Activity of Spray-dried Microparticles Containing Pomegranate Peel Extract against Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microparticle Preparation
| Microparticle composition | Extract:polymer ratio | Theoretical extract content (%) | Actual extract content (%) | Encapsulation efficiency (%) | Production yield (%) | Mean diameter (µm) |
|---|---|---|---|---|---|---|
| Alginate | 1:2 | 33.3 | 27.3 ± 2.2 | 81.9 ± 6.5 | 40 ± 1.2 | 2.45 ± 1.37 |
| Chitosan | 1:2 | 33.3 | 24.9 ± 1.4 | 74.7 ± 7.2 | 41 ± 1.0 | 2.80 ± 1.28 |
2.2. Scanning Electron Microscopy
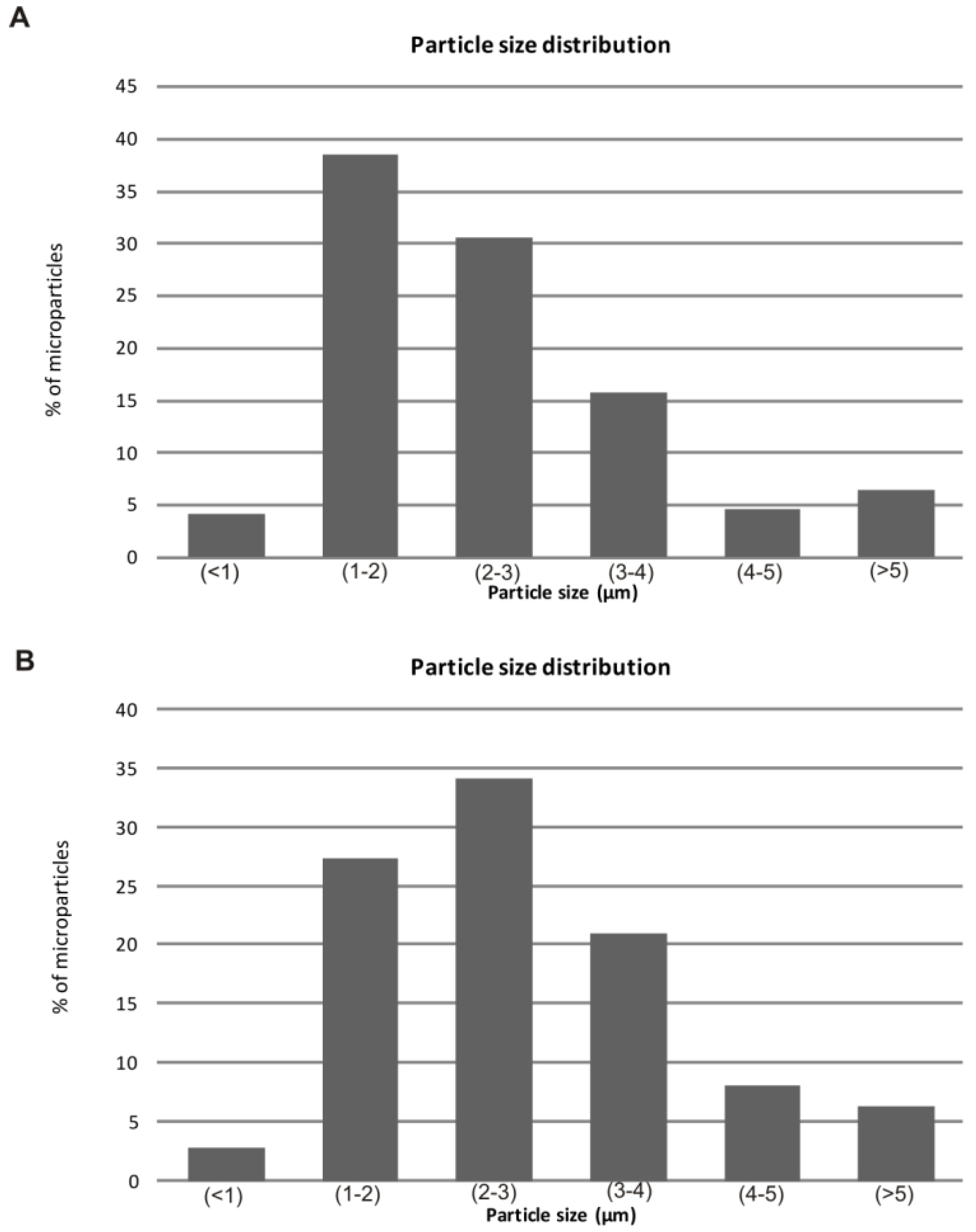
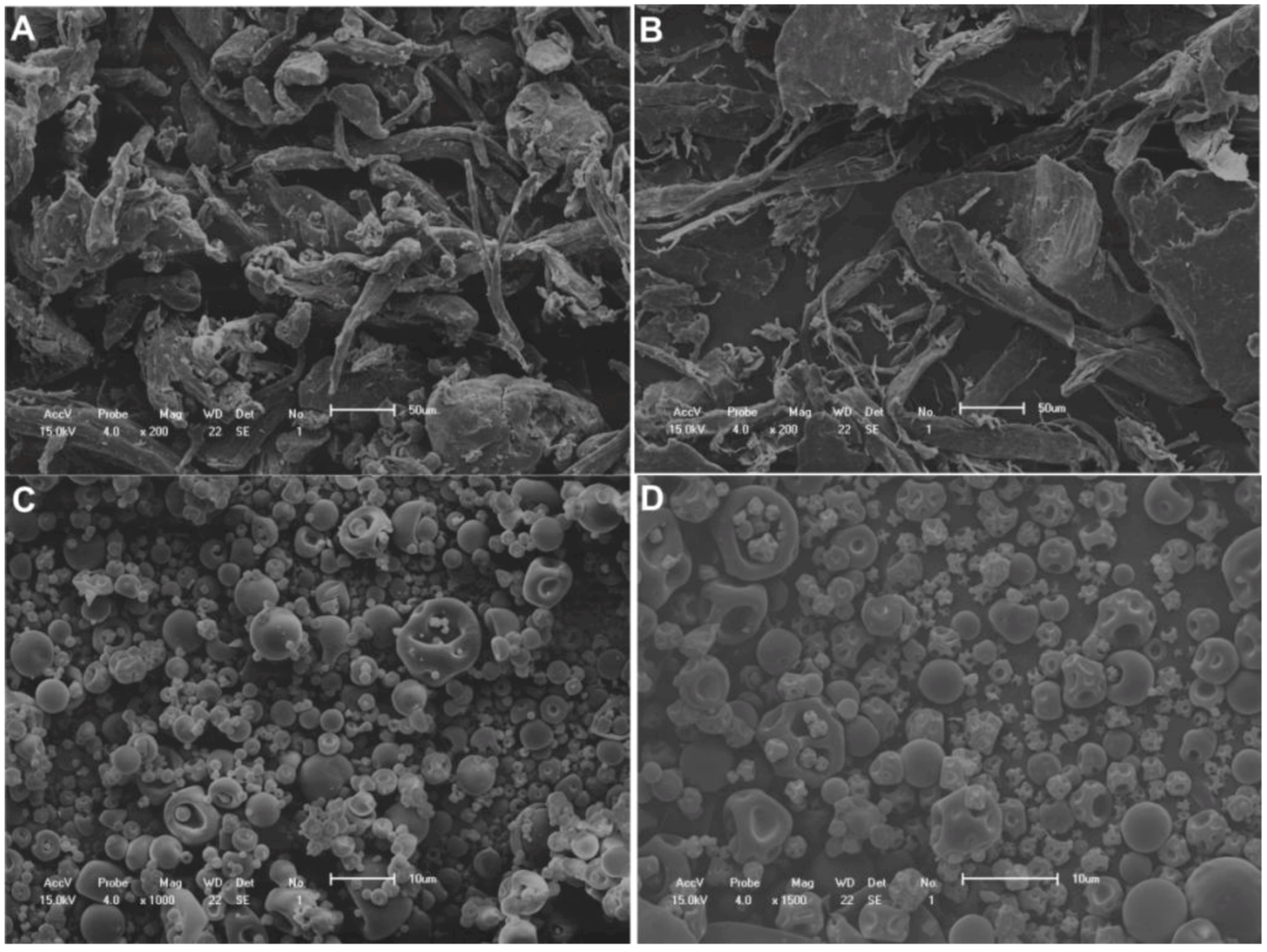
2.3. Determination of Extract Content on Microparticles
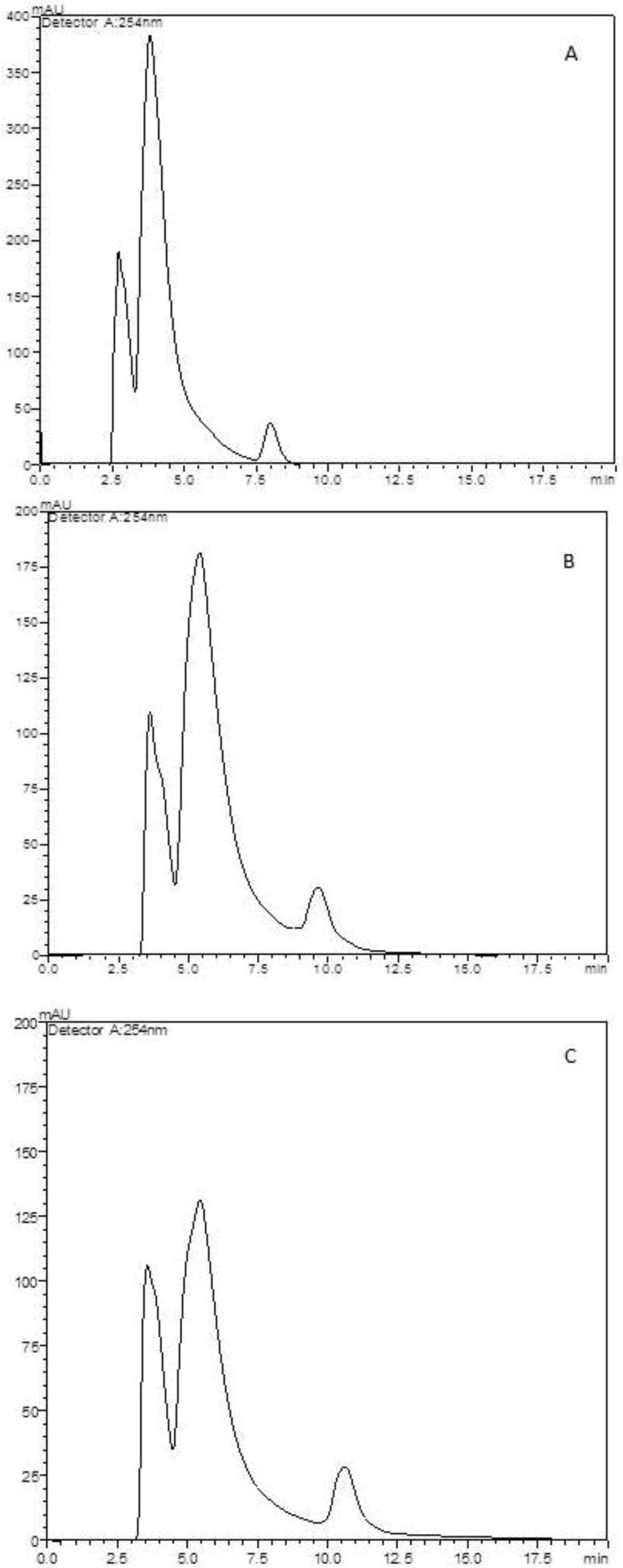
2.4. In vitro Extract Release Test
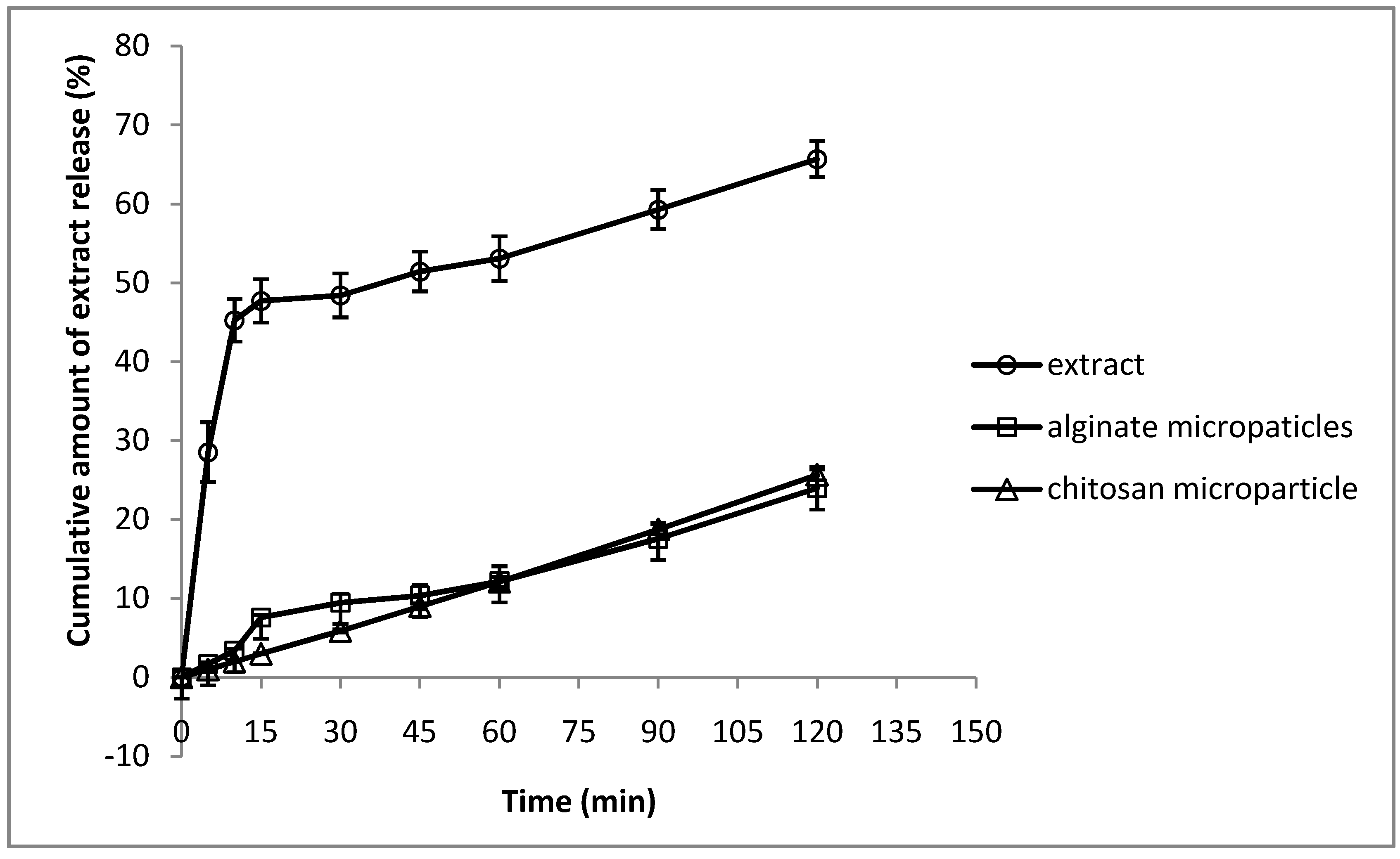
2.5. Antifungal in Vitro Assay
2.6. In Vitro Biofilm Assay
| MIC (µg/mL) | |||
|---|---|---|---|
| Planktonic cells | Biofilm at 48 h | ||
| CV50 | MTT50 | ||
| Pomegranate Extract | 3.9 | 62.5 | >1000 |
| Fluconazole | 7.8 | 250 | >1000 |
| Nystatin | 3.1 | 7.8 | 31.2 |
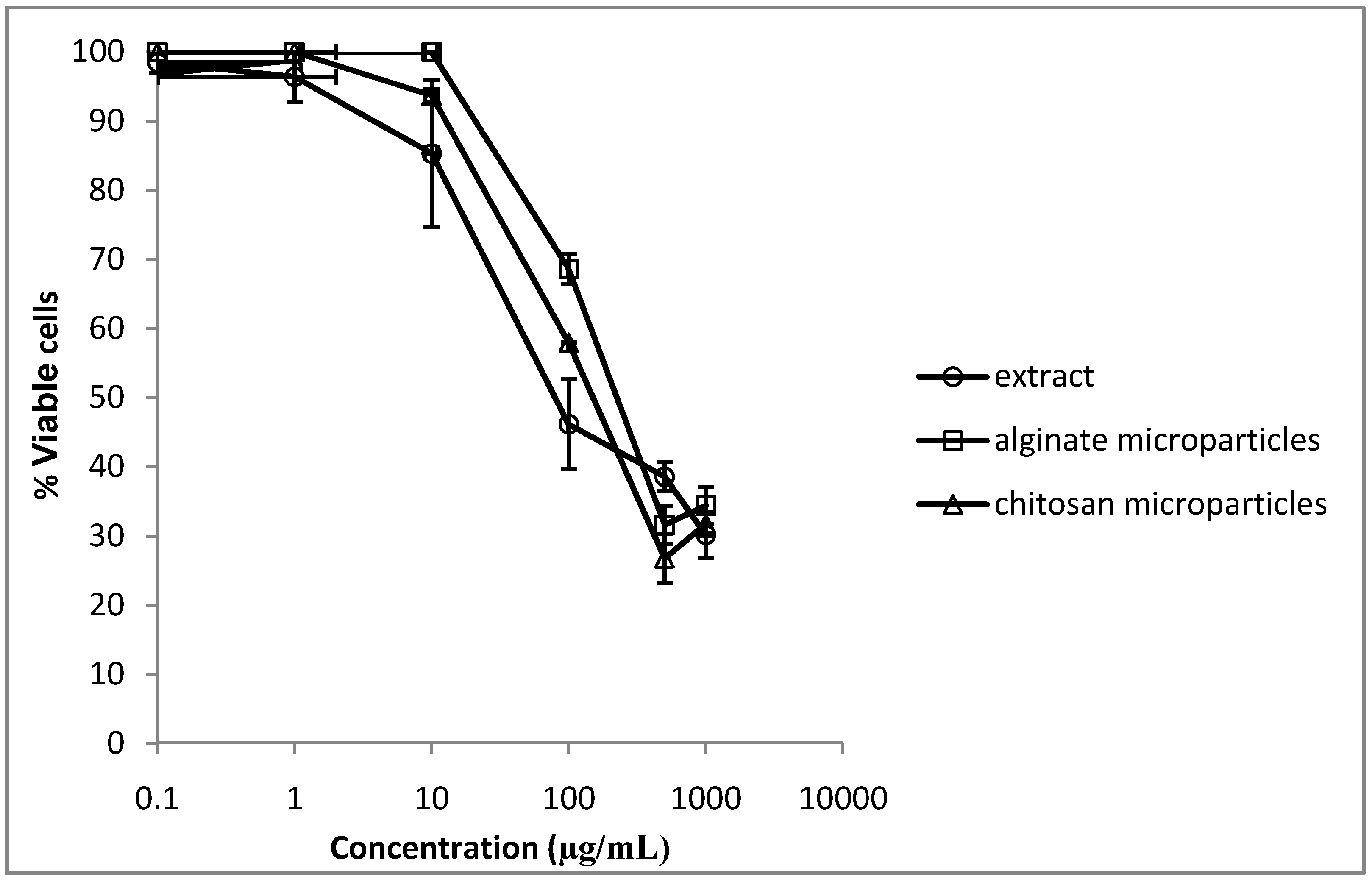
2.7. Cytotoxicity
3. Experimental
3.1. Extract Preparation
3.2. Alginate Microparticles Preparation
3.3. Chitosan Microparticles Preparation
3.4. Microparticles Characterization
3.4.1. Scanning Electron Microscopy
3.4.2. Determination of Extract Content on Microparticles
3.4.3. In Vitro Extract Release Test
3.5. Antifungal in Vitro Assay
3.6. In Vitro Biofilm Assay
3.7. Cytotoxicity
4. Conclusions
Acknowledgements
References
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.S.; Heber, D.D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar]
- Ajaikumar, K.B.; Asheef, M.; Babu, B.H.; Padikkala, J. The inhibition of gastric mucosal injury by Punica granatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005, 96, 171–176. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Al-Mustafa, A.H.; Tarawneh, K.; Khleifat, K.M.; Ridzwan, B.H.; Qaralleh, H.N. Protective role of Punica granatum L. peel extract against oxidative damage in experimental diabetic rats. Process Biochem. 2010, 45, 581–585. [Google Scholar]
- Malik, A.; Afaq, F.; Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Mukhtar, H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 14823–14818. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Al-Zoreky, P.N. Antimicrobial activity of pomegranate (Punica granatum) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef]
- Braga, L.C.; Cummings, C.; Jett, M.; Takahashi, J.A.; Carmo, L.S.; Chartone-Souza, E.; Nascimento, A.M.A. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J. Ethnopharmacol. 2005, 96, 335–339. [Google Scholar] [CrossRef]
- Braga, L.C.; Leite, A.A.M.; Xavier, K.G.S.; Takahashi, J.A.; Bemquerer, M.P.; Chartone-Souza, E.; Nascimento, A.M.A. Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can. J. Microbiol. 2005, 51, 541–547. [Google Scholar]
- Endo, E.H.; Cortez, D.A.G.; Nakamura, T.U.; Nakamura, C.V.; Dias Filho, B.P. Potent antifungal activity of extract and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef]
- Haidari, M.; Ali, M.; Casscels, S.W., III; Madjef, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza vírus and hás a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar]
- Machado, T.B.; Leal, I.C.R.; Amaral, A.C.F.; Santos, K.R.N.; Silva, M.G.; Kuster, R.M. Antimicrobial allagitannin of Punica granatum fruits. J. Braz. Chem. Soc. 2002, 13, 606–610. [Google Scholar]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- Heber, D.; Seeram, N.P.; Wyatt, H.; Henning, S.M.; Zhang, Y.; Ogden, L.G.; Dreher, M.; Hill, J.O. Safety and antiosidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased wais size. J. Agric. Food Chem. 2007, 55, 10050–10054. [Google Scholar]
- Kulkarni, A.P.; Mahal, H.S.; kapoor, S.; Aradhya, S.M. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. Food Chem. 2007, 55, 1491–1500. [Google Scholar] [CrossRef]
- Cruz, M.C.; Goldstein, A.L.; Blakenship, J.R.; Del Poeta, M.; Davis, D.; Cardenas, M.E.; Perfect, J.R.; McCuster, J.H.; Heitman, J. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002, 24, 546–559. [Google Scholar]
- Morschauser, J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 2002, 1587, 240–246. [Google Scholar] [CrossRef]
- Pereira-Cenci, T.; Delbelcury, A.A.; Crielaard, W.; Tencate, J.M. Development of Candida-associated denture stomatitis: New insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray-drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar] [CrossRef]
- Giunchedi, P.; Juliano, C.; Gavini, E.; Cossu, M.; Sorrenti, M. Formulation and in vivo evaluation of chlorexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur. J. Pharm. Biopharm. 2002, 53, 233–239. [Google Scholar] [CrossRef]
- Tran, V.T.; Benoît, J.P.; Julienne, M.C.V. Why and how to prepare biodegradable, monodispersed, polymeric microparticles in the field of pharmacy? Int. J. Pharm. 2011, 407, 1–11. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Bernabei, M.T.; Cameroni, R. Alginate microparticles for enzyme peroral administration. Int. J. Pharm. 2002, 242, 263–266. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Cardoso, M.L.C.; Lucchesi, M.B.; Gremião, M.P.D. Gelatin microparticles containing propolis prepared by spray-drying technique: preparation and characterization. Int. J. Pharm. 2003, 264, 45–55. [Google Scholar]
- Hawser, S.P.; Douglas, L.J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 1995, 39, 2128–2131. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Van Vuuren, S.F.; Viljoen, A.M. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. S. Afr. J. Bot. 2011, 77, 80–85. [Google Scholar]
- Consterton, J.W.; Stewart, S.; Greenberg, E.P. Baterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar]
- Melo, A.C.; Bizerra, F.C.; Freymuller, E.; Arthington-Skaggs, B.A.; Colombo, A.L. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. Isolates, including strains of the Candida parapsilosis complex. Med. Mycol 2011, 49, 253–262. [Google Scholar]
- Clinical And Laboratory Standards Institute. Reference method for Broth Dilution Antifungal Susceptibility testing of yeasts, Approved Standard. In In CLSI Document; CLSI: Wayne, PA, USA, 2002; pp. M27–A2.
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Stefano, V.D. In vitro anti-biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar]
- Sample Availability: Samples of the extract are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Activity of Spray-dried Microparticles Containing Pomegranate Peel Extract against Candida albicans. Molecules 2012, 17, 10094-10107. https://doi.org/10.3390/molecules170910094
Endo EH, Ueda-Nakamura T, Nakamura CV, Filho BPD. Activity of Spray-dried Microparticles Containing Pomegranate Peel Extract against Candida albicans. Molecules. 2012; 17(9):10094-10107. https://doi.org/10.3390/molecules170910094
Chicago/Turabian StyleEndo, Eliana Harue, Tânia Ueda-Nakamura, Celso Vataru Nakamura, and Benedito Prado Dias Filho. 2012. "Activity of Spray-dried Microparticles Containing Pomegranate Peel Extract against Candida albicans" Molecules 17, no. 9: 10094-10107. https://doi.org/10.3390/molecules170910094
APA StyleEndo, E. H., Ueda-Nakamura, T., Nakamura, C. V., & Filho, B. P. D. (2012). Activity of Spray-dried Microparticles Containing Pomegranate Peel Extract against Candida albicans. Molecules, 17(9), 10094-10107. https://doi.org/10.3390/molecules170910094




