Promising Therapeutics with Natural Bioactive Compounds for Improving Learning and Memory — A Review of Randomized Trials
Abstract
:1. Introduction
2. Bioactive Compounds That Improve Learning and Memory
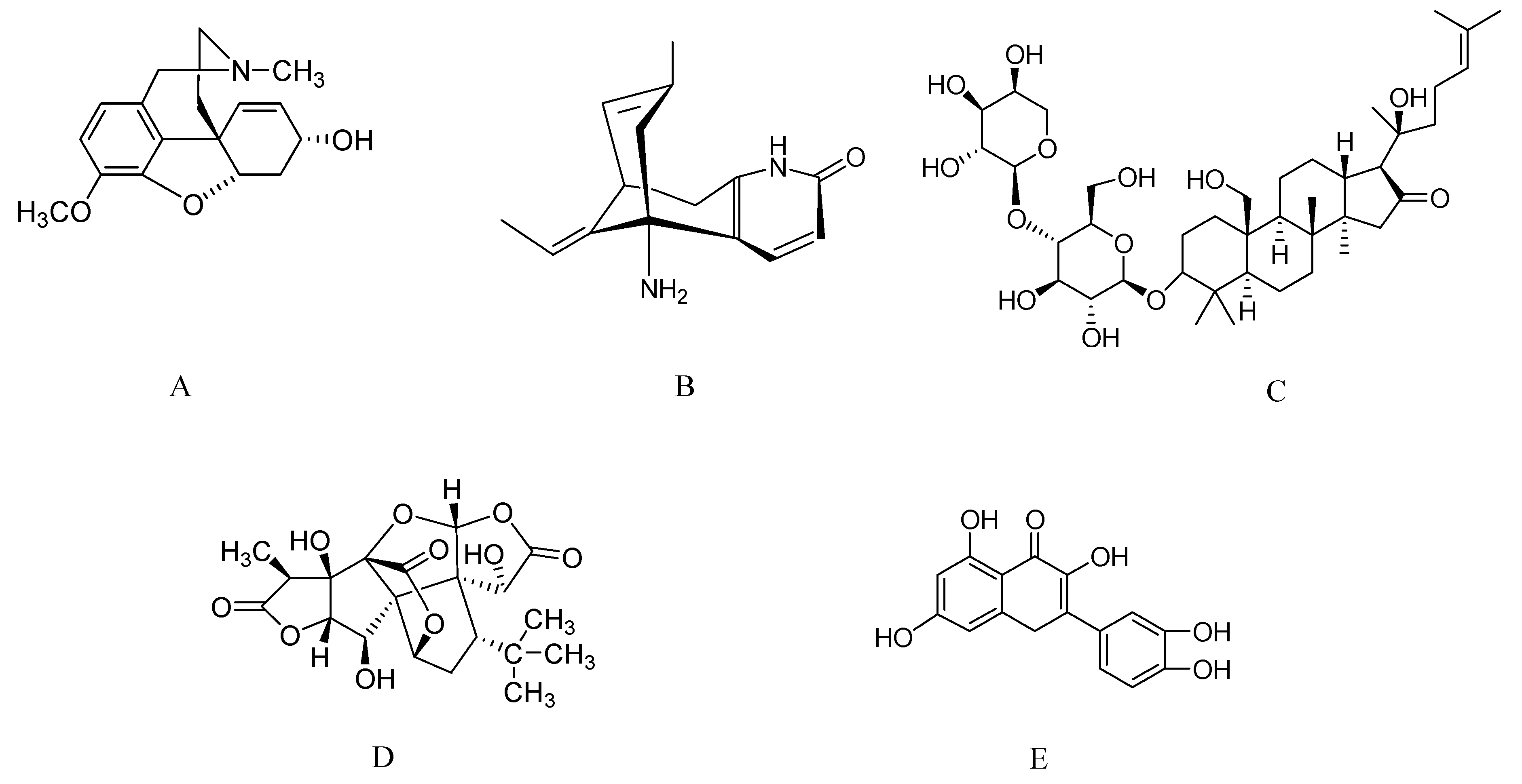
2.1. Galantamine from Galantus, Narcissus, and Leucojum spp.
| Bioactive Compound/Intervention | Study design | Memory test | Result/Activity | Ref. |
|---|---|---|---|---|
| Galantamine | Patients with Mild to moderate AD, R, DB, PC (3 months/n = 386) | CIBIC-plus and ADAS-cog | Improved cognitive function and basic activities of normal living than placebo in AD patients. | [13] |
| Galantamine (8, 16 & 24 mg/day) | Patients with Mild to moderate AD, R, DB, PC, M (5-month) | ADAS-cog and CIBIC-plus | Benefits the cognitive, functional, and behavioral symptoms of AD as compared with placebo. | [14] |
| Galantamine (24 or 32 mg/day) | Patients with Mild to moderate AD, M, DB, (6-month/n = 636) | ADAS-cog and CIBIC-plus | Improved cognition and global function, better outcome on CIBIC-plus and ADAS-cog. | [15] |
| Galantamine (24 or 32 mg) | Patients with Mild to moderate AD, R, DB, PC blind, parallel group, trial (6-month/n = 653) | ADAS-cog | Better scores on the disability assessment for dementia, slowed the decline of functional ability as well as cognition in subjects with mild to moderate AD as compared with placebo. | [16] |
| Galantamine (24 mg/day) | Patients with Vascular dementia, M, DB, (n = 592) | ADAS-cog and CIBIC-plus | Therapeutic effect on all key areas of cognitive and non-cognitive abilities with improved activities of daily living and behavioral symptoms were also significantly improved in dementia patients. | [17] |
| Galantamine (24 mg/day) | Patient with severe AD, R, DB, PC, blind (n = 207) | SIB and MDS-ADL | Improvement in memory, praxis, and visuospatial ability. | [18] |
| Huperzine A (0.2 mg twice daily) | R, DB, PC with AD (n = 28) | MMSE, ADL, HDS and WMI | Improved memory test scores over the individuals receiving the placebo. | [20] |
| Huperzine A (0.4 mg daily) | Subjects with benign vascular dementia, and AD (n = 80) | MQ test | Significant improvement in MQ test as compared to control group. | [21] |
| Huperzine Alpha (400 μg/day) | Subjects with diagnosis of possible or probable AD (n = 100/12 weeks) | ADL &ADAS-Cog | Remarkably improves the cognition, behavior, ADL, and mood of AD patients as assessed by ADAS-Cog. | [22] |
| Huperzine A (200 & 400 μg; twice daily) | Patients with mild to moderate AD, M, R (16 weeks/n = 210) | ADAS-Cog | Huperzine A 400 μg and not at 200 μg has cognitive effect in patients with mild to moderate AD. | [23] |
| Huperzine A (0.1 mg twice daily) | Patients with mild to moderate VaD, R, DB ,PC (12 weeks/n = 78) | CDR, MMSE, and ADL | Huperzine A showed significant improvement in cognitive functions of all test. | [24] |
| Bacognize® (300mg; twice daily) | Patients with AD, R, DB, PC and M (6 months) | MMSE | Improvement in attention, language, reading, writing & comprehension. | [25] |
| Bacopa monniera | Healthy individuals, DB, PC (n = 46) | Well-validated neuropsychological tests | Significant improvement in information processing and memory consolidation, & in state anxiety. | [26] |
| Bacopa monniera (2 × 150 mg) | Healthy individuals, DB, PC (90 days/n = 127) | Neuropsychological testing using the Cognitive Drug Research cognitive assessment system | Improves partial working memory and reduced number of false positives in the rapid visual information processing task. | [27] |
| Bacopa monniera (300 mg) | 65 or older year individuals, R, DB, PC (12weeks/n = 54) | AVLT, DAT, and WAIS | Enhances AVLT delayed word recall memory scores and also improves ability to ignore irrelevant information as assessed by Stroop test. | [28] |
| BT-11 (extracted from roots of Polygala tenuifolia) | Healthy elderly individuals, R, DB, PC (n = 28) | CERAD and MMSE | Treatment by BT-11, increased CERAD scores, word list recognition, constructional recall and praxis, and modified Boston naming test. | [29] |
| BT-11 | Healthy individuals, R, DB, PC (4 weeks) | K-CVLTSOPT | Improvement in verbal memory and working memory. | [30] |
| EGb 761 (240 mg) | Patients with AD and multi-infarct dementia, R, DB, PC (24 weeks/n = 216) | CGI and NAB | Effective in Alzheimer and multi-infarct dementia. | [31] |
| EGb 761 (240 mg/day or 160 mg/day) | Patient with AD or VaD or AAMI, R, DB, PC (n = 214/24 weeks) | SKT, CGI and NAI-NAA | EGb 761 is not beneficial for dementia patients. | [32] |
| EGb 761 (Ginkgo extract - 180 mg/day) | Aged subjects with no history of significant neurocognitive dysfunction (6 weeks) | Stroop Color and Word Test color-naming task | Significantly showed improvement on a task assessing speed of processing abilities. | [33] |
| Standardized extract of Ginkgo biloba (GK501) & Standardized extract of Panax ginseng (G115) (60 mg - Capsule) | Healthy individuals, DB, PC (14 week/n = 256) | Tests for attention and memory from the Cognitive Drug Research computerized cognitive assessment system | Significantly to improve an Index of Memory Quality, memory, including long-term and working memory. | [34] |
| Capsulated aqueous extract of C. asiatica (250, 500, and 750 mg) | Healthy individuals, R, DB, PC (2 months/n = 28) | Computer assisted technique | Cognitive enhancing effect observed. | [35] |
| Ginseng (400 mg) | Healthy young volunteer, R, DB, PC, balanced, cross-over (n = 20) | CDR two serial subtraction mental arithmetic tasks | Improvement in the speed and accuracy of memory and attentional tasks. | [36] |
| Panax ginseng extract (G115) (400 mg) | Healthy middle aged individuals, DB, PC balanced trail (n = 30) | Cognitive and mood performance test | Improvement speed of attention and tasks associated with episodic memory performance. | [37] |
| Cereboost(P. quinquefolius standardized to 10.65% ginsenosides) | Healthy young volunteer, R, DB, PC, crossover trial (n = 32) | Parameters for mood and neurocognitive effect | Improvement working memory performance, reaction time accuracy and calmness. | [38] |
| G115 (200 mg & 400 mg) | Healthy young volunteer, R, DB, PC, crossover trail (n = 30) | COGNITIVE BATTERY (Bond-Lader visual analogue scales, Computerised Corsi block tapping task, N-back task and Random number generation task | Modulation of cognitive function and mood. | [39] |
| HT100 (proprietary North American ginseng extract) | Individuals with schizophrenia, DB, PC (4 weeks/n = 64) | Letter-Number Span Test and Visual Pattern Test | Significant improvement in visual working memory. | [40] |
| LGNC-07 (combination of green tea extract and L-Theanine/1,680 mg) | MCI subjects, DB, PC (16 weeks/n = 91) | Rey–Kim memory test and Stroop color-word test | Significant improvement in selective attention, cognitive alertness, memory and verbal reading. | [41] |
| Green Tea | Cross-sectional trial (n = 1003) | MMSE | Higher consumption of green tea lowers prevalence of cognitive impairment. | [42] |
| Total Tea | Cross-sectional and longitudinal (n = 2501) | MMSE | Total tea consumption lowers risk of cognitive impairment and cognitive decline. | [43] |
| Total Tea | Cross-sectional trial (n = 716) | MMSE | Total tea consumption improves global cognition, memory, executive function, and information processing speed. | [44] |
| Salvia officinalis extract (60 drops/day) | Patients with mild to moderate AD, DB, R, PC (n = 424 months) | ADAS-cog & CDR | Improvement in cognitive functions. | [45] |
| Extract of Salvia officinalis (167, 333, 666 and 1332 mg) | Patients with mild to moderate AD, DB, R, PC (n = 20) | CDR | Enhancement of secondary memory performance. | [46] |
| S. officinalis aroma | Healthy individuals, SB, one factor, independent group trial (n = 135) | CDR & Bond-Lader mood scale | Significantly increases the alertness in mood and quality of memory. | [47] |
| S. lavandulaefolia (25 and 50 μL) | Healthy individuals, PC, DB, balanced, crossover trail (n = 24) | CDR & Bond-Lader mood scale | Improvement on the speed of memory and secondary memory. | [48] |
| Tofu | Honolulu-Asia Aging Study (n = 3734) | All task included in Cognitive abilities screening instrument(CASI) | Higher midlife tofu consumption was independently associated with indicators of cognitive impairment and brain atrophy in late life. | [49] |
| Dietary phytoestrogens | Cross-sectional study (n = 301) | Functions like, memory, processing capacity, speed and executive function | High lignan intake improves capacity, speed and executive function. | [50] |
2.2. Huperzine A from Huperzia serrata
2.3. Bacosides from Bacopa monnieri
2.4. Ginkgolides from Ginkgo Biloba
2.5. Asiatic Acid from Centella asiatica
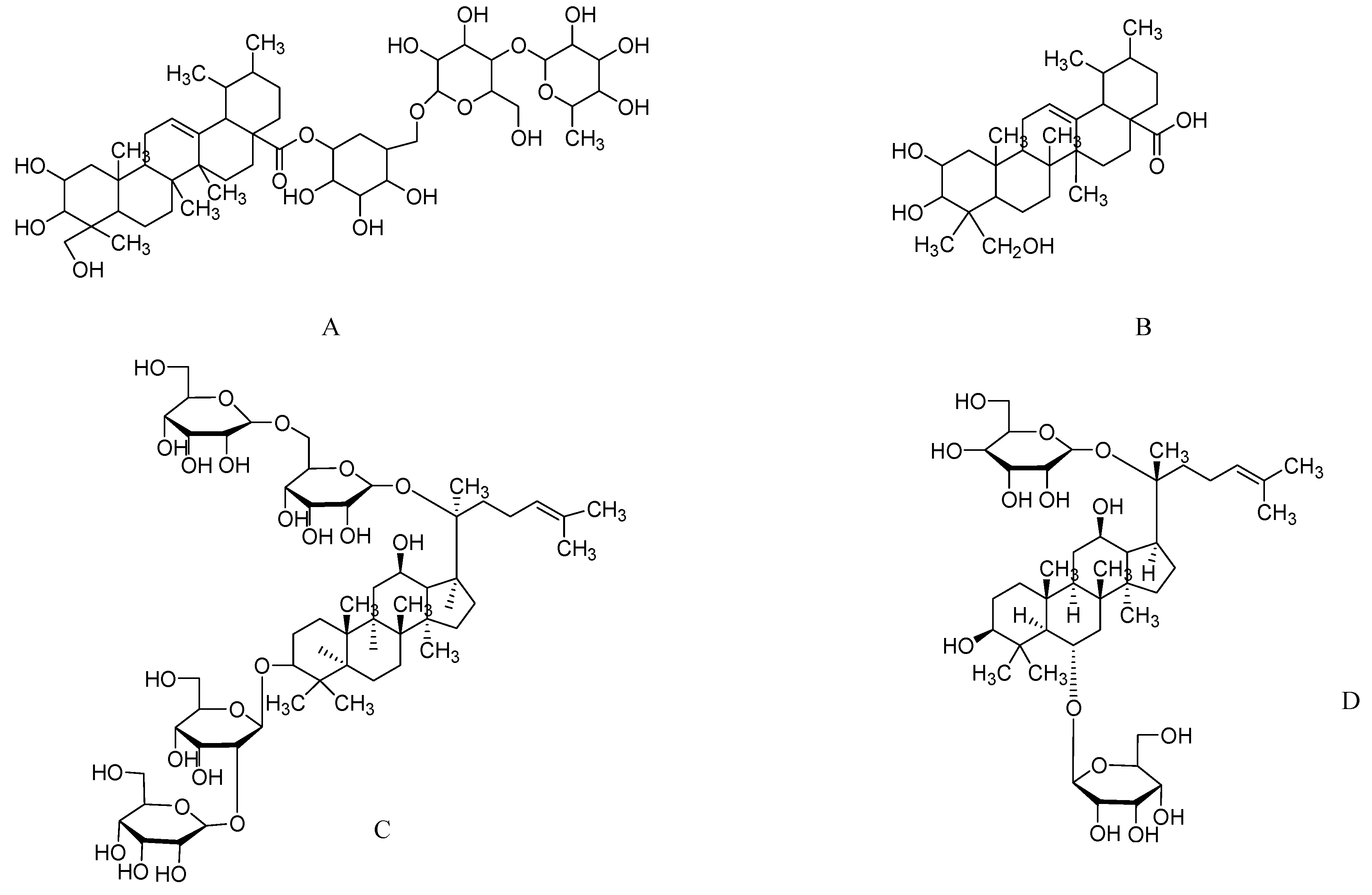
2.6. Ginsenosides from Ginseng
2.7. Epigallocatechin-3-gallate from Green Tea
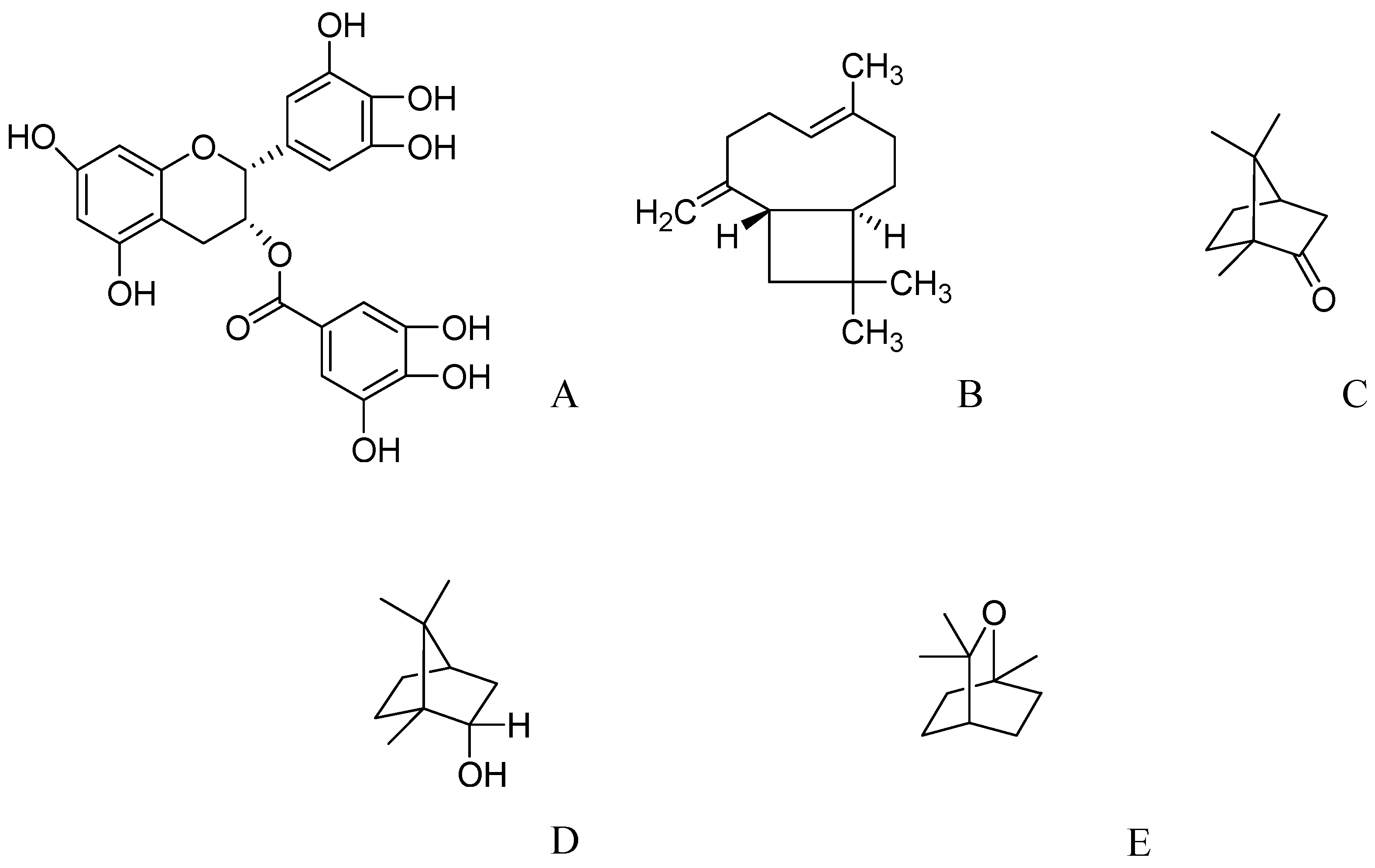
2.8. Essential Oils from Salvia Species
2.9. Phytoestrogens from Tofu
2.10. Triterpenoid Saponins from Polygala tenuifolia
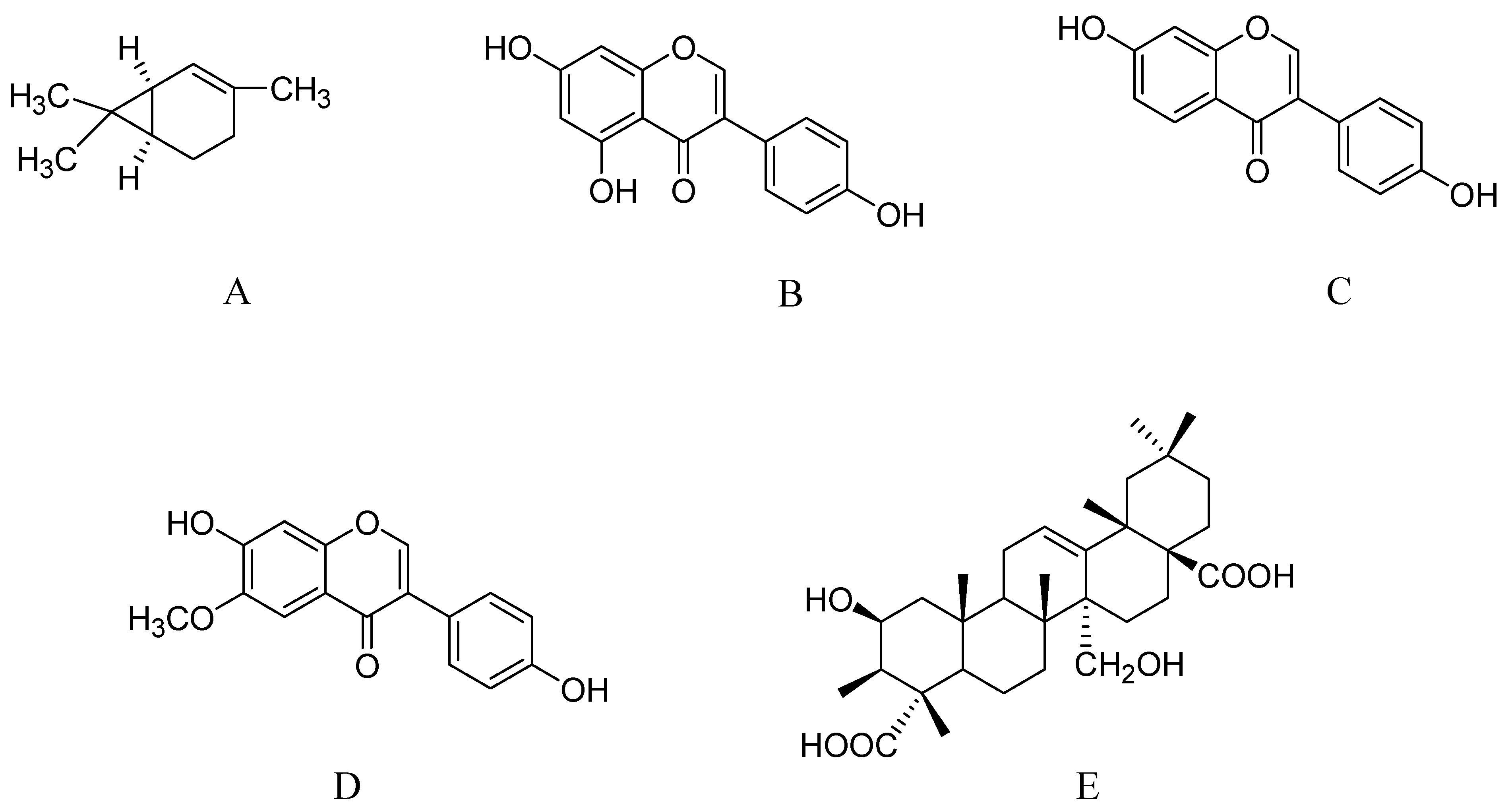
3. Potential Promising Compounds
4. Conclusions
Acknowledgments
References
- Lye, T.C.; Piguet, O.; Grayson, D.A.; Creasey, H.; Ridley, L.J.; Bennett, H.P.; Broe, G.A. Hippocampal size and memory function in the ninth and tenth decades of life: The Sydney Older Persons Study. J. Neurol. Neurosurg. Psychiat. 2004, 75, 548–554. [Google Scholar] [CrossRef]
- Rusinek, H.; De Santi, S.; Frid, D.; Tsui, W.H.; Tarshish, C.Y.; Convit, A.; de Leon, M.J. Regional brain atrophy rate predicts future cognitive decline: 6-Year longitudinal MR imaging study of normal aging. Radiology 2003, 229, 691–696. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Harigaya, Y.; Tomidokoro, Y.; Kanai, M.; Ikeda, M.; Matsubara, E.; Kawarabayashi, T.; Kuribara, H.; Younkin, S.G.; Maruyama, Y.; et al. Decreased level of brain acetylcholine and memory disturbance in APPsw mice. Neurobiol. Aging 2004, 25, 483–490. [Google Scholar]
- Schliebs, R.; Arendt, T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural. Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Bergmann, I.; Priestley, J.V.; McMahon, S.B.; Brocker, E.B.; Toyka, K.V.; Koltzenburg, M. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur. J. Neurosci. 1997, 9, 18–28. [Google Scholar] [CrossRef]
- Adams, M.; Gmunder, F.; Hamburger, M. Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [CrossRef]
- Gijtenbeek, J.M.; van den Bent, M.J.; Vecht, C.J. Cyclosporine neurotoxicity: A review. J. Neurol. 1999, 246, 339–346. [Google Scholar] [CrossRef]
- Johnson, W.C.; Williford, W.O. Benefits, Morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: A prospective randomized study. J. Vasc. Surg. 2002, 35, 413–421. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Alkondon, M.; Pereira, E.F.; Castro, N.G.; Schrattenholz, A.; Barbosa, C.T.; Bonfante-Cabarcas, R.; Aracava, Y.; Eisenberg, H.M.; Maelicke, A. Properties of neuronal nicotinic acetylcholine receptors: Pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. 1997, 280, 1117–1136. [Google Scholar]
- Schrattenholz, A.; Pereira, E.F.; Roth, U.; Weber, K.H.; Albuquerque, E.X.; Maelicke, A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol. Pharmacol. 1996, 49, 1–6. [Google Scholar]
- Bores, G.M.; Huger, F.P.; Petko, W.; Mutlib, A.E.; Camacho, F.; Rush, D.K.; Selk, D.E.; Wolf, V.; Kosley, R.W., Jr.; Davis, L.; et al. Pharmacological evaluation of novel Alzheimer’s disease therapeutics: Acetylcholinesterase inhibitors related to galanthamine. J. Pharmacol. Exp. Ther. 1996, 277, 728–738. [Google Scholar]
- Rockwood, K.; Mintzer, J.; Truyen, L.; Wessel, T.; Wilkinson, D. Effects of a flexible galantamine dose in Alzheimer’s disease: A randomised, controlled trial. J. Neurol. Neurosurg. Psychiat. 2001, 71, 589–595. [Google Scholar] [CrossRef]
- Tariot, P.N.; Solomon, P.R.; Morris, J.C.; Kershaw, P.; Lilienfeld, S.; Ding, C. A 5-month, Randomized, Placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 2000, 54, 2269–2276. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R.; Wessel, T.; Yuan, W. Galantamine in AD: A 6-month randomized, Placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000, 54, 2261–2268. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Lilienfeld, S.; Gaens, E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: Multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 2000, 321, 1445–1449. [Google Scholar] [CrossRef]
- Erkinjuntti, T.; Kurz, A.; Gauthier, S.; Bullock, R.; Lilienfeld, S.; Damaraju, C.V. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: A randomised trial. Lancet 2002, 359, 1283–1290. [Google Scholar] [CrossRef]
- Burns, A.; Bernabei, R.; Bullock, R.; Cruz Jentoft, A.J.; Frolich, L.; Hock, C.; Raivio, M.; Triau, E.; Vandewoude, M.; Wimo, A.; et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer’s disease (the SERAD study): A randomised, Placebo-controlled, Double-blind trial. Lancet Neurol. 2009, 8, 39–47. [Google Scholar] [CrossRef]
- Rockwood, K.; Fay, S.; Gorman, M. The ADAS-cog and clinically meaningful change in the VISTA clinical trial of galantamine for Alzheimer’s disease. Int. J. Geriatr. Psychiat. 2010, 25, 191–201. [Google Scholar] [CrossRef]
- Liu, F.G.; Fang, Y.S.; Gao, Z.X.; Zuo, J.D.; Sou, M.L. Double-blind control treatment of huperzine-A and placebo in 28 patients with Alzheimer disease. Chin. J. Pharmacoepidemiol. 1995, 4, 196–198. [Google Scholar]
- Ma, Y.X.; Zhu, Y.; Gu, Y.D.; Yu, Z.Y.; Yu, S.M.; Ye, Y.Z. Double-blind trial of huperzine-A (HUP) on cognitive deterioration in 314 cases of benign senescent forgetfulness, vascular dementia, and Alzheimer’s disease. Ann. NY Acad. Sci. 1998, 854, 506–507. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Chen, Q.; Shu, L.; Wang, J.; Shan, G. Clinical efficacy and safety of huperzine Alpha in treatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial. Zhonghua Yi Xue Za Zhi 2002, 82, 941–944. [Google Scholar]
- Rafii, M.S.; Walsh, S.; Little, J.T.; Behan, K.; Reynolds, B.; Ward, C.; Jin, S.; Thomas, R.; Aisen, P.S. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 2011, 76, 1389–1394. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Liang, X.M.; Juan, W.; Zhang, Y.F.; Zhu, C.X.; Jiang, X.J. Treatment with Huperzine A improves cognition in vascular dementia patients. Cell. Biochem. Biophys. 2012, 62, 55–58. [Google Scholar] [CrossRef]
- Shishir, G.; Anand, S.; Navneet, K.; Vijay, T.; Meenal, T.; Manasi, T. Effect of Bacopa monnieri on Cognitive functions in Alzheimer’s disease patients. Int. J. Collab. Res. Intern. Med. Public Health 2011, 3, 285–293. [Google Scholar]
- Stough, C.; Lloyd, J.; Clarke, J.; Downey, L.A.; Hutchison, C.W.; Rodgers, T.; Nathan, P.J. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl) 2001, 156, 481–484. [Google Scholar] [CrossRef]
- Stough, C.; Downey, L.A.; Lloyd, J.; Silber, B.; Redman, S.; Hutchison, C.; Wesnes, K.; Nathan, P.J. Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90 Day double-blind placebo-controlled randomized trial. Phytother. Res. 2008, 22, 1629–1634. [Google Scholar] [CrossRef]
- Calabrese, C.; Gregory, W.L.; Leo, M.; Kraemer, D.; Bone, K.; Oken, B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: A randomized, Double-blind, Placebo-controlled trial. J. Altern. Complement Med. 2008, 14, 707–713. [Google Scholar] [CrossRef]
- Shin, K.Y.; Lee, J.Y.; Won, B.Y.; Jung, H.Y.; Chang, K.A.; Koppula, S.; Suh, Y.H. BT-11 is effective for enhancing cognitive functions in the elderly humans. Neurosci. Lett. 2009, 465, 157–159. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, K.Y.; Shin, K.Y.; Won, B.Y.; Jung, H.Y.; Suh, Y.H. Effects of BT-11 on memory in healthy humans. Neurosci. Lett. 2009, 454, 111–114. [Google Scholar] [CrossRef]
- Kanowski, S.; Herrmann, W.M.; Stephan, K.; Wierich, W.; Horr, R. Proof of efficacy of the ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry 1996, 29, 47–56. [Google Scholar] [CrossRef]
- Dongen, M.V.; van Rossum, E.; Kessels, A.; Sielhorst, H.; Knipschild, P. Ginkgo for elderly people with dementia and age-associated memory impairment: A randomized clinical trial. J. Clin. Epidem. 2003, 56, 367–376. [Google Scholar] [CrossRef]
- Mix, J.A.; Crews, W.D., Jr. An examination of the efficacy of Ginkgo biloba extract EGb761 on the neuropsychologic functioning of cognitively intact older adults. J. Altern. Complement Med. 2000, 6, 219–229. [Google Scholar] [CrossRef]
- Wesnes, K.A.; Ward, T.; McGinty, A.; Petrini, O. The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle-aged volunteers. Psychopharmacology (Berl) 2000, 152, 353–361. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Mator, L.; Muchimapura, S.; Tongun, T.; Pasuriwong, O.; Piyawatkul, N.; Yimtae, K.; Sripanidkulchai, B.; Singkhoraard, J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008, 116, 325–332. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol. Behav. 2002, 75, 739–751. [Google Scholar] [CrossRef]
- Sünram-Lea, S.I.; Birchall, R.J.; Wesnes, K.A.; Petrini, O. The Effect Of Acute Administration of 400 mg of Panax gingseng on Cognitive Performance and mood in Healthy Young Volunteers. Curr. Top. Nutraceu. Res. 2005, 3, 65–74. [Google Scholar]
- Scholey, A.; Ossoukhova, A.; Owen, L.; Ibarra, A.; Pipingas, A.; He, K.; Roller, M.; Stough, C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: An acute, Randomised, Double-blind, Placebo-controlled, Crossover study. Psychopharmcology (Berl) 2010, 212, 345–356. [Google Scholar] [CrossRef]
- Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum. Psychopharmacol. 2010, 25, 462–471. [Google Scholar] [CrossRef]
- Chen, E.Y.; Hui, C.L. HT1001, A Proprietary North American Ginseng Extract, Improves Working Memory in Schizophrenia: A Double-blind, Placebo-Controlled Study. Phytother. Res. 2012, 26, 1166–1172. [Google Scholar] [CrossRef]
- Park, S.K.; Jung, I.C.; Lee, W.K.; Lee, Y.S.; Park, H.K.; Go, H.J.; Kim, K.; Lim, N.K.; Hong, J.T.; Ly, S.Y.; et al. A combination of green tea extract and l-theanine improves memory and attention in subjects with mild cognitive impairment: A double-blind placebo-controlled study. J. Med. Food 2011, 14, 334–343. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar]
- Ng, T.P.; Feng, L.; Niti, M.; Kua, E.H.; Yap, K.B. Tea consumption and cognitive impairment and decline in older Chinese adults. Am. J. Clin. Nutr. 2008, 88, 224–231. [Google Scholar]
- Feng, L.; Gwee, X.; Kua, E.H.; Ng, T.P. Cognitive function and tea consumption in community dwelling older Chinese in Singapore. J. Nutr. Health Aging 2010, 14, 433–438. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Scholey, A.B.; Tildesley, N.T.; Ballard, C.G.; Wesnes, K.A.; Tasker, A.; Perry, E.K.; Kennedy, D.O. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology (Berl) 2008, 198, 127–139. [Google Scholar] [CrossRef]
- Moss, L.; Rouse, M.; Wesnes, K.A.; Moss, M. Differential effects of the aromas of Salvia species on memory and mood. Hum. Psychopharmacol. 2010, 25, 388–396. [Google Scholar] [CrossRef]
- Tildesley, N.T.; Kennedy, D.O.; Perry, E.K.; Ballard, C.G.; Wesnes, K.A.; Scholey, A.B. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol. Behav. 2005, 83, 699–709. [Google Scholar] [CrossRef]
- White, L.R.; Petrovitch, H.; Ross, G.W.; Masaki, K.; Hardman, J.; Nelson, J.; Davis, D.; Markesbery, W. Brain aging and midlife tofu consumption. J. Am. Coll. Nutr. 2000, 19, 242–255. [Google Scholar]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; de Haan, E.H.; Aleman, A.; van der Schouw, Y.T. Dietary phytoestrogen intake and cognitive function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 556–562. [Google Scholar] [CrossRef]
- Gaudig, M.; Richarz, U.; Han, J.; Van Baelen, B.; Schauble, B. Effects of galantamine in Alzheimer’s disease: Double-blind withdrawal studies evaluating sustained versus interrupted treatment. Curr. Alzheimer Res. 2011, 8, 771–780. [Google Scholar] [CrossRef]
- Kavanagh, S.; Van Baelen, B.; Schauble, B. Long-term effects of galantamine on cognitive function in Alzheimer’s disease: A large-scale international retrospective study. J. Alzheimer Dis. 2011, 27, 521–530. [Google Scholar]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane. Database Syst. Rev. 2006, 25, CD001747. [Google Scholar]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–928. [Google Scholar] [CrossRef]
- Thal, L.J.; Schwartz, G.; Sano, M.; Weiner, M.; Knopman, D.; Harrell, L.; Bodenheimer, S.; Rossor, M.; Philpot, M.; Schor, J.; Goldberg, A. A multicenter double-blind study of controlled-release physostigmine for the treatment of symptoms secondary to Alzheimer’s disease. Physostigmine Study Group. Neurology 1996, 47, 1389–1395. [Google Scholar] [CrossRef]
- Rogers, S.L.; Doody, R.S.; Mohs, R.C.; Friedhoff, L.T. Donepezil improves cognition and global function in Alzheimer disease: A 15-week, Double-blind, Placebo-controlled study. Donepezil Study Group. Arch. Intern. Med. 1998, 158, 1021–1031. [Google Scholar] [CrossRef]
- Liu, J.S.; Zhu, Y.L.; Yu, C.M.; Zhou, Y.Z.; Han, Y.Y.; Wu, F.W.; Qi, B.F. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 1986, 64, 837–839. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tang, X.C. Neuroprotective effects of huperzine A: New therapeutic targets for neurodegenerative disease. Trends. Pharmacol. Sci. 2006, 27, 619–625. [Google Scholar] [CrossRef]
- Tang, X.C. Huperzine A (shuangyiping): A promising drug for Alzheimer’s disease. Zhongguo Yao Li Xue Bao 1996, 17, 481–484. [Google Scholar]
- Wang, R.; Yan, H.; Tang, X.C. Progress in studies of huperzine A, A natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006, 27, 1–26. [Google Scholar] [CrossRef]
- Chiu, H.F.; Zhang, M. Dementia research in China. Int. J. Geriatr. Psychiat. 2000, 15, 947–953. [Google Scholar] [CrossRef]
- Xu, S.S.; Cai, Z.; Cai, Z.Y.; Qu, Z.W.; Yang, R.M.; Cai, Y.L.; Wang, G.Q.; Su, X.Q.; Zhong, X.S.; Cheng, R.Y.; et al. Huperzine-A in capsules and tablets for treating patients with Alzheimer’s disease. Acta Pharmacol. Sin. 1999, 20, 486–490. [Google Scholar]
- Singh, H.; Dhawan, B. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa Monniera Linn. (Brahmi) Indian J. Pharmacol. 1997, 29, 359–365. [Google Scholar]
- Ganguly, D.K.; Malhtora, C.L. Some neuropharmacological and behavioural effects of an active fraction from Herpestis monniera, Linn (Brahmi). Indian J. Physiol. Pharmacol. 1967, 11, 33–43. [Google Scholar]
- Chatterji, N.; Rastogi, R.P.; Dhar, M.L. Chemical examination of Bacopa monniera Wettst.: Part I – isolation of chemical constituents. Indian. J. Chem. 1965, 3, 24–29. [Google Scholar]
- Singh, H.K.; Rastogi, R.P.; Sriman, R.C.; Dhawan, B.N. Effect of bacoside A and B on avoidance response in rats. Phytother. Res. 1988, 2, 70–75. [Google Scholar] [CrossRef]
- Prakash, J.C.; Sirsi, M. Comparative study of the effects of Brahmi and chlorpromazine on motor learning in rats. J. Sci. Ins. Res. 1962, 21, 93–96. [Google Scholar]
- Saraf, M.K.; Prabhakar, S.; Khanduja, K.L.; Anand, A. Bacopa monniera Attenuates Scopolamine-Induced Impairment of Spatial Memory in Mice. Evid. Based Complement Alternat. Med. 2011, 2011, 236186. [Google Scholar]
- Russo, A.; Borrelli, F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomed 2005, 12, 305–317. [Google Scholar] [CrossRef]
- Vohora, D.; Pal, S.N.; Pillai, K.K. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J. Ethnopharmacol. 2000, 71, 383–390. [Google Scholar] [CrossRef]
- Uabundit, N.; Wattanathorn, J.; Mucimapura, S.; Ingkaninan, K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J. Ethnopharmacol. 2010, 127, 26–31. [Google Scholar] [CrossRef]
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kumar, A.; Ghosal, S. Effect of Bacopa Monniera on Animal Models of Alzheimer’s Disease and Perturbed Central Cholinergic Markers of Cognition in Rats; PJD: New York, NY, USA, 2000; pp. 21–32. [Google Scholar]
- Bhattacharya, S.K.; Bhattacharya, A.; Kumar, A.; Ghosal, S. Antioxidant activity of Bacopa monniera in rat frontal cortex, Striatum and hippocampus. Phytother. Res. 2000, 14, 174–179. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Chaurasia, S.; Tripathi, E.; Upadhyay, A.; Dubey, G.P. Bacopa monniera Linn. as an antioxidant: Mechanism of action. Indian J. Exp. Biol. 1996, 34, 523–526. [Google Scholar]
- del Cerro, S.; Arai, A.; Lynch, G. Inhibition of long-term potentiation by an antagonist of platelet-activating factor receptors. Behav. Neural. Biol. 1990, 54, 213–217. [Google Scholar] [CrossRef]
- Pravina, K.; Ravindra, K.R.; Goudar, K.S.; Vinod, D.R.; Joshua, A.J.; Wasim, P.; Venkateshwarlu, K.; Saxena, V.S.; Amit, A. Safety evaluation of BacoMind in healthy volunteers: A phase I study. Phytomed 2007, 14, 301–308. [Google Scholar] [CrossRef]
- Sharma, R.; Chaturvedi, C.; Tewari, P.V. Efficacy of Bacopa in revitalizing intellectual functions in children. J. Res. Edu. Indian Med. 1987, 1, 12. [Google Scholar]
- Singh, R.H.; Singh, L. Studies on the anti-anxiety effect of the medyha rasayana drug Brahmi (Bacopa monniera Wettst.). Res. Ayur. Siddha. 1980, 1, 133–148. [Google Scholar]
- Nathan, P.J.; Clarke, J.; Lloyd, J.; Hutchison, C.W.; Downey, L.; Stough, C. The acute effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy normal subjects. Hum. Psychopharmacol. 2001, 16, 345–351. [Google Scholar] [CrossRef]
- Roodenrys, S.; Booth, D.; Bulzomi, S.; Phipps, A.; Micallef, C.; Smoker, J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology 2002, 27, 279–281. [Google Scholar] [CrossRef]
- Morgan, A.; Stevens, J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, Placebo-controlled, Double-blind trial. J. Altern. Complement Med. 2010, 16, 753–759. [Google Scholar] [CrossRef]
- Stough, C.K.; Pase, M.P.; Cropley, V.; Myers, S.; Nolidin, K.; King, R.; Camfield, D.; Wesnes, K.; Pipingas, A.; Croft, K.; et al. A randomized controlled trial investigating the effect of Pycnogenol and Bacopa CDRI08 herbal medicines on cognitive, cardiovascular, and biochemical functioning in cognitively healthy elderly people: The Australian Research Council Longevity Intervention (ARCLI) study protocol (ANZCTR12611000487910). Nutr. J. 2012, 11, 11. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: Chemistry, Efficacy, Safety, and Uses. J. Food Sci. 2008, 73, R14–R19. [Google Scholar]
- Xie, J.; Ding, C.; Ge, Q.; Zhou, Z.; Zhi, X. Simultaneous determination of ginkgolides A, B, C and bilobalide in plasma by LC-MS/MS and its application to the pharmacokinetic study of Ginkgo biloba extract in rats. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2008, 864, 87–94. [Google Scholar] [CrossRef]
- Kleijnen, J.; Knipschild, P. Ginkgo biloba. Lancet 1992, 340, 1136–1139. [Google Scholar] [CrossRef]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and Alternative Medicine Use Among Adults and Children: Unites States, 2007. Natl. Health Stat. Report 2008, 1–23. [Google Scholar]
- Itil, T.; Martorano, D. Natural substances in psychiatry (Ginkgo biloba in dementia). Psychopharmacol. Bull. 1995, 31, 147–158. [Google Scholar]
- Hoyer, S.; Lannert, H.; Noldner, M.; Chatterjee, S.S. Damaged neuronal energy metabolism and behavior are improved by Ginkgo biloba extract (EGb 761). J. Neural. Transm. 1999, 106, 1171–1188. [Google Scholar] [CrossRef]
- Guidetti, C.; Paracchini, S.; Lucchini, S.; Cambieri, M.; Marzatico, F. Prevention of neuronal cell damage induced by oxidative stress in vitro: Effect of different Ginkgo biloba extracts. J. Pharm. Pharmacol. 2001, 53, 387–392. [Google Scholar]
- Oyama, Y.; Fuchs, P.A.; Katayama, N.; Noda, K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca(2+)-loaded brain neurons. Brain Res. 1994, 635, 125–129. [Google Scholar] [CrossRef]
- Tendi, E.A.; Bosetti, F.; Dasgupta, S.F.; Stella, A.M.; Drieu, K.; Rapoport, S.I. Ginkgo biloba extracts EGb 761 and bilobalide increase NADH dehydrogenase mRNA level and mitochondrial respiratory control ratio in PC12 cells. Neurochem. Res. 2002, 27, 319–323. [Google Scholar] [CrossRef]
- van Dongen, M.; van Rossum, E.; Kessels, A.; Sielhorst, H.; Knipschild, P. Ginkgo for elderly people with dementia and age-associated memory impairment: A randomized clinical trial. J. Clin. Epidemiol. 2003, 56, 367–376. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 300, 2253–2262. [Google Scholar]
- Napryeyenko, O.; Sonnik, G.; Tartakovsky, I. Efficacy and tolerability of Ginkgo biloba extract EGb 761 by type of dementia: Analyses of a randomised controlled trial. J. Neurol. Sci. 2009, 283, 224–229. [Google Scholar] [CrossRef]
- Kaschel, R. Specific memory effects of Ginkgo biloba extract EGb 761 in middle-aged healthy volunteers. Phytomed 2011, 18, 1202–1207. [Google Scholar] [CrossRef]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: A randomised, Placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef]
- Kapoor, L. Handbook of Ayurvedic Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Newall, C.A.; Anderson, L.; Phillipson, J.D. Herbal Medicines: A Guide for Healthcare Professionals; In Pharmaceutical Press: London, UK, 1996. [Google Scholar]
- Pan, J.; Kai, G.; Yuan, C.; Zhou, B.; Jin, R.; Yuan, Y. Separation and determination of madecassic acid in extracts of Centella asiatica using high performance liquid chromatography with β-cyclodextrin as mobile phase additive. Chi. J. Chromatogr. 2007, 25, 316–318. [Google Scholar] [CrossRef]
- Williamson, E. Major Herbs of Ayurveda; Elsevier Science: London, UK, 2002; pp. 102–110. [Google Scholar]
- Sharma, P.V. Dravyaguna Vignana, 13th ed; Chaukhamba Vishwa Bharati Academy: New Delhi, India, 1992; pp. 3–5. [Google Scholar]
- Rao, S.B.; Chetana, M.; Uma Devi, P. Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol. Behav. 2005, 86, 449–457. [Google Scholar] [CrossRef]
- Veerendra Kumar, M.H.; Gupta, Y.K. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J. Ethnopharmacol. 2002, 79, 253–260. [Google Scholar] [CrossRef]
- Veerendra Kumar, M.H.; Gupta, Y.K. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 336–342. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Houghton, P.J. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother. Res. 2007, 21, 1142–1145. [Google Scholar] [CrossRef]
- Gadahad, M.R.; Rao, M.; Rao, G. Enhancement of hippocampal CA3 neuronal dendritic arborization by Centella asiatica (Linn) fresh leaf extract treatment in adult rats. J. Chi. Med. Assoc. 2008, 71, 6–13. [Google Scholar] [CrossRef]
- Mohandas Rao, K.G.; Muddanna Rao, S.; Gurumadhva Rao, S. Enhancement of Amygdaloid Neuronal Dendritic Arborization by Fresh Leaf Juice of Centella asiatica (Linn) During Growth Spurt Period in Rats. Evid. Based Compl. Alternat. Med. 2009, 6, 203–210. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Holcomb, L.A.; Hitt, A.R.; Tharakan, B.; Porter, J.W.; Young, K.A.; Manyam, B.V. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother. Res. 2009, 23, 14–19. [Google Scholar] [CrossRef]
- Shobi, V.; Goel, H.C. Protection against radiation-induced conditioned taste aversion by Centella asiatica. Physiol. Behav. 2001, 73, 19–23. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.; Dogra, S. Centella asiatica Attenuates D-Galactose-Induced Cognitive Impairment, Oxidative and Mitochondrial Dysfunction in Mice. Int. J. Alzheimer. Dis. 2011, 2011, 347569. [Google Scholar]
- De Souza, N.D.; Shah, V.; Desai, P.D.; Inamdar, P.K.; Sa, A.D.; Ammonamanchi, R. 2,3,23-trihydroxy-urs-12-ene and its derivatives, Processes for their preparation and their use. EP0383171, 22 August 1990. [Google Scholar]
- Mook-Jung, I.; Shin, J.E.; Yun, S.H.; Huh, K.; Koh, J.Y.; Park, H.K.; Jew, S.S.; Jung, M.W. Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. J. Neurosci. Res. 1999, 58, 417–425. [Google Scholar] [CrossRef]
- Dev, R.D.O.; Mohamed, S.; Hambali, Z.; Samah, B.A. Comparison on cognitive effects of Centella asiatica in healthy middle age female and male volunteers. Eur. J. Sci. Res. 2009, 31, 553–565. [Google Scholar]
- Tiwari, S.; Singh, S.; Patwardhan, K.; Gehlot, S.; Gambhir, I.S. Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. Dig. J. Nanomater. Bios. 2008, 3, 215–220. [Google Scholar]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.R.; Bae, C.S.; Kim, D.; Hong, H.; Nah, S. Protective effect of ginsenosides, Active ingredients of Panax ginseng, On kainic acid-induced neurotoxicity in rat hippocampus. Neurosci. Lett. 2002, 325, 129–133. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, J.T. New achievements in ginseng research and its future prospects. Chin. J. Integr. Med. 2009, 15, 403–408. [Google Scholar] [CrossRef]
- Yang, J.H.; Han, S.J.; Ryu, J.H.; Jang, I.S.; Kim, D.H. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol. Pharm. Bull. 2009, 32, 1710–1715. [Google Scholar] [CrossRef]
- Nishiyama, N.; Cho, S.I.; Kitagawa, I.; Saito, H. Malonylginsenoside Rb1 potentiates nerve growth factor (NGF)-induced neurite outgrowth of cultured chick embryonic dorsal root ganglia. Biol. Pharm. Bull. 1994, 17, 509–513. [Google Scholar] [CrossRef]
- Xue, J.F.; Liu, Z.J.; Hu, J.F.; Chen, H.; Zhang, J.T.; Chen, N.H. Ginsenoside Rb1 promotes neurotransmitter release by modulating phosphorylation of synapsins through a cAMP-dependent protein kinase pathway. Brain Res. 2006, 1106, 91–98. [Google Scholar] [CrossRef]
- Liu, L.; Hoang-Gia, T.; Wu, H.; Lee, M.R.; Gu, L.; Wang, C.; Yun, B.S.; Wang, Q.; Ye, S.; Sung, C.K. Ginsenoside Rb1 improves spatial learning and memory by regulation of cell genesis in the hippocampal subregions of rats. Brain Res. 1382, 147–154. [Google Scholar]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutr. Neurosci. 2001, 4, 295–310. [Google Scholar]
- More, S.V.; Koppula, S.; Kim, I.S.; Kumar, H.; Kim, B.W.; Choi, D.K. The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 2012, 17, 6728–6753. [Google Scholar] [CrossRef]
- Mandel, S.; Weinreb, O.; Amit, T.; Youdim, M.B. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J. Neurochem. 2004, 88, 1555–1569. [Google Scholar] [CrossRef]
- Hong, J.T.; Ryu, S.R.; Kim, H.J.; Lee, J.K.; Lee, S.H.; Kim, D.B.; Yun, Y.P.; Ryu, J.H.; Lee, B.M.; Kim, P.Y. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res. Bull. 2000, 53, 743–749. [Google Scholar] [CrossRef]
- Hong, J.T.; Ryu, S.R.; Kim, H.J.; Lee, J.K.; Lee, S.H.; Yun, Y.P.; Lee, B.M.; Kim, P.Y. Protective effect of green tea extract on ischemia/reperfusion-induced brain injury in Mongolian gerbils. Brain Res. 2001, 888, 11–18. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef]
- Choi, Y.T.; Jung, C.H.; Lee, S.R.; Bae, J.H.; Baek, W.K.; Suh, M.H.; Park, J.; Park, C.W.; Suh, S.I. The green tea polyphenol (−)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Takeda, A.; Sakamoto, K.; Tamano, H.; Fukura, K.; Inui, N.; Suh, S.W.; Won, S.J.; Yokogoshi, H. Facilitated neurogenesis in the developing hippocampus after intake of theanine, an amino acid in tea leaves, and object recognition memory. Cell. Mol. Neurobiol. 2011, 31, 1079–1088. [Google Scholar] [CrossRef]
- Assuncao, M.; Santos-Marques, M.J.; Carvalho, F.; Lukoyanov, N.V.; Andrade, J.P. Chronic green tea consumption prevents age-related changes in rat hippocampal formation. Neurobiol. Aging 2011, 32, 707–717. [Google Scholar] [CrossRef]
- Mandel, S.A.; Amit, T.; Weinreb, O.; Youdim, M.B. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J. Alzheimers Dis. 2011, 25, 187–208. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of flavonoid-rich wine, Tea, and Chocolate by elderly men and women is associated with better cognitive test performance. J. Nutr. 2009, 139, 120–127. [Google Scholar]
- Huang, C.Q.; Dong, B.R.; Zhang, Y.L.; Wu, H.M.; Liu, Q.X. Association of cognitive impairment with smoking, Alcohol consumption, Tea consumption, and Exercise among Chinese nonagenarians/centenarians. Cogn. Behav. Neurol. 2009, 22, 190–196. [Google Scholar] [CrossRef]
- Tabernaemontanus, D.I.T. Kräuterbuch; Johann Ludwig König/Johann Brandmüller: Basel, Switzerland, 1987. [Google Scholar]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef]
- Sfikas, G. Heilpflanzen Griechenlands; Efstathiadis Group: Athen, Greece, 1980. [Google Scholar]
- Imanshahidi, M.; Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phytother. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef]
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef]
- COT report, Phytoestrogens and Health, Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Food Standard Agency: London, UK, 2003; pp. 1–444.
- United Soybean Board. 16th Annual Survey: Consumer Attitudes About Nutrition. Available online: http://www.soyconnection.com/health_nutrition/consumer_attitudes.php (accessed on 1 July 2009).
- Lund, T.D.; West, T.W.; Tian, L.Y.; Bu, L.H.; Simmons, D.L.; Setchell, K.D.; Adlercreutz, H.; Lephart, E.D. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001, 2, 20. [Google Scholar] [CrossRef]
- Chen, J.; Quan, M.H.; Cheng, Y.Q.; Sun, J.; Li, L.T. Acetylcholinesterase inhibitory activity of Chinese sufu (fermented tofu) ethanol-extract. Food Chem. 2012, 134, 1263–1266. [Google Scholar] [CrossRef]
- Hogervorst, E.; Sadjimim, T.; Yesufu, A.; Kreager, P.; Rahardjo, T.B. High tofu intake is associated with worse memory in elderly Indonesian men and women. Dement. Geriatr. Cogn. Disord. 2008, 26, 50–57. [Google Scholar] [CrossRef]
- Henderson, V.W.; St John, J.A.; Hodis, H.N.; Kono, N.; McCleary, C.A.; Franke, A.A.; Mack, W.J. Long-term soy isoflavone supplementation and cognition in women: A randomized, controlled trial. Neurology 2012, 78, 1841–1848. [Google Scholar] [CrossRef]
- Greendale, G.A.; Huang, M.H.; Leung, K.; Crawford, S.L.; Gold, E.B.; Wight, R.; Waetjen, E.; Karlamangla, A.S. Dietary phytoestrogen intakes and cognitive function during the menopausal transition: Results from the Study of Women’s Health Across the Nation Phytoestrogen Study. Menopause 2012, 19, 894–903. [Google Scholar]
- Liu, W.B.; Liu, W.Z. Disciplinarian investigation of Chinese complex prescription with promoting intelligence in past dynasties. Jiangxi. J. Trad. Chin. Med. 2005, 36, 62–63. [Google Scholar]
- Ikeya, Y.; Takeda, S.; Tunakawa, M.; Karakida, H.; Toda, K.; Yamaguchi, T.; Aburada, M. Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol. Pharm. Bull. 2004, 27, 1081–1085. [Google Scholar] [CrossRef]
- Sun, X.L.; Ito, H.; Masuoka, T.; Kamei, C.; Hatano, T. Effect of Polygala tenuifolia root extract on scopolamine-induced impairment of rat spatial cognition in an eight-arm radial maze task. Biol. Pharm. Bull. 2007, 30, 1727–1731. [Google Scholar] [CrossRef]
- Zhang, H.; Han, T.; Zhang, L.; Yu, C.H.; Wan, D.G.; Rahman, K.; Qin, L.P.; Peng, C. Effects of tenuifolin extracted from radix polygalae on learning and memory: A behavioral and biochemical study on aged and amnesic mice. Phytomedicine 2008, 15, 587–594. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, S.H.; Koo, J.W.; Seo, J.H.; Kim, H.S.; Jeong, S.J.; Suh, Y.H. Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT-11. J. Neurosci. Res. 2002, 70, 484–492. [Google Scholar] [CrossRef]
- Shin, K.Y.; Won, B.Y.; Heo, C.; Kim, H.J.; Jang, D.P.; Park, C.H.; Kim, S.; Kim, H.S.; Kim, Y.B.; Lee, H.G.; et al. BT-11 improves stress-induced memory impairments through increment of glucose utilization and total neural cell adhesion molecule levels in rat brains. J. Neurosci. Res. 2009, 87, 260–268. [Google Scholar] [CrossRef]
- Xu, S.P.; Yang, Y.Y.; Xue, D.; Liu, J.X.; Liu, X.M.; Fan, T.P.; le Pan, R.; Li, P. Cognitive-enhancing effects of polygalasaponin hydrolysate in abeta(25–35)-induced amnesic mice. Evid. Based Complement Alternat. Med. 2011, 2011, 839720. [Google Scholar]
- Tohda, C.; Matsumoto, N.; Zou, K.; Meselhy, M.R.; Komatsu, K. Abeta(25–35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology 2004, 29, 860–868. [Google Scholar] [CrossRef]
- Jia, H.; Jiang, Y.; Ruan, Y.; Zhang, Y.; Ma, X.; Zhang, J.; Beyreuther, K.; Tu, P.; Zhang, D. Tenuigenin treatment decreases secretion of the Alzheimer’s disease amyloid beta-protein in cultured cells. Neurosci. Lett. 2004, 367, 123–128. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, K.; Heo, H.; Lee, M.; Kim, J.W.; Whang, W.W.; Kwon, Y.K.; Kwon, H. Effects of Polygala tenuifolia root extract on proliferation of neural stem cells in the hippocampal CA1 region. Phytother. Res. 2008, 22, 1324–1329. [Google Scholar] [CrossRef]
- Xue, W.; Hu, J.F.; Yuan, Y.H.; Sun, J.D.; Li, B.Y.; Zhang, D.M.; Li, C.J.; Chen, N.H. Polygalasaponin XXXII from Polygala tenuifolia root improves hippocampal-dependent learning and memory. Acta Pharmacol. Sin. 2009, 30, 1211–1219. [Google Scholar] [CrossRef]
- Limon, I.D.; Mendieta, L.; Diaz, A.; Chamorro, G.; Espinosa, B.; Zenteno, E.; Guevara, J. Neuroprotective effect of alpha-asarone on spatial memory and nitric oxide levels in rats injected with amyloid-beta((25–35)). Neurosci. Lett. 2009, 453, 98–103. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007, 73, 283–285. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, B.W.; Song, S.Y.; Kim, J.S.; Kim, I.S.; Kwon, Y.S.; Koppula, S.; Choi, D.K. Cognitive Enhancing Effects of Alpha Asarone in Amnesic Mice by Influencing Cholinergic and Antioxidant Defense Mechanisms. Biosci. Biotechnol. Biochem. 2012, 76, 1518–1522. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Reeta, K.H.; Mehla, J.; Gupta, Y.K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009, 1301, 52–60. [Google Scholar]
- Ali, E.H.; Arafa, N.M. Comparative protective action of curcumin, memantine and diclofenac against scopolamine-induced memory dysfunction. Fitoterapia 2011, 82, 601–608. [Google Scholar] [CrossRef]
- Jaques, J.A.; Rezer, J.F.; Carvalho, F.B.; da Rosa, M.M.; Gutierres, J.M.; Goncalves, J.F.; Schmatz, R.; de Bairros, A.V.; Mazzanti, C.M.; Rubin, M.A.; et al. Curcumin protects against cigarette smoke-induced cognitive impairment and increased acetylcholinesterase activity in rats. Physiol. Behav. 2012, 106, 664–669. [Google Scholar] [CrossRef]
- Yadav, R.S.; Chandravanshi, L.P.; Shukla, R.K.; Sankhwar, M.L.; Ansari, R.W.; Shukla, P.K.; Pant, A.B.; Khanna, V.K. Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology 2011, 32, 760–768. [Google Scholar] [CrossRef]
- Awasthi, H.; Tota, S.; Hanif, K.; Nath, C.; Shukla, R. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci. 2010, 86, 87–94. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, D.; Li, S.; Li, G.; Shyamala, S.G.; Barish, P.A.; Vernon, M.M.; Pan, J.; Ogle, W.O. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology 2009, 57, 463–471. [Google Scholar] [CrossRef]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar]
- Pitsikas, N.; Sakellaridis, N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav. Brain Res. 2006, 173, 112–115. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Shafiee Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. A 22-week, Multicenter, Randomized, Double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology (Berl) 2010, 207, 637–643. [Google Scholar]
- Wheatley, D. Medicinal plants for insomnia: A review of their pharmacology, efficacy and tolerability. J. Psychopharmacol. 2005, 19, 414–421. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Tildesley, N.T.; Perry, E.K.; Wesnes, K.A. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol. Biochem. Behav. 2002, 72, 953–964. [Google Scholar] [CrossRef]
- Ballard, C.G.; O’Brien, J.T.; Reichelt, K.; Perry, E.K. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: The results of a double-blind, placebo-controlled trial with Melissa. J. Clin. Psychiat. 2002, 63, 553–558. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiat. 2003, 74, 863–866. [Google Scholar] [CrossRef]
- Duarte, J.M.; Agostinho, P.M.; Carvalho, R.A.; Cunha, R.A. Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS One 2012, 7, e21899. [Google Scholar]
- Smith, A.P.; Clark, R.; Gallagher, J. Breakfast cereal and caffeinated coffee: Effects on working memory, attention, mood, and cardiovascular function. Physiol. Behav. 1999, 67, 9–17. [Google Scholar] [CrossRef]
- Hawkes, C.; Harris, J.L. An analysis of the content of food industry pledges on marketing to children. Public Health Nutr. 2011, 14, 1403–1414. [Google Scholar] [CrossRef]
- Kaur, T.; Pathak, C.M.; Pandhi, P.; Khanduja, K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008, 67, 25–30. [Google Scholar]
- Unno, K.; Takabayashi, F.; Yoshida, H.; Choba, D.; Fukutomi, R.; Kikunaga, N.; Kishido, T.; Oku, N.; Hoshino, M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology 2007, 8, 89–95. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kumar, H.; More, S.V.; Han, S.-D.; Choi, J.-Y.; Choi, D.-K. Promising Therapeutics with Natural Bioactive Compounds for Improving Learning and Memory — A Review of Randomized Trials. Molecules 2012, 17, 10503-10539. https://doi.org/10.3390/molecules170910503
Kumar H, More SV, Han S-D, Choi J-Y, Choi D-K. Promising Therapeutics with Natural Bioactive Compounds for Improving Learning and Memory — A Review of Randomized Trials. Molecules. 2012; 17(9):10503-10539. https://doi.org/10.3390/molecules170910503
Chicago/Turabian StyleKumar, Hemant, Sandeep Vasant More, Sang-Don Han, Jin-Yong Choi, and Dong-Kug Choi. 2012. "Promising Therapeutics with Natural Bioactive Compounds for Improving Learning and Memory — A Review of Randomized Trials" Molecules 17, no. 9: 10503-10539. https://doi.org/10.3390/molecules170910503
APA StyleKumar, H., More, S. V., Han, S.-D., Choi, J.-Y., & Choi, D.-K. (2012). Promising Therapeutics with Natural Bioactive Compounds for Improving Learning and Memory — A Review of Randomized Trials. Molecules, 17(9), 10503-10539. https://doi.org/10.3390/molecules170910503





