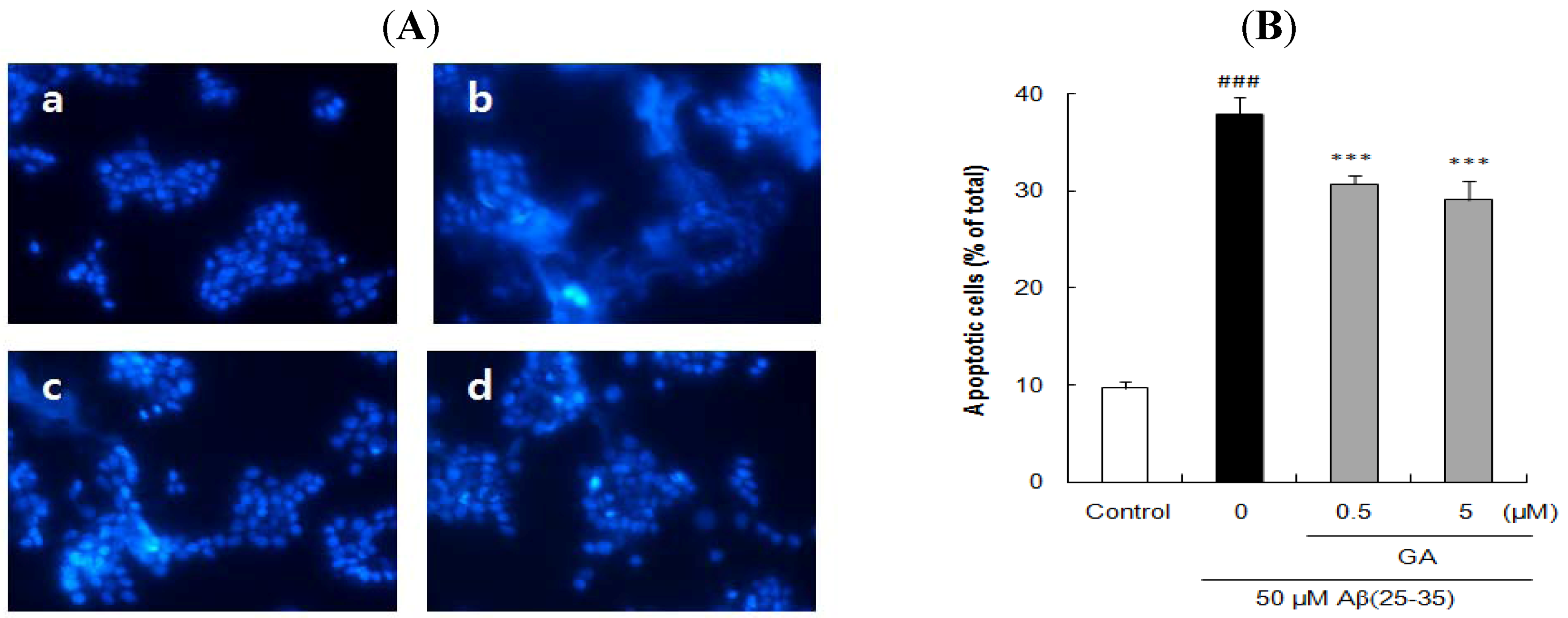
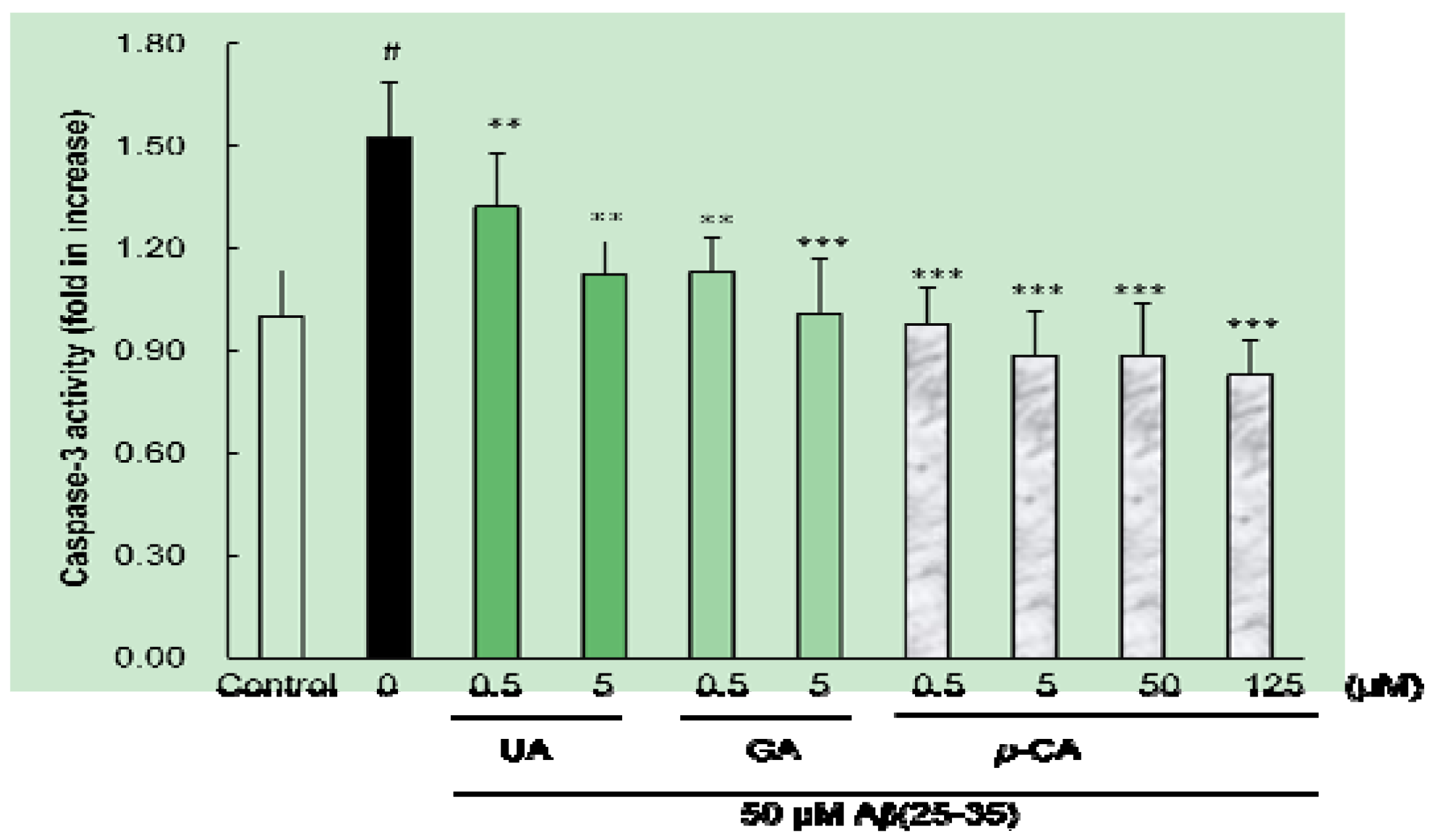
2.4. Ursolic Acid, p-Coumaric Acid and Gallic Acid Inhibited Aβ(25–35)-Induced Caspase-3 Activity
3. Experimental
3.1. General
3.2. Plant Material, Extraction, Fractionation, and Isolation of Key Compounds from Corni fructus
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Intracellular Reactive Oxygen Species Production Determination
3.6. Apoptotic Morphology Observation by Hoechst 33342 Staining
3.7. Measurement of Caspase-3 Activity
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Liu, Q.; Kou, J.P.; Yu, B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-kB activation. Neurochem. Int. 2011, 58, 119–125. [Google Scholar] [CrossRef]
- Xie, X.; Wang, H.T.; Li, C.L.; Gao, X.H.; Ding, J.L.; Zhao, H.H.; Lu, Y.L. Ginsedoside Rb1 protects PC12 cells against β-amyloid-induced cell injury. Mol. Med. Rep. 2010, 3, 635–639. [Google Scholar]
- Li, M.H.; Jang, J.H.; Sun, B.; Surh, Y.J. Protective effects of oligomers of grape seed polyphenols against β-amyloid-induced oxidative cell death. Ann. NY Acad. Sci. 2004, 1030, 317–329. [Google Scholar]
- Gray, C.W.; Patel, A.J. Neurodegeneration mediated by glutamate and β-amyloid peptide: A comparison and possible interaction. Brain Res. 1995, 691, 169–179. [Google Scholar] [CrossRef]
- Ueda, K.; Shinohara, S.; Yagami, T.; Asakura, K.; Kawasaki, K. Amyloid β protein potentiates Ca2+ influx though L-type voltage-sensitive Ca2+ channels: A possible involvement of free radicals. J. Neurochem. 1997, 68, 265–271. [Google Scholar]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef]
- Floyd, R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar]
- Calabrese, V.; Guagliano, E.; Sapienza, M.; Panebianco, M.; Calafato, S.; Puleo, E.; Pennisi, G.; Mancuso, C.; Allan, B.D.; Stella, A.G. Redox regulation of cellular stress response in aging and neurodegenerative disorders: Role of vitagenes. Neurochem. Res. 2006, 32, 757–773. [Google Scholar]
- Ban, J.Y.; Jeon, S.Y.; Nguyen, T.T.H.; Bae, K. Neuroprotective effect of oxyresveratrol from Smilacis Chinae Rhizome of amyloid beta-protein (25–35) induced neurotoxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424. [Google Scholar] [CrossRef]
- Conte, A.; Pellegrini, S.; Tagliazucchi, D. Synergistic protection of PC12 cells from β-amyloid toxicity by resveratrol and catechin. Brain Res. Bull. 2003, 62, 29–38. [Google Scholar] [CrossRef]
- Yokozawa, T.; Yamabe, N.; Kim, H.Y.; Kang, K.S.; Hur, J.M.; Park, C.H.; Tanaka, T. Protective effects of Morroniside isolated from Corni fructus against renal damage in streptozotosin-induced diabetic rats. Biol. Pharm. Bull. 2008, 31, 1422–1428. [Google Scholar] [CrossRef]
- Sung, Y.H.; Chang, H.K.; Kim, S.E.; Kim, Y.M.; Seo, J.H.; Shin, M.C.; Shin, M.S.; Yi, J.W.; Shin, D.H.; Kim, H.; et al. Anti-inflammatory and analgesic effects of the aqueous extract of Corni fructus in murine RAW 264.7 macrophage cells. J. Med. Food 2009, 12, 788–795. [Google Scholar] [CrossRef]
- Lee, S.O.; Kim, S.Y.; Han, S.M.; Kim, H.M.; Ham, S.S.; Kang, I.J. Corni fructus scavenges hydroxy radicals and decreases oxidative stress in endothelial cells. J. Med. Food 2006, 9, 594–598. [Google Scholar] [CrossRef]
- Chang, J.S.; Chiang, L.C.; Hsu, F.F.; Lin, C.C. Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro. Am. J. Chin. Med. 2004, 32, 717–725. [Google Scholar] [CrossRef]
- Youn, K.; Jun, M. Inhibitory effects of key compounds isolated from Corni fructus on BACE1 activity. Phytother. Res. 2012. [Google Scholar] [CrossRef]
- Song, K.S.; Jeong, W.S.; Jun, M. Inhibition of β-Amyloid peptide-induced neurotoxicity by kaempferol-3-O-(6"-acetyl)-β-glucopyranoside from butterbur leaves in B103 cells. Food Sci. Biotechnol. 2012, 21, 845–852. [Google Scholar] [CrossRef]
- Ban, J.Y.; Nguyen, H.T.T.; Lee, H.J.; Cho, S.O.; Ju, H.S.; Kim, J.Y.; Bae, K.H.; Song, K.S.; Seong, Y.H. Neuroprotective properties of gallic acid from Sanguisorbae radix on amyloid β protein (25–35)-induced toxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2008, 31, 149–153. [Google Scholar] [CrossRef]
- Selkoe, D.J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu. Rev. Cell Biol. 1994, 10, 373–403. [Google Scholar]
- Cotman, C.W.; Tenner, A.J.; Cummings, B.J. β-Amyloid converts an acute phase injury response to chonic injury responses. Neurobiol. Aging 1996, 17, 723–731. [Google Scholar] [CrossRef]
- Kokoszka, J.E.; Coskun, P.; Esposito, L.A.; Wallace, D.C. Increased mitochondrial oxidative stress in the SOD2(+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc. Natl. Acad. Sci. USA 2001, 98, 2278–2283. [Google Scholar]
- Behl, C.; Davis, J.B.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Castegna, A.; Lauderback, C.M.; Drake, J. Evidence that amyloid beta-peptide-induced lipid peroxidation in Alzheimer’s disease brain contributes to neuronal death. Neurobiol. Aging 2002, 5, 655–664. [Google Scholar]
- Lee, H.J.; Yoon, M.Y.; Kim, J.Y.; Kim, Y.S.; Park, H.R.; Park, E.J. Antioxidant activity of Glycyrrhiza uralensis fisch extracts on hydrogen peroxide-induced DNA damage in human leucocytes and cell death in PC12 cells. Food Sci. Biotechnol. 2008, 17, 343–348. [Google Scholar]
- Lee, S.M.; Yoon, M.Y.; Park, H.R. Protective effects of Paeonia lactiflora pall on hydrogen peroxide-induced apoptosis in PC12 cells. Biosci. Biotechnol. Biochem. 2008, 72, 1272–1277. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ha, T.Y.; Son, D.J.; Kim, S.R.; Hong, J.T. Effect of sesaminol glucosides on β-amyloid-induced PC12 cell death though antioxidant mechanisms. Neurosci. Res. 2005, 52, 330–341. [Google Scholar] [CrossRef]
- Ge, Y.S.; Teng, W.Y.; Zhang, C.D. Protective effect of cyclophilin A against Alzheimer’s amyloid beta-peptide (25–35)-induced oxidative stress in PC12 cells. Chin. Med. J. 2009, 122, 716–724. [Google Scholar]
- Peng, Q.L.; Bu’Zard, A.R.; Lau, B.H.S. Pycnogenol protects neurons from amyloid-β peptide-induced apoptosis. Mol. Brain Res. 2002, 104, 55–65. [Google Scholar] [CrossRef]
- Hoi, C.P.; Ho, Y.P.; Baum, L.; Chow, A.H.L. Neuroprotective effect of Honokiol and Magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010, 24, 1538–1542. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, H.Y.; Cho, E.J.; Baek, S.H.; Kwon, S.W.; Park, J.H. Resveratrol oilgomers from Vitis amurensis attenuate β-amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull. 2007, 30, 1130–1134. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Alzheimer’s amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, Y.H.; Wang, H.Q.; Xu, J.H.; Hu, H.T. Puerarin protects PC12 cells against β-amyloid-induced cell injury. Cell Biol. Int. 2008, 32, 1230–1237. [Google Scholar] [CrossRef]
- Fukui, K.; Takatsu, H.; Shinkai, T.; Suzuki, S.; Abe, K.; Urano, S. Appearance of amyloid beta-like substances and delayed-type apoptosis in rat hippocampus CA1 region though aging and oxidative stress. J. Alzheimers Dis. 2005, 8, 299–309. [Google Scholar]
- Heo, H.J.; Cho, H.Y.; Hong, B.S.; Kim, H.K.; Heo, T.R.; Kim, E.K.; Kim, S.K.; Kim, C.J.; Shin, D.H. Ursolic acid of Origanum majorana L. reduces Aβ-induced oxidative injury. Mol. Cells 2002, 13, 5–11. [Google Scholar]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti-and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranoval, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell Physiol. 2000, 279, 954–960. [Google Scholar]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef]
- Shin, K.Y.; Lee, G.H.; Park, C.H.; Kim, H.J.; Park, S.H.; Kim, S.H.; Kim, H.S.; Lee, K.S.; Won, B.Y.; Lee, H.G.; et al. A novel compound, maltolyl p-coumarate, attenuates cognitive deficits and shows neuroprotective effects in vitro and in vivo dementia models. J. Neurosci. Res. 2007, 85, 2500–2522. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Protective effects of Quercetin and Viatamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef]
- Polewski, K.; Kniat, S.; Siawinska, D. Gallic acid, a natural antioxidant, in aqueous and micellar environment: Spectroscopic studies. Curr. Top. Biophys. 2002, 26, 217–227. [Google Scholar]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-Atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar]
- Liu, Y.M.; Jiang, B.; Bao, Y.M.; An, L.J. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol. In Vitro 2008, 22, 430–437. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds used in this study are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hong, S.-Y.; Jeong, W.-S.; Jun, M. Protective Effects of the Key Compounds Isolated from Corni fructus against β-Amyloid-Induced Neurotoxicity in PC12 Cells. Molecules 2012, 17, 10831-10845. https://doi.org/10.3390/molecules170910831
Hong S-Y, Jeong W-S, Jun M. Protective Effects of the Key Compounds Isolated from Corni fructus against β-Amyloid-Induced Neurotoxicity in PC12 Cells. Molecules. 2012; 17(9):10831-10845. https://doi.org/10.3390/molecules170910831
Chicago/Turabian StyleHong, Seung-Young, Woo-Sik Jeong, and Mira Jun. 2012. "Protective Effects of the Key Compounds Isolated from Corni fructus against β-Amyloid-Induced Neurotoxicity in PC12 Cells" Molecules 17, no. 9: 10831-10845. https://doi.org/10.3390/molecules170910831
APA StyleHong, S.-Y., Jeong, W.-S., & Jun, M. (2012). Protective Effects of the Key Compounds Isolated from Corni fructus against β-Amyloid-Induced Neurotoxicity in PC12 Cells. Molecules, 17(9), 10831-10845. https://doi.org/10.3390/molecules170910831




