Abstract
To gain further insight into the structural requirements of the aliphatic group at position 2 for their antimycobacterial activity, some N-alkyl-4-(1H)-quinolones bearing position 2 alkynyls with various chain length and triple bond positions were prepared and tested for in vitro antibacterial activity against rapidly-growing strains of mycobacteria, the vaccine strain Mycobacterium bovis BCG, and methicillin-resistant Staphylococcus aureus strains, EMRSA-15 and -16. The compounds were also evaluated for inhibition of ATP-dependent MurE ligase of Mycobacterium tuberculosis. The lowest MIC value of 0.5 mg/L (1.2–1.5 µM) was found against M. fortuitum and M. smegmatis. These compounds displayed no or only weak toxicity to the human lung fibroblast cell line MRC-5 at 100 µM concentration. The quinolone derivatives exhibited pronounced activity against the epidemic MRSA strains (EMRSA-15 and -16) with MIC values of 2–128 mg/L (5.3–364.7 µM), and M. bovis BCG with an MIC value of 25 mg/L (66.0–77.4 µM). In addition, the compounds inhibited the MurE ligase of M. tuberculosis with moderate to weak activity showing IC50 values of 200–774 µM. The increased selectivity towards mycobacterial bacilli with reference to MRC-5 cells observed for 2-alkynyl quinolones compared to their corresponding 2-alkenyl analogues serves to highlight the mycobacterial specific effect of the triple bond. Exploration of a terminal bromine atom at the side chain of N-alkyl-2-(E)-alkenyl-4-(1H)-quinolones showed improved antimycobacterial activity whereas a cyclopropyl residue at N-1 was suggested to be detrimental to antibacterial activity.
1. Introduction
Since the discovery of the bacterial ethiology of tuberculosis (TB) by Robert Koch in 1882, various attempts have been devised to treat infections caused by Mycobacterium tuberculosis. Streptomycin and p-aminosalicylic acid were the first drugs introduced to treat TB, followed by isoniazid, pyrazinamide, rifampicin and ethambutol, which were championed as the first-line agents [1,2]. However, the emergence of strains that are resistant to these weapons necessitated further drug options that led to the second- and third-generation antibiotics. In the early 1990’s fluoroquinolones were introduced to tackle the ever increasing danger of bacterial resistance and have enjoyed great success against both Gram-positive and Gram-negative bacteria and certain anaerobes. Unfortunately, mutations resulting from spontaneous chromosomal alterations, and their widespread overuse and misuse have contributed significantly to quinolone resistance [3]. Currently there is increasing concern regarding the paucity of new antimycobacterial therapeutic agents that are coming onto the market in spite of increased mycobacterial resistance. Therefore, new drugs with new modes of action are required in view of worldwide-emerging multi drug resistant (MDR-) and extensively drug resistant (XDR) M. tuberculosis strains [4].
Cell wall biosynthesis is one of the prime targets for drug discovery, because it is an essential process in the bacterial life cycle. Enzymes involved in the later stage of bacterial cell wall biosynthesis have been the targets of successfully marketed antibacterial drugs such as β-lactams including pencillins, carbapenems and cephalosporins, and vancomycin as well as several promising agents in advanced clinical study such as telavancin and capuramycin [5]. In contrast, except for fosfomycin no successful antibacterial agents targeting the first cytoplasmic steps of murein biosynthesis have appeared on the market.
Mur ligases are enzymes that catalyze in a stepwise fashion the early cytoplasmic reactions of bacterial cell wall biosynthesis, and one of these are the ATP-dependent amide forming ligases of M. tuberculosis, being MurE the enzyme responsible for catalyzing the addition of meso-diaminopimelic acid (m-DAP) to the nucleotide precursor UDP-MurNAc-L-Ala-D-Glu to form UDP-MurNAc-L-Ala-D-Glu-m-DAP [6]. We have recently disclosed N-methyl-4(1H)-quinolones bearing an alkenyl moiety at position 2 as a novel class of M. tuberculosis MurE inhibitors [7]. The compounds inhibited M. tuberculosis MurE in vitro in the micromolar range, making them successful anti-TB scaffolds for further rounds of medicinal chemistry improvement.
Our investigations into the design and synthesis of this class of antimycobacterials started with the initial discovery of the potent antimycobacterial properties of quinolone alkaloids isolated from the Chinese medicinal plant Euodia rutaecarpa [8,9]. In recent reports, we have described the synthesis, antimycobacterial and cytotoxicity evaluation of a series of N-methyl-2-alkenyl-4(1H)-quinolones [10,11]. We observed that quinolones having alkenyl moieties at C-2 displayed superior inhibitory effect compared to their alkyl analogues and subsequent mechanistic investigations indicated that the antimycobacterial activity of these compounds is related to inhibition of M. tuberculosis MurE ligase [7]. We are particularly interested in the synthesis and biological evaluation of other N-alkyl-4(1H)-quinolones, since the antimycobacterial mechanism of action is assumed to be different from the currently used anti-TB agents, and also different from DNA gyrase/topoisomerase inhibition of clinically-used fluoroquinolones that have an essential free carboxylic group at position C-3 [12], absent in active N-alkyl-4(1H)-quinolones. Based on these findings we decided to continue our investigation on modification of the aliphatic side chain at position 2, and the present study was principally undertaken to explore the impact of the triple bond on the antibacterial and cytotoxic activities of the quinolones at C-2. Here we report the synthesis and biological evaluation of N-alkyl-4(1H)-quinolones having alkynyls at C-2 with the intent of elucidating the role that these groups may play in the in vitro antibacterial profile against fast and slow growing strains of mycobacteria, and methicillin-resistant S. aureus strains keeping in mind potential correlations with the M. tuberculosis MurE enzyme inhibitory data. In addition, the influence of a terminal bromine atom at the side chain on the antimycobacterial activity was evaluated. The cytotoxicity profile of the synthetic derivatives was assessed against the human lung fibroblast cell line MRC-5.
2. Results and Discussion
2.1. Synthesis
As shown in Scheme 1, our rational design for the synthesis of N-alkyl-2-alkynyl-4(1H)-quinolones was based on the replacement of the double bond with a triple bond, while maintaining the basic quinolone skeleton. The synthetic route targeting compounds 8a–t is given in Scheme 1. The key intermediates, 3a–e and 6a–d, which are essential for the synthesis of our target quinolone derivatives were obtained in two ways. Pd(OAc)2 and PMe3 mediated direct coupling of methyl vinyl ketone (2) and 1-alkynes 1 in acetone afforded 5-alkynyl-2-ones 3a–e [13]. The 3-alkynyl-2-ones 6a–d, on the other hand, were prepared according to the Xing and O’Doherty procedure [14] by treatment of the terminal alkynes 1 with acetaldehyde in the presence of n-BuLi in THF and subsequent oxidation with MnO2. The synthesis of this series of 4(1H)-quinolones was accomplished by the well-known condensation of methyl alkynyl ketones 3a–e and 6a–d with N-alkyl isatoic acid anhydrides 7a–d in presence of LDA in THF at −78 °C.
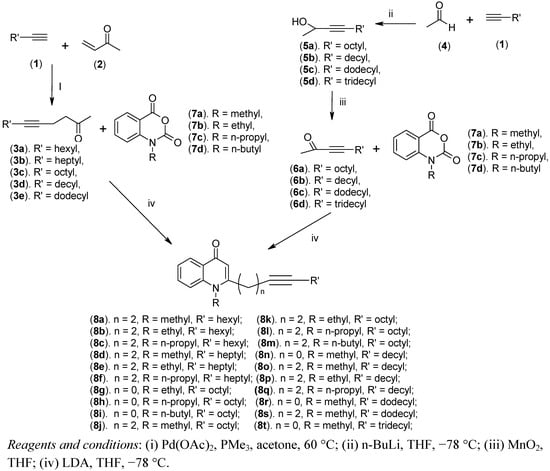
Scheme 1.
Synthesis of N-alkyl-2-alkynyl-4(1H)-quinolones.
In order to study the biological effect of the cyclopropyl moiety which is the constituent of many therapeutically important fluoroquinolones, we explored the possibility of introducing a cyclopropyl group at N-1. N-Cyclopropyl isatoic acid anhydride (12) was synthesized from 2-iodobenzoic acid (9) as shown in Scheme 2. Copper (0) mediated substitution of the iodine of 9 with cyclopropyl amine in DMF gave 2-(cyclopropylamino)benzoic acid (11). Compound 11 was then treated with NEt3 and bis-(trichloromethyl) carbonate in the presence of catalytic N,N-dimethylaminopyridine in CH2Cl2 to afford 12.
The intermediate, ω-bromo-α,β-unsaturated methyl alkenyl ketones 17a–b, required to incorporate a terminal bromine atom in the aliphatic side chain, were prepared from commercially available α,ω-diols 13a–b. Bromination of the diols with HBr and subsequent oxidation with pyridinium chlorochromate (PCC) gave ω-bromoaldehydes 15a–b. Compounds 17a–b were prepared as previously reported [11] by refluxing 15a–b with the ylide methylcarbonylmethylenephosphorane (16) obtained from chloroacetone and triphenylphosphine.
To further assess the biological role of the cyclopropyl moiety at N-1 and compare their antimycobacterial properties with our previously reported N-methyl-2-(E)-alkenyl-4(1H)-quinolones, compounds 22a–b were prepared from the commercially available aldehydes 20a–b following the same methods described before [10]. Similarly, analogue 23 containing cyclopropyl and undecynyl groups at positions 1 and 2, respectively (Scheme 2), was prepared from compounds 3b and 12 in order to examine their impact on biological potency. The identity of the synthetic intermediates and the 4(1H)-quinolone derivatives was confirmed by analysis of 1D and 2D-NMR spectroscopic and LC-ESI-MS data. The compounds 8a–8t, 18a–b, 19a–b, 22a–b and 23 are reported here for the first time.
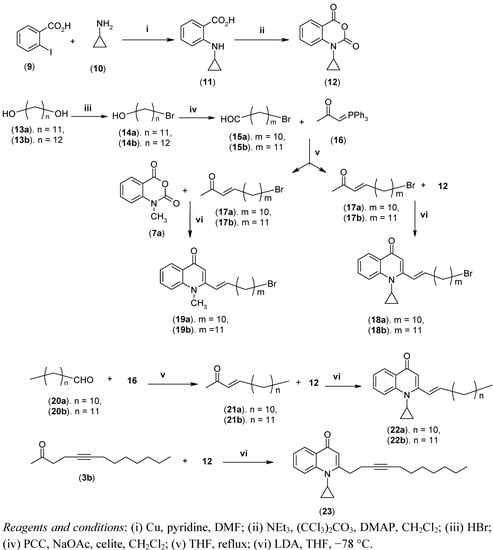
Scheme 2.
Synthesis of N-cyclopropyl-2-(E)-alkenyl-4(1H)-quinolones and N-cyclo- propyl-2-alkynyl-4(1H)-quinolones.
2.2. Biological Evaluation
All of the novel synthetic compounds were screened for their in vitro antimycobacterial activity against M. smegmatis using a microbroth dilution method [10] and the most active compounds were further tested against M. fortuitum, M. phlei, M. smegmatis mc2155 and M. bovis BCG. Enzymatic inhibition of the MurE ligase of M. tuberculosis was also determined.
In our microtiter broth dilution assay, the quinolone derivatives displayed significant inhibitory effect against M. smegmatis, with MIC values ranging from 0.5 to 128 mg/L (1.2–350.7 µM). The most active compounds were 8n, 8r, 19a and 19b, with an MIC value of 0.5 mg/L (1.2–1.5 µM).
As shown in Table 1, compound 8a with the shortest aliphatic chain (3-decynyl and methyl groups at position 2 and 1, respectively), displayed the lowest growth inhibition (MIC value of 32 mg/L). As the aliphatic chain length increased, an increase in potency was observed with maximum activity achieved for 1-tetradecynyl (compound 8r) and 1-dodecynyl (compound 8n) groups. Further elongation of the aliphatic chain resulted in a gradual loss of activity. The antimycobacterial data in Table 1 also indicated that the potency of the quinolone derivatives was related to chain length of the aliphatic group at position 1 and 2. As shown in Table 2, among the rapidly-growing strains of mycobacteria, M. smegmatis mc2155 was found to be the most susceptible to the test compounds. In the spot culture growth inhibition assay (SPOTi), the tested compounds were found to be moderate growth inhibitors of M. bovis BCG (MIC value of 25 mg/L). Against the epidemic strains of MRSA (EMRSA-15 and -16) the most active quinolone derivatives, 8l, 8m and 8n displayed pronounced growth inhibition with MIC values ranging from 2 to 8 mg/L (5.5–22.8 µM).

Table 1.
MIC values against M. smegmatis and cytotoxicity against MRC-5 cells of the N-alkyl-2-alkynyl/(E)-alkenyl-4(1H)-quinolone derivatives. 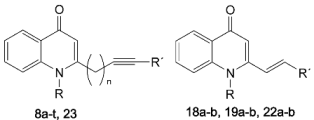

| Comp. | Substituent | MIC against M. smegmatis (ATCC 14468) | Metabolic active MRC-5 cells (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | R | R' | mg/L | µM | 100 µM | 60 µM | 30 µM | 10 µM | |
| 8a | 2 | –CH3 | hexyl | 32 | 108.5 | 89.3 ± 2.94 | 97.5 ± 0.74 | 98.4 ± 1.07 | 100 ± 1.05 |
| 8b | 2 | –C2H5 | hexyl | 16 | 51.8 | 85.7 ± 1.96 | 98.4 ± 0.77 | 100 ± 0.73 | 102 ± 0.85 |
| 8c | 2 | –C3H7 | hexyl | 8 | 24.8 | 100 ± 1.45 | 100 ± 0.55 | 102 ± 0.42 | 103 ± 1.22 |
| 8d | 2 | –CH3 | heptyl | 8 | 25.9 | 97.3 ± 0.66 | 98.7 ± 0.8 | 99.8 ± 0.52 | 101 ± 1.08 |
| 8e | 2 | –C2H5 | heptyl | 8 | 24.8 | 100 ± 1.05 | 100 ± 0.56 | 101 ± 1.42 | 102 ± 1.05 |
| 8f | 2 | –C3H7 | heptyl | 4 | 11.9 | 70.9 ± 8.50 | 91.7 ± 2.77 | 101 ± 1.28 | 100 ± 1.05 |
| 8g | 0 | –C2H5 | octyl | 2 | 6.5 | 12.1 ± 4.79 | 56.5 ± 2.03 | 98.7 ± 2.97 | 101 ± 0.97 |
| 8h | 0 | –C3H7 | octyl | 4 | 12.4 | 14.1 ± 7.83 | 39.5 ± 3.59 | 101 ± 2.47 | 103 ± 1.38 |
| 8i | 0 | C4H9 | octyl | 4 | 11.9 | 28.7 ± 8.95 | 67.2 ± 9.90 | 101 ± 0.81 | 103 ± 3.13 |
| 8j | 2 | –CH3 | octyl | 4 | 12.4 | 95.84 ± 0.96 | 95.1 ± 0.81 | 96.7 ± 1.49 | 97.6 ± 1.03 |
| 8k | 2 | –C2H5 | octyl | 4 | 11.9 | 74.8 ± 1.99 | 94.7 ± 0.92 | 95.1 ± 1.04 | 97.6 ± 1.69 |
| 8l | 2 | –C3H7 | octyl | 1 | 2.8 | 9.0 ± 6.85 | 69.1 ± 8.04 | 99.1 ± 2.32 | 100 ± 0.31 |
| 8m | 2 | –C4H9 | octyl | 2 | 5.5 | 0.4 ± 0.02 | 67.5 ± 3.19 | 89.7 ± 2.46 | 94.9 ± 3.18 |
| 8n | 0 | –CH3 | decyl | 0.5 | 1.5 | 100 ± 0.82 | 100 ± 0.81 | 100 ± 0.72 | 101 ± 0.85 |
| 8o | 2 | –CH3 | decyl | 2 | 5.7 | 90.7 ± 3.48 | 95.8 ± 2.54 | 97.9 ± 0.88 | 100 ± 0.73 |
| 8p | 2 | –C2H5 | decyl | 1 | 2.7 | 8.75 ± 9.35 | 93.3 ± 1.13 | 95.4 ± 0.76 | 97.3 ± 1.37 |
| 8q | 2 | –C3H7 | decyl | 1 | 2.6 | 0.2 ± 0.09 | 19.8 ± 9.85 | 67.9 ± 2.65 | 92.7 ± 4.62 |
| 8r | 0 | –CH3 | dodecyl | 0.5 | 1.5 | 95.7 ± 1.14 | 97.1 ± 1.69 | 98.1 ± 1.65 | 99.7 ± 2.03 |
| 8s | 2 | –CH3 | dodecyl | 128 | 337.7 | 41.5 ± 9.49 | 58.9 ± 3.65 | 92.2 ± 0.70 | 93.9 ± 0.61 |
| 8t | 0 | –CH3 | tridecyl | 128 | 350.7 | 93.8 ± 0.96 | 98.8 ± 1.28 | 100 ± 1.38 | 100 ± 1.01 |
| 18a | - | Cp | bromodecyl | 8 | 18.6 | ND | ND | ND | ND |
| 18b | - | Cp | bromoundecyl | 8 | 18.1 | ND | ND | ND | ND |
| 19a | - | –CH3 | bromodecyl | 0.5 | 1.2 | ND | ND | ND | ND |
| 19b | - | –CH3 | bromoundecyl | 0.5 | 1.2 | ND | ND | ND | ND |
| 22a | - | Cp | undecyl | 128 | 350.7 | ND | ND | ND | ND |
| 22b | - | Cp | dodecyl | 128 | 337.8 | ND | ND | ND | ND |
| 23 | 2 | Cp | hexyl | 8 | 24.3 | ND | ND | ND | ND |
| Isoniazid | - | - | - | 4 | 29.2 | ND | ND | ND | ND |
| Ethambutol | - | - | - | 2 | 9.8 | ND | ND | ND | ND |
| Vinblastin * | 51.5 ± 2.36 | ||||||||
ND: not determined; * 0.12 µM.

Table 2.
In vitro biological activity of selected N-alkyl-4(1H)-quinolone derivatives and positive controls.
| Compound | MIC mg/L(µM) | IC50 (µM) | |||||
|---|---|---|---|---|---|---|---|
| M. smegmatis mc2155 | M. fortuitum | M. phlei | M. bovis BCG | S. aureus EMRSA-15 | S. aureus EMRSA-16 | Mtb MurE | |
| 8g | 4 (12.9) | 8 (25.9) | 4 (12.9) | ND | ND | ND | ND |
| 8l | 2 (5.7) | 2 (5.7) | 4 (11.4) | 25 (71.2) | 2 (5.7) | 8 (22.8) | 263 |
| 8m | 2 (5.5) | 2 (5.5) | 4 (10.9) | 25 (68.5) | 2 (5.5) | 4 (10.9) | 235 |
| 8n | 1 (3.1) | 0.5 (1.5) | 1 (3.1) | 25 (77.4) | 2 (6.2) | 4 (12.4) | 251 |
| 8o | 1 (2.8) | 2 (5.7) | 2 (5.7) | 25 (71.2) | 8 (22.8) | 8 (22.8) | 774 |
| 8p | 1 (2.7) | 4 (10.9) | 2 (5.5) | ND | ND | ND | ND |
| 8q | 1 (2.6) | 2 (5.3) | 2 (5.3) | 25 (66.0) | 2 (5.3) | 128 (337.7) | 200 |
| 8r | 1 (2.8) | 0.5 (1.5) | 2 (5.7) | 25 (71.2) | 16 (45.6) | 128 (364.7) | 631 |
| 19a | 1 (2.5) | 1 (2.5) | 1 (2.5) | ND | ND | ND | ND |
| 19b | 1 (2.4) | 1 (2.4) | 1 (2.4) | ND | ND | ND | ND |
| Isoniazid | 4 (29.2) | 2 (14.6) | 8 (58.3) | 0.1 (0.7) | ND | ND | ND |
| Ethambutol | 4 (19.6) | 8 (39.2) | 4 (19.6) | ND | ND | ND | ND |
| Norfloxacin | ND | ND | ND | ND | 0.5 (1.6) | 128 (400.9) | ND |
| Tetracycline | ND | ND | ND | ND | 0.25 (0.6) | 0.25 (0.6) | ND |
| 1-Methyl-2-[(5Z)-tetradecenyl]-4(1H)-quinolone | ND | ND | ND | ND | ND | ND | 36 [7] |
ND: Not determined.
When assayed against M. tuberculosis ATP-dependent MurE using a colorimetric method the tested compounds displayed moderate to low activity, with IC50 values ranging from 200 µM to 774 µM. Our biological results further strengthen our previous finding that optimal activity is achieved when the 4(1H)-quinolones possess an aliphatic chain at C-2 which is 12–14 carbons in length. When the length of the aliphatic side chain at position 2 of the quinolone nucleus was further increased, for instance compounds 8s and 8t, a marked reduction of the antimycobacterial activity was noted. A significant decrease in antimycobacterial potency was also encountered upon introduction of a cyclopropyl group at N-1. However, it should be pointed out that compounds 8c and 23, both having a dec-3-ynyl group at position 2 of the quinolone nucleus and n-propyl and cyclopropyl groups at N-1, respectively, were found to be equipotent against M. smegmatis. The most active compounds 8n and 8r are of particular interest because they are devoid of toxicity to the human diploid embryonic lung cell line (MRC-5) up to a concentration of 100 µM.
The antimycobacterial activity of compounds having the same substituent at N-1 and an alkynyl group with the same chain length at position 2, but differing on the position of the triple bond, such as 8b and 8g (N-Et and decynyl), 8c and 8h (N-propyl and decynyl), 8j and 8n (N-Me and dodecynyl) and 8o and 8r (N-Me and tetradecynyl), was compared in order to assess the effect of the position of the triple bond on the activity. It was realized that compounds 8g, 8h, 8n and 8r exhibited two to four fold greater potencies than 8b, 8c, 8j and 8o. In general, a higher antimycobacterial activity was observed for compounds having the triple bond in position α,β to the quinolone nucleus compared to compounds having the triple bond in a position distant from the quinolone nucleus. However, this tendency was inverted for N-propyl or N-cyclopropyl substitution, where compounds having the triple bond distant from the quinolone 8l and 8m were slightly more active than compounds having the triple bond in position α,β, such as 8h and 8i. This SAR suggests that antimycobacterial potency depends on different structural parameters in a non-linear way and a relation valid for a set of compounds might not be valid for a different set, and thus point out on a complementary approach for obtaining meaningful SAR.
Compounds 18a–b and 19a–b were prepared in order to explore the effects of a terminal bromine atom on the antimycobacterial activity. It is worth mentioning that compound 19b showed a two-fold increase in antimycobacterial potency against M. smegmatis compared to its analogue lacking a bromine atom reported in our previous work [10,11]. This result is quite interesting and we are aiming to explore the possibility of the same effect for other active anti-TB scaffolds having long aliphatic chains. The presence of a bromine at the terminal position may increase the probability of covalent bonding to a particular protein by nucleophilic attack, an hypothesis which deserves further attention.
On the other hand, compounds 18a–b and 22a–b, bearing a cyclopropyl moiety at N-1 appeared to be less potent than their analogues 19a–b having a methyl group at N-1, again suggesting that a cyclopropyl group is detrimental to the activity.
In order to assess the cytotoxicity of these novel quinolone derivatives, the effect on the viability of a human lung fibroblast cell line MRC-5, was measured using XTT proliferation assay. The compounds displayed little or no toxicity at 100 µM test concentration. By comparing the cytotoxicity results of this study with that of our previous reports [10,11], one can notice a remarkable reduction in cytotoxicity to the MRC-5 cell line, indicating that the triple bond was important in reducing the toxicity of the quinolones. This finding finds support in other studies which revealed that 5-decynyl, 5-dodecynyl and 5-tetradecynyl pyrimidine nucleoside derivatives have enhanced antimycobacterial activity while being devoid of cytotoxic activity up to a high concentration [15]. In addition, the position of the triple bond in the side chain seems to have a significant influence on cytotoxcity which is increased in compounds with a triple bond more distant to the quinolone nucleus. This can be concluded by comparing results of 8l, 8n, 8p, and 8r having similar antimycobacterial activity on M. smegmatis but different cytotoxcity depending on triple bond position.
3. Experimental
3.1. General
The strains M. smegmatis (ATCC 14468), M. smegmatis mc2155, M. fortuitum (ATCC 6841), M. phlei (ATCC 11758) and M. bovis BCG (ATCC 35734) were obtained from American Type Culture Collection or German Collection of Microorganisms and Cell Culture (DSMZ). EMRSA-15 and 16 were gifts from Dr Paul Stapleton (UCL School of Pharmacy, UK). M. tuberculosis MurE ligase was over-expressed in E. coli BL21(DE3)pLysS cells (New England Biolabs, Hitchin, UK) and purified according to our previous report [7].
All chemicals were purchased from Sigma-Aldrich (Munich, Germany). Reactions were carried out using oven-dried glassware under an atmosphere of argon. THF was distilled from sodium and stored in molecular sieve (4 Å). DMF was distilled from CaH2. Melting points were recorded using a KOFLER hot plate microscope and are uncorrected. IR spectra obtained on a Perkin-Elmer 281 B spectrometer, were recorded in KBr unless otherwise noted. 1H and 13C-NMR spectra were recorded on a Varian 400 MHz spectrometer (at 400 and 100 MHz, respectively) using deuterated chloroform as solvent with TMS as internal standard. Mass spectra were obtained by LC-ESI-MS analysis in positive mode on a Thermo Finnigan LCQ Deca XP Plus mass spectrometer connected to a Surveyor LC-system (Thermo-Finnigan, West Palm Beach, FL, USA). Precoated Si gel 60 F254 plates (Merck, Darmstadt, Germany) were used to monitor the progress of the reactions and column fractions. Spots were detected by UV/254 nm and spraying with molybdato-phosphoric acid and subsequent heating. Compounds were purified by column chromatography on silica gel 60 (0.063–0.200 mm) using cyclohexane/ethyl acetate mixtures as eluent.
3.2. Synthesis
3.2.1. General Procedure for Synthesis of Alkynones 3a–e
Compounds 3a–e were obtained according to the procedure described previously [13] with some modifications. To a stirred solution of Pd(OAc)2 (0.05 equiv.) in P(CH3)3 (1.0 M in toluene, 0.2 equiv.) at 60 °C, acetone (~2 mL) and a mixture of 1-alkyne 1 (1 equiv.) and methyl vinyl ketone (2, 3.0 equiv.) were added. The reaction mixture was stirred for 24 h at 60 °C. Then the catalyst was filtered off on celite, extracted with ether, dried over Na2SO4 and concentrated. The crude product was purified by silica gel CC eluting with cyclohexane/ethyl acetate = 95:5 to afford 3a–e.
5-Dodecyn-2-one (3a) was prepared from 1-octyne (5.0 g, 45.5 mmol), 2 (9.6 g, 136.4 mmol), Pd(OAc)2 (0.51 g, 2.3 mmol) and P(CH3)3 (9.1 mL, 9.1 mmol) as a colourless oil (75%) [16].
5-Tridecyn-2-one (3b) was prepared from 1-nonyne (5.0 g, 40.3 mmol), 2 (8.5 g, 121 mmol), Pd(OAc)2 (0.45 g, 2.0 mmol) and P(CH3)3 (8.1 mL, 8.1 mmol) as a colourless oil (82%). 1H-NMR δ: 2.60 (t, J = 7.6 Hz, 2H, H-3), 2.39 (t, J = 7.6 Hz, 2H, H-4), 2.16 (s, 3H, H-1), 2.10 (t, J = 7.6 Hz, 2H, H-7), 1.43 (quint, J = 6.8 Hz, 2H, H-8), 1.30–1.25 (m, 8H, H-9-12), 0.86 (t, J = 6.8 Hz, 3H, H-13). 13C-NMR δ: 207.2 (C-2), 80.7 (C-6), 78.4 (C-5), 43.0 (C-3), 31.8, 29.4, 29.3, 29.1, 28.9, 22.6, 18.6, 14.0, 13.5.
5-Tetradecyn-2-one (3c) was prepared from 1-decyne (5.0 g, 36.2 mmol), 2 (7.6 g, 108.7 mmol), Pd(OAc)2 (0.41 g, 1.8 mmol) and P(CH3)3 (7.2 mL, 7.2 mmol) as a colourless oil (79%) [13].
5-Hexadecyn-2-one (3d) was prepared from 1-dodecyne (5.0 g, 30.1 mmol), 2 (6.3 g, 90.4 mmol), Pd(OAc)2 (0.34 g, 1.5 mmol) and P(CH3)3 (6.0 mL, 6.0 mmol) as a colourless oil (73%). 1H-NMR δ: 2.61 (t, J = 7.2 Hz, 2H, H-3), 2.40 (t, J = 7.2 Hz, 2H, H-4), 2.15 (s, 3H, H-1), 2.09 (t, J = 7.2 Hz, 2H, H-7), 1.43 (quint, J = 6.8 Hz, 2H, H-8), 1.30–1.23 (m, 14H, H-9-15), 0.87 (t, J = 6.8 Hz, 3H, H-16). 13C-NMR δ: 207.1 (C-2), 80.9 (C-6), 78.3 (C-5), 42.9 (C-3), 31.9, 29.8, 29.5, 29.5, 29.3, 29.1, 29.0, 28.8, 22.6, 18.6, 14.0, 13.5.
5-Octadecyn-2-one (3e) was prepared from 1-tetradecyne (4.0 g, 20.6 mmol), 2 (4.3 g, 61.9 mmol), Pd(OAc)2 (0.23 g, 1.0 mmol) and P(CH3)3 (4.1 mL, 4.1 mmol) as a colourless oil (73%). 1H-NMR δ: 2.59 (t, J = 7.6 Hz, 2H, H-3), 2.41 (t, J = 7.6 Hz, 2H, H-4), 2.14 (s, 3H, H-1), 2.08 (t, J = 7.6 Hz, 2H, H-7), 1.44 (quint, J = 6.8 Hz, 2H, H-8), 1.33–1.21 (m, 18H, H-9-17), 0.85 (t, J = 6.8 Hz, 3H, H-18). 13C-NMR δ: 207.3 (C-2), 81.0 (C-6), 78.5 (C-5), 42.8 (C-3), 31.8, 29.8, 29.8, 29.5, 29.5, 29.4, 29.3, 29.1, 29.0, 28.8, 22.6, 18.6, 14.0, 13.5.
3.2.2. General Procedure for Synthesis of Alkynols 5a–d
To a stirred solution of 1-alkyne (1 equiv.) in THF at −78 °C, n-BuLi (1.6 M in hexane) (1.1 equiv.) was added dropwise and stirring was continued for 2 h. To this solution acetaldehyde (2.0 equiv.) was added and further stirred for about 1 h until the temperature dropped to room temperature. The reaction was quenched by the addition of NH4Cl solution and extracted with CH2Cl2 (3×), washed with brine, dried over Na2SO4 and concentrated. The crude product was purified by silica gel CC eluting with cyclohexane/ethyl acetate = 9:1.
3-Dodecyn-2-ol (5a) was prepared from 1-decyne (10.0 g, 72.5 mmol) in THF (150 mL), n-BuLi (49.8 mL, 79.7 mmol), acetaldehyde (6.4 g, 145.1 mmol) as a yellow oil (77%). 1H-NMR δ: 4.46 (qd, J = 6.4, 1.6 Hz, 1H, H-2), 2.14 (td, J = 6.8, 1.6 Hz, 2H, H-5), 1.45 (quint, J = 7.6 Hz, 2H, H-6), 1.37 (d, J = 6.4 Hz, 3H, H-1), 1.30–1.21 (m, 10H, H-7-11), 0.85 (t, J = 6.8 Hz, 3H, H-12). 13C-NMR δ: 84.4 (C-4), 82.2 (C-3), 58.4 (C-2), 31.2, 29.1, 29.0, 28.5, 28.4, 24.6, 22.4, 18.5, 13.9.
3-Tetradecyn-2-ol (5b) was prepared from 1-dodecyne (4.0 g, 24.1 mmol) in THF (75 mL), n-BuLi (16.6 mL, 26.5 mmol), acetaldehyde (2.1 g, 48.2 mmol) as a yellow oil (69%) [17].
3-Hexadecyn-2-ol (5c) was prepared from 1-tetradecyne (5.0 g, 25.8 mmol) in THF (80 mL), n-BuLi (17.7 mL, 28.4 mmol), acetaldehyde (2.27 g, 51.6 mmol) as a yellow oil (81%) [18].
3-Heptadecyn-2-ol (5d) was prepared from 1-pentadecyne (4.0 g, 19.2 mmol) in THF (75 mL), n-BuLi (13.2 mL, 21.2 mmol), acetaldehyde (1.69 g, 38.5 mmol) as a yellow oil (65%). 1H-NMR δ: 4.49 (qd, J = 6.8, 1.6 Hz, 1H, H-2), 2.17 (td, J = 6.8, 1.6 Hz, 2H, H-5), 1.48 (quint, J = 7.2 Hz, 2H, H-6), 1.40 (d, J = 6.4 Hz, 3H, H-1), 1.33–1.21 (m, 20H, H-7-16), 0.87 (t, J = 6.8 Hz, 3H, H-17). 13C-NMR δ: 84.7 (C-4), 82.2 (C-3), 58.5 (C-2), 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.3, 29.1, 28.8, 28.6, 24.7, 22.6, 18.6, 14.1.
3.2.3. General Procedure for the Synthesis of Alkynones 6a–d
A mixture of 5 (1.0 equiv.) in THF and MnO2 (0.67 equiv.) was stirred at room temperature for 24 h. The reaction mixture was filtered through celite to give 6.
3-Dodecyn-2-one (6a) was prepared from 5a (10.0 g, 54.9 mmol), in THF (50 mL) and MnO2 (3.2 g, 36.8 mmol) as a colorless oil (79%). 1H-NMR δ: 2.32 (t, J = 7.2 Hz, 2H, H-5), 2.29 (s, 3H, H-1), 1.55 (quint, J = 7.6 Hz, 2H, H-6), 1.30–1.23 (m, 10H, H-7-11), 0.87 (t, J = 6.8 Hz, 3H, H-12). 13C-NMR δ: 184.8 (C-2), 94.1 (C-4), 81.3 (C-3), 32.7, 31.1, 29.3, 29.0, 28.4, 27.6, 22.5, 18.9, 14.0.
3-Tetradecyn-2-one (6b) was prepared from 5b (3.0 g, 14.3 mmol), in THF (15 mL) and MnO2 (0.83 g, 9.6 mmol) as a colourless oil (87%). 1H-NMR δ: 2.33 (t, J = 6.8 Hz, 2H, H-5), 2.30 (s, 3H, H-1), 1.56 (quint, J = 7.2 Hz, 2H, H-6), 1.31–1.23 (m, 14H, H-7-13), 0.87 (t, J = 6.8 Hz, 3H, H-14). 13C-NMR δ: 184.8 (C-2), 94.1 (C-4), 81.4 (C-3), 32.7, 31.8, 29.5, 29.4, 29.2, 29.0, 28.8, 27.6, 22.6, 18.9, 14.0.
3-Hexadecyn-2-one (6c) was prepared from 5c (4.5 g, 18.9 mmol), in THF (20 mL) and MnO2 (1.1 g, 12.7 mmol) as a colourless oil (82%). 1H-NMR δ: 2.32 (t, J = 6.8 Hz, 2H, H-5), 2.29 (s, 3H, H-1), 1.55 (quint, J = 7.2 Hz, 2H, H-6), 1.33–1.19 (m, 18H, H-7-15), 0.86 (t, J = 6.8 Hz, 3H, H-16). 13C-NMR δ: 184.7 (C-2), 94.0 (C-4), 81.3 (C-3), 32.6, 31.8, 29.6, 29.6, 29.5, 29.4, 29.2, 29.0, 28.8, 27.6, 22.6, 18.9, 14.0.
3-Heptadecyn-2-one (6d) was prepared from 5d (3.0 g, 11.9 mmol), in THF (15 mL) and MnO2 (0.69 g, 7.9 mmol) as a colourless oil (83%). 1H-NMR δ: 2.34 (t, J = 6.8 Hz, 2H, H-5), 2.31 (s, 3H, H-1), 1.57 (quint, J = 7.6 Hz, 2H, H-6), 1.33–1.21 (m, 20H, H-7-16), 0.87 (t, J = 6.8 Hz, 3H, H-17). 13C-NMR δ: 184.9 (C-2), 94.1 (C-4), 81.4 (C-3), 32.7, 31.9, 29.6, 29.6, 29.6, 29.6, 29.4, 29.3, 29.0, 28.8, 27.7, 22.7, 18.9, 14.1.
3.2.4. Preparation of 2-(Cyclopropylamino)benzoic acid (11)
2-Iodobenzoic acid (10.0 g, 40.3 mmol, 1.0 equiv.) dissolved in DMF (50 mL) was poured into an argon purged two mouth flask containing freshly prepared Cu (512 mg, 8.1 mmol, 0.2 equiv.), pyridine (4.8 g, 60.5 mmol, 1.5 equiv.) and cyclopropylamine (5.1 g, 88.7 mmol, 2.2 equiv.). The mixture was stirred for 24 h at room temperature and poured into 500 mL of acidified water (pH 4.5). Filtration and drying of the slurry gave a white precipitate of 11 (83% yield). 1H-NMR δ:7.98 (d, J = 8.0 Hz, 1H, H-6), 7.72 (bs, 1H, –NH–), 7.44 (t, J = 7.6 Hz, 1H, H-4), 7.17 (d, J = 8.0 Hz, 1H, H-3), 6.68 (t; J = 7.6 Hz, 1H, H-5), 2.49 (sept, J = 3.2 Hz, 1H, N–CH–), 0.83 (m, 2H, N–CH–CH2–), 0.60 (m, 2H, N–CH–CH2–). 13C-NMR δ: 174.0 (–COOH), 152.4 (C-2), 135.4 (C-4), 132.3 (C-6), 115.3 (C-5), 112.9 (C-3), 108.8 (C-1), 24.2 (N–CH–), 7.6 (2 × N–CH–CH2–).
3.2.5. Preparation of N-Cyclopropylisatoic Anhydride (12)
2-(Cyclopropylamine)benzoic acid (4.0 g, 22.6 mmol, 1 equiv.) and N(Et)3 (2.2 g, 21.5 mmol, 0.95 equiv.) were dissolved in CH2Cl2 (50 mL) and cooled to 0 °C with ice. Bis(trichloromethyl) carbonate (2.0 g, 6.8 mmol, 0.3 equiv.) dissolved in CH2Cl2 (25 mL) was added to the mixture using a syringe followed by N,N-dimethylaminopyridine (0.4 g, 3.3 mmol, 0.15 equiv.) in CH2Cl2 (15 mL). After 2 h of stirring at the same temperature the reaction was quenched by adding 25 mL of HCl (1.0 M), extracted with CH2Cl2, dried over Na2SO4 and concentrated to give a light yellow powder of 12 (94%). 1H-NMR δ: 8.11 (d, J = 8.0 Hz, 1H, H-5), 7.79 (t, J = 7.6 Hz, 1H, H-7), 7.65 (d, J = 8.0 Hz, 1H, H-8), 7.31 (t, J = 7.6 Hz, 1H, H-6), 2.93 (quint, J = 3.2 Hz, 1H, N–CH–), 1.33 (m, 2H, N–CH–CH2–), 0.99 (m, 2H, N–CH–CH2–). 13C-NMR δ: 158.7 (C-4), 147.7 (C-2), 142.6 (C-8a), 136.8 (C-7), 130.3 (C-5), 124.1 (C-6), 115.4 (C-8), 111.8 (4a), 27.1 (N–CH–), 9.9 (2 × N–CH–CH2–).
3.2.6. Synthesis of ω-Bromoalcohols 14a–b
Treatment of the diols 13a–b with 48% of HBr according to a previously described method [10] provided 11-bromoundecan-1-ol (14a) [19] and 12-bromododecan-1-ol (14b) [20].
3.2.7. Synthesis of ω-Bromoaldehydes 15a–b
Both 11-bromoundecanal (15a) and 12-bromodecanal were obtained from 14a and 14b, respectively, by the action of PCC in the presence of NaOAc in CH2Cl2 according to Houghton et al. [21], spectral data are in accordance with [22].
3.2.8. Synthesis of ω-Bromo-(3E)-ketones 17a–b
14-Bromo-(3E)-tetradecen-2-one (17a) was prepared from 15a and ylide 16 according to Wube et al. [10] as a colorless oil (78% yield). 1H-NMR δ: 6.81 (dt, J = 16.0, 6.8 Hz, 1H, H-4), 6.06 (d, J = 16.0 Hz, 1H, H-3), 3.41 (t, J = 6.8 Hz, 2H, H-14), 2.25 (s, 3H, H-1), 2.21 (q, J = 7.2 Hz, 2H, H-5), 1.84 (quint, J = 6.8 Hz, 2H, H-13), 1.44 (m, 4H, H-12, 6), 1.32–1.23 (m, 10H, H-7-11). 13C-NMR δ: 198.6 (C-2), 148.5 (C-4), 131.1 (C-3), 33.9 (C-14), 32.6 (C-13), 32.3 (C-5), 29.2 (C-10), 29.2 (C-9), 29.0 (C-8), 28.6 (C-6), 28.0 (C-7), 27.9 (C-11), 26.7 (C-12), 26.7 (C-1).
15-Bromo-(3E)-pentadecen-2-one (17b) was prepared from 15b and ylide 16 as a colourless oil (84% yield). 1H-NMR δ: 6.81 (dt, J = 16.0, 6.4 Hz, 1H, H-4), 6.06 (d, J = 16.0 Hz, 1H, H-3), 3.41 (t, J = 6.8 Hz, 2H, H-15), 2.25 (s, 3H, H-1), 2.22 (q, J = 6.8 Hz, 2H, H-5), 1.85 (quint, J = 6.8 Hz, 2H, H-14), 1.43 (m, 4H, H-13, 6), 1.32–1.23 (m, 12H, H-7-12). 13C-NMR δ: 198.9 (C-2), 148.7 (C-4), 131.2 (C-3), 33.9 (C-15), 32.4 (C-14), 32.4 (C-5), 29.4 (C-10), 29.4 (C-9), 29.3 (C-11), 29.3 (C-8), 29.1 (C-7), 28.6 (C-6), 28.0 (C-12), 26.8 (C-13), 26.7 (C-1).
3.2.9. General Procedure for the Synthesis of 8a–t, 18a–b, 19a–b, 22a–b and 23
Compounds 8a–t, 18a–b, 19a–b, 22a–b and 23 were prepared according to procedure described previously [10] from methyl alkynyl ketones (1.0 equiv.) in THF, LDA (1.8 M in THF/heptane/ethylbenzene) (1.0 equiv.) and N-alkyl isatoic anhydride (0.75 equiv.) in THF at −78 °C. The purity of the quinolones was determined by LC-MS (88–95%).
1-Methyl-2-(3′-decynyl)-4(1H)-quinolone (8a) was prepared from 3a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-methylisatoic anhydride (7a) (0.74 g, 4.2 mmol) in THF (10 mL) as a yellow oil (63%). IR (KBr, cm−1): 3406, 2929, 2857, 1627, 1600, 1500, 1469, 1177, 759. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.68 (t, J = 7.6 Hz, 1H, H-7), 7.52 (d, J = 8.0 Hz, 1H, H-8), 7.39 (t, J = 7.2 Hz, 1H, H-6), 6.32 (s, 1H, H-3), 3.78 (s, 3H, N–CH3), 2.95 (t, J = 7.2 Hz, 2H, H-1'), 2.57 (t, J = 6.8 Hz, 2H, H-2'), 2.12 (t, J = 6.8 Hz, 2H, H-5'), 1.44 (quint, J = 7.2 Hz, 2H, H-6'), 1.32–1.20 (m, 6H, H-7'-9'), 0.86 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 177.5 (C-4), 152.6 (C-2), 140.6 (C-8a), 132.2 (C-7), 126.8 (C-5), 126.6 (C-4a), 123.5 (C-6), 115.5 (C-8), 110.7 (C-3), 82.8 (C-4'), 77.2 (C-3'), 35.3 (N–CH3), 32.9 (C-1'), 31.6 (C-8'), 28.6 (C-7'), 28.4 (C-6'), 22.6 (C-9'), 18.7 (C-2'), 18.5 (C-5'), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 296 (100).
1-Ethyl-2-(3'-decynyl)-4(1H)-quinolone (8b) was prepared from 3a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-ethylisatoic anhydride (7b) (0.8 g, 4.2 mmol) in THF (10 mL) as a yellow oil (55%). IR (KBr, cm−1): 3428, 2929, 2854, 1624, 1600, 1490, 1468, 1430, 1308, 759. 1H-NMR δ: 8.46 (d, J = 8.0 Hz, 1H, H-5), 7.63 (t, J = 7.6 Hz, 1H, H-7), 7.55 (d, J = 8.0 Hz, 1H, H-8), 7.37 (t, J = 7.6 Hz, 1H, H-6), 6.34 (s, 1H, H-3), 4.33 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.96 (t, J = 7.2 Hz, 2H, H-1'), 2.61 (t, J = 6.8 Hz, 2H, H-2'), 2.13 (t, J = 6.8 Hz, 2H, H-5'), 1.45 (t, J = 7.2 Hz, 3H, N–CH2–CH3), 1.42 (quint, J = 6.8 Hz, 2H, H-6'), 1.30–1.21 (m, 6H, H-7'-9'), 0.86 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 177.4 (C-4), 152.6 (C-2), 140.5 (C-8a), 132.2 (C-7), 126.9 (C-5), 126.7 (C-4a), 123.4 (C-6), 115.4 (C-8), 110.9 (C-3), 82.7 (C-4'), 77.1 (C-3'), 41.2 (N–CH2–CH3), 33.0 (C-1'), 31.5 (C-8'), 28.7 (C-7'), 28.5 (C-6'), 22.5 (C-9'), 18.8 (C-2'), 18.7 (C-5'), 14.2 (N–CH2–CH3), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 310 (100).
1-(n-Propyl)-2-(3′-decynyl)-4(1H)-quinolone (8c) was prepared from 3a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.85 g, 4.2 mmol) in THF (10 mL) as a yellow oil (56%). IR (KBr, cm−1): 3420, 2929, 2856, 1627, 1600, 1489, 1468, 1428, 1177, 759. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.64 (t, J = 8.0 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.29 (s, 1H, H-3), 4.13 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.91 (t, J = 7.6 Hz, 2H, H-1'), 2.58 (t, J = 7.6 Hz, 2H, H-2'), 2.12 (t, J = 7.6 Hz, 2H, H-5'), 1.84 (m, 2H, N–CH2–CH2–CH3), 1.44 (quint, J = 7.6 Hz, 2H, H-6'), 1.35–1.23 (m, 6H, H-7'-9'), 1.08 (t, J = 7.6 Hz, 3H, N–CH2–CH2–CH3), 0.85 (t, J = 7.2 Hz, 3H, H-10'). 13C-NMR δ: 175.4 (C-4), 152.7 (C-2), 140.7 (C-8a), 132.1 (C-7), 126.8 (C-5), 126.6 (C-4a), 123.3 (C-6), 115.5 (C-8), 110.9 (C-3), 82.7 (C-4'), 77.1 (C-3'), 47.8 (N–CH2–CH2–CH3), 33.2 (C-1'), 31.4 (C-8'), 28.7 (C-7'), 28.5 (C-6'), 22.5 (C-9'), 22.1 (N–CH2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.0 (C-10'), 11.0 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-Methyl-2-(3'-undecynyl)-4(1H)-quinolone (8d) was prepared from 3b (1.0 g, 5.2 mmol) in THF (15 mL), LDA (2.9 mL, 5.2 mmol) and N-methylisatoic anhydride (7a) (0.69 g, 3.9 mmol) in THF (10 mL) as a yellow semi solid (58%). IR (KBr, cm−1): 3420, 2928, 2855, 1628, 1600, 1500, 1469, 1177, 759. 1H-NMR δ: 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.65 (t, J = 8.0 Hz, 1H, H-7), 7.49 (d, J = 8.4 Hz, 1H, H-8), 7.36 (t, J = 7.6 Hz, 1H, H-6), 6.27 (s, 1H, H-3), 3.76 (s, 3H, N–CH3), 2.92 (t, J = 7.2 Hz, 2H, H-1'), 2.55 (t, J = 6.8 Hz, 2H, H-2'), 2.11 (t, J = 6.8 Hz, 2H, H-5'), 1.44 (quint, J = 7.2 Hz, 2H, H-6'), 1.33–1.21 (m, 8H, H-7'-10'), 0.86 (t, J = 6.8 Hz, 3H, H-11'). 13C-NMR δ: 177.4 (C-4), 153.1 (C-2), 141.8 (C-8a), 132.2 (C-7), 126.6 (C-5), 126.4 (C-4a), 123.5 (C-6), 115.4 (C-8), 111.1 (C-3), 82.8 (C-4'), 76.9 (C-3'), 34.4 (N–CH3), 34.0 (C-1'), 31.7 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.7 (C-6'), 22.6 (C-10'), 18.6 (C-2'), 18.5 (C-5'), 14.1 (C-11'). ESI-MS m/z (rel. int.): [M+H]+ 310 (100).
1-Ethyl-2-(3'-undecynyl)-4(1H)-quinolone (8e) was prepared from 3b (1.0 g, 5.2 mmol) in THF (15 mL), LDA (2.9 mL, 5.2 mmol) and N-ethylisatoic anhydride (7b) (0.74 g, 3.9 mmol) in THF (10 mL) as a yellow semi solid (62%). IR (KBr, cm−1): 3434, 2930, 2851, 1621, 1600, 1490, 1468, 1431, 1309, 759. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.65 (t, J = 8.0 Hz, 1H, H-7), 7.51 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.2 Hz, 1H, H-6), 6.28 (s, 1H, H-3), 4.27 (q, J = 7.6 Hz, 2H, N–CH2–CH3), 2.91 (t, J = 7.2 Hz, 2H, H-1'), 2.59 (t, J = 6.8 Hz, 2H, H-2'), 2.12 (t, J = 6.8 Hz, 2H, H-5'), 1.44 (t, J = 7.2 Hz, 2H, N–CH2–CH3), 1.42 (quint, J = 6.8 Hz, 2H, H-6'), 1.31–1.21 (m, 8H, H-7'-10'), 0.85 (t, J = 6.8 Hz, 3H, H-11'). 13C-NMR δ: 177.1 (C-4), 151.9 (C-2), 141.1 (C-8a), 132.1 (C-7), 126.9 (C-5), 126.7 (C-4a), 123.2 (C-6), 115.4 (C-8), 110.8 (C-3), 82.4 (C-4'), 77.4 (C-3'), 41.3 (N–CH2–CH3), 33.0 (C-1'), 31.6 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.6 (C-6'), 22.6 (C-10'), 18.8 (C-2'), 18.7 (C-5'), 14.2 (N–CH2–CH3), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-(n-Propyl)-2-(3'-undecynyl)-4(1H)-quinolone (8f) was prepared from 3b (1.0 g, 5.2 mmol) in THF (15 mL), LDA (2.9 mL, 5.2 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.79 g, 3.9 mmol) in THF (10 mL) as a yellow oil (59%). IR (KBr, cm−1): 3426, 2929, 2856, 1627, 1600, 1488, 1468, 1427, 1177, 759. 1H-NMR δ: 8.46 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.6 Hz, 1H, H-7), 7.48 (d, J = 8.0 Hz, 1H, H-8), 7.37 (t, J = 7.6 Hz, 1H, H-6), 6.36 (s, 1H, H-3), 4.13 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.93 (t, J = 7.6 Hz, 2H, H-1'), 2.61 (t, J = 7.2 Hz, 2H, H-2'), 2.13 (t, J = 7.2 Hz, 2H, H-5'), 1.85 (sext, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 1.45 (quint, J = 7.2 Hz, 2H, H-6'), 1.33–1.21 (m, 8H, H-7'-10'), 1.08 (t, J = 7.2 Hz, 2H, N–CH2–CH2–CH3), 0.86 (t, J = 6.8 Hz, 3H, H-11'). 13C-NMR δ: 177.3 (C-4), 152.7 (C-2), 140.5 (C-8a), 132.3 (C-7), 126.7 (C-5), 126.5 (C-4a), 123.2 (C-6), 115.6 (C-8), 111.0 (C-3), 82.5 (C-4'), 77.0 (C-3'), 47.7 (N–CH2–CH2–CH3), 32.9 (C-1'), 31.5 (C-9'), 29.0 (C-8'), 28.7 (C-7'), 28.4 (C-6'), 22.6 (C-10'), 22.3 (N–CH2–CH2–CH3), 18.7 (C-2'), 18.6 (C-5'), 14.0 (C-11'), 11.1 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 338 (100).
1-Ethyl-2-(1'-decynyl)-4(1H)-quinolone (8g) was prepared from 6a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-ethylisatoic anhydride (7b) (0.80 g, 4.2 mmol) in THF (10 mL) as a light yellow semi solid (54%). IR (KBr, cm−1): 3372, 2928, 2855, 2234, 1625, 1598, 1488, 1421, 758. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.69 (t, J = 7.6 Hz, 1H, H-7), 7.50 (d, J = 8.0, Hz, 1H, H-8), 7.38 (t, J = 7.6 Hz, 1H, H-6), 6.58 (s, 1H, H-3), 4.53 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.53 (t, J = 7.2 Hz, 2H, H-3'), 1.67 (quint, J = 7.2 Hz, 2H, H-4'), 1.49 (t, J = 7.2 Hz, 2H, N–CH2–CH3), 1.33–1.22 (m, 10H, H-5'-9'), 0.89 (t, J = 7.2 Hz, 3H, H-10'). 13C-NMR δ: 177.1 (C-4), 141.3 (C-8a), 137.5 (C-2), 132.5 (C-7), 126.8 (C-4a), 126.6 (C-5), 123.5 (C-6), 115.5 (C-8), 115.1 (C-3), 111.3 (C-2'), 74.5 (C-1'), 42.4 (N–CH2–CH3), 31.8 (C-8'), 29.2 (C-7'), 29.1 (C-6'), 29.0 (C-5'), 28.1 (C-4'), 22.6 (C-9'), 19.3 (C-3'), 14.2 (N–CH2–CH3), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 310 (100).
1-(n-Propyl)-2-(1'-decynyl)-4(1H)-quinolone (8h) was prepared from 6a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.85 g, 4.2 mmol) in THF (10 mL) as a light yellow semi solid (51%). IR (KBr, cm−1): 3430, 2927, 2855, 2235, 1624, 1598, 1488, 1420, 1177, 758. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.2 Hz, 1H, H-7), 7.44 (d, J = 8.0 Hz, 1H, H-8), 7.36 (t, J = 7.2 Hz, 1H, H-6), 6.56 (s, 1H, H-3), 4.40 (t, J = 7.6 Hz, 2H, N–CH2–CH2–CH3), 2.51 (t, J = 6.8 Hz, 2H, H-3'), 1.86 (m, 2H, N–CH2–CH2–CH3), 1.65 (quint, J = 6.8 Hz, 2H, H-4'), 1.33–1.21 (m, 10H, H-5'-9'), 1.03 (t, J = 7.2 Hz, 3H, N–CH2–CH2–CH3), 0.89 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 177.1 (C-4), 141.0 (C-8a), 138.2 (C-2),132.5 (C-7), 126.7 (C-4a), 126.5 (C-5), 123.7 (C-6), 115.4 (C-8), 115.2 (C-3), 111.0 (C-2'), 75.1 (C-1'), 47.9 (N–CH2–CH2–CH3), 31.7 (C-8'), 29.1 (C-7'), 29.0 (C-6'), 29.0 (C-5'), 28.1 (C-4'), 22.6 (C-9'), 22.3 (N–CH2–CH2–CH3), 19.8 (C-3'), 14.0 (C-10'), 11.0 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-(n-Butyl)-2-(1'-decynyl)-4(1H)-quinolone (8i) was prepared from 6a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-(n-butyl)isatoic anhydride (7d) (0.91 g, 4.2 mmol) in THF (10 mL) as a light yellow oil (54%). IR (KBr, cm−1): 3428, 2928, 2856, 2233, 1622, 1597, 1490, 1466, 1422, 759. 1H-NMR δ: 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.6 Hz, 1H, H-7), 7.45 (d, J = 8.0, Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.58 (s, 1H, H-3), 4.38 (t, J = 7.6 Hz, 2H, N–CH2–CH2–CH2–CH3), 2.53 (t, J = 6.8 Hz, 2H, H-3'), 1.78 (quint, J = 7.2 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.65 (quint, J = 6.8 Hz, 2H, H-4'), 1.48 (m, 2H, N–CH2–CH2–CH2–CH3), 1.31–1.24 (m, 10H, H-5'-9'), 1.04 (t, J = 7.6 Hz, 3H, N–CH2–CH2–CH2–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-10’). 13C-NMR δ: 177.1 (C-4), 141.3 (C-8a), 137.3 (C-2),132.5 (C-7), 126.7 (C-4a), 126.5 (C-5), 123.7 (C-6), 115.7 (C-8), 115.0 (C-3), 111.0 (C-2'), 74.4 (C-1'), 48.4 (N–CH2–CH2–CH2–CH3), 31.8 (C-8'), 31.4 (N–CH2–CH2–CH2–CH3), 29.1 (C-7'), 29.0 (C-6'), 29.0 (C-5'), 28.1 (C-4'), 22.6 (C-9'), 20.9 (N–CH2–CH2–CH2–CH3), 19.3 (C-3'), 14.3 (N–CH2–CH2–CH2–CH3), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 338 (100).
1-Methyl-2-(3'-dodecynyl)-4(1H)-quinolone (8j) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-methylisatoic anhydride (7a) (0.64 g, 3.6 mmol) in THF (10 mL) as a light yellow semi solid (60%). IR (KBr, cm−1): 3420, 2927, 2855, 1628, 1600, 1499, 1469, 759. 1H-NMR δ: 8.38 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.61 (t, J = 8.4 Hz, 1H, H-7), 7.45 (d, J = 8.4 Hz, 1H, H-8), 7.32 (t, J = 7.6 Hz, 1H, H-6), 6.19 (s, 1H, H-3), 3.71 (s, 3H, N–CH3), 2.87 (t, J = 7.6 Hz, 2H, H-1'), 2.52 (t, J = 7.6 Hz, 2H, H-2'), 2.07 (t, J = 7.6 Hz, 2H, H-5'), 1.43 (quint, J = 7.2 Hz, 2H, H-6'), 1.31–1.20 (m, 10H, H-7'-11'), 0.84 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.6 (C-4), 152.9 (C-2), 141.8 (C-8a), 132.1 (C-7), 126.5 (C-5), 126.3 (C-4a), 123.3 (C-6), 115.4 (C-8), 111.1 (C-3), 82.6 (C-4'), 77.0 (C-3'), 34.3 (N–CH3), 33.9 (C-1'), 31.8 (C-10'), 29.1 (C-9'), 29.1 (C-8'), 29.0 (C-7'), 28.8 (C-6'), 22.6 (C-11'), 18.6 (C-2'), 18.4 (C-5'), 14.0 (C-12'). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-Ethyl-2-(3'-dodecynyl)-4(1H)-quinolone (8k) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-ethylisatoic anhydride (7b) (0.69 g, 3.6 mmol) in THF (10 mL) as a white solid (55%). m.p. 66–68 °C. IR (KBr, cm−1): 3440, 2919, 2850, 1621, 1600, 1490, 1468, 1308, 769. 1H-NMR δ: 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.64 (t, J = 8.0 Hz, 1H, H-7), 7.50 (d, J = 8.4 Hz, 1H, H-8), 7.34 (t, J = 7.2 Hz, 1H, H-6), 6.28 (s, 1H, H-3), 4.26 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.90 (t, J = 7.6 Hz, 2H, H-1'), 2.57 (t, J = 7.2 Hz, 2H, H-2'), 2.10 (t, J = 6.8 Hz, 2H, H-5'), 1.43 (t, J = 7.6 Hz, N–CH2–CH3), 1.41 (quint, J = 7.2 Hz, 2H, H-6'), 1.31–1.20 (m, 10H, H-7'-11'), 0.85 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.5 (C-4), 152.8 (C-2), 140.5 (C-8a), 132.1 (C-7), 126.9 (C-5), 126.7 (C-4a), 123.2 (C-6), 115.4 (C-8), 110.9 (C-3), 82.7 (C-4'), 77.0 (C-3'), 41.2 (N–CH2–CH3), 33.0 (C-1'), 31.7 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.8 (C-6'), 22.5 (C-11'), 18.8 (C-2'), 18.6 (C-5'), 14.1 (N–CH2–CH3), 14.0 (C-12'). ESI-MS m/z (rel. int.): [M+H]+ 338 (100).
1-(n-Propyl)-2-(3'-dodecynyl)-4(1H)-quinolone (8l) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.74 g, 3.6 mmol) in THF (10 mL) as a light yellow semi solid (51%). IR (KBr, cm−1): 3426, 2928, 2855, 1628, 1600, 1488, 1467, 1427, 759. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 8.0 Hz, 1H, H-7), 7.47 (d, J = 8.0 Hz, 1H, H-8), 7.36 (t, J = 7.6 Hz, 1H, H-6), 6.28 (s, 1H, H-3), 4.16 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.90 (t, J = 7.6 Hz, 2H, H-1'), 2.59 (t, J = 7.2 Hz, 2H, H-2'), 2.13 (t, J = 7.6 Hz, 2H, H-5'), 1.85 (sext, J = 7.2 Hz, 2H, N–CH2–CH2–CH3), 1.43 (quint, J = 7.6 Hz, 2H, H-6'), 1.33–1.21 (m, 10H, H-7'-11'), 1.07 (t, J = 7.2 Hz, 3H, N–CH2–CH2–CH3), 0.86 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 175.8 (C-4), 152.5 (C-2), 140.9 (C-8a), 132.2 (C-7), 126.7 (C-5), 126.5 (C-4a), 123.1 (C-6), 115.4 (C-8), 110.8 (C-3), 82.6 (C-4'), 77.1 (C-3'), 47.8 (N–CH2–CH2–CH3), 33.1 (C-1'), 31.6 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 29.0 (C-7'), 28.6 (C-6'), 22.6 (C-11'), 22.0 (N–CH2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.0 (C-14’), 10.9 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 352 (100).
1-(n-Butyl)-2-(3'-dodecynyl)-4(1H)-quinolone (8m) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-(n-butyl)isatoic anhydride (7d) (0.79 g, 3.6 mmol) in THF (10 mL) as a yellow oil (58%). IR (KBr, cm−1): 3425, 2927, 2855, 1628, 1600, 1488, 1467, 1427, 759. 1H-NMR δ: 8.44 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.65 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.48 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.27 (s, 1H, H-3), 4.13 (t, J =8.0 Hz, 2H, N–CH2–(CH2)2–CH3), 2.91 (t, J = 7.2 Hz, 2H, H-1'), 2.58 (t, J = 7.2 Hz, 2H, H-2'), 2.11 (m, 2H, H-5'), 1.80 (quint, J = 7.2 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.46 (m, 2H, N-(CH2)2–CH2–CH3), 1.41 (quint, J = 7.2 Hz, 2H, H-6'), 1.32–1.23 (m, 10H, H-7'-11'), 1.03 (t, J = 7.2 Hz, 3H, N-(CH2)3–CH3), 0.85 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.4 (C-4), 152.8 (C-2), 140.7 (C-8a), 132.1 (C-7), 126.8 (C-5), 126.6 (C-4a), 123.3 (C-6), 115.6 (C-8), 110.8 (C-3), 82.7 (C-4'), 77.1 (C-3'), 47.6 (N–CH2–(CH2)2–CH3), 33.2 (C-1'), 31.8 (C-10'), 30.8 (N–CH2–CH2–CH2–CH3), 29.1 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.8 (C-6'), 22.6 (C-11'), 20.1 (N–(CH2)2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.1 (C-14’), 13.8 (N–(CH2)3–CH3). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-Methyl-2-(1'-dodecynyl)-4(1H)-quinolone (8n) was prepared from 6b (1.5 g, 7.2 mmol) in THF (25 mL), LDA (4.0 mL, 7.2 mmol) and N-methylisatoic anhydride (7a) (0.96 g, 5.4 mmol) in THF (15 mL) as a light yellow semi-solid (51%). IR (KBr, cm−1): 3421, 2925, 2854, 2234, 1625, 1599, 1496, 1469, 757. 1H-NMR δ: 8.41 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.6 Hz, 1H, H-7), 7.44 (d, J = 8.0, Hz, 1H, H-8), 7.36 (t, J = 7.6 Hz, 1H, H-6), 6.55 (s, 1H, H-3), 3.94 (s, 3H, N–CH3), 3.17 (t, J = 7.2 Hz, 2H, H-1'), 2.51 (t, J = 6.8 Hz, 2H, H-3'), 1.64 (quint, J = 6.8 Hz, 2H, H-4'), 1.46 (m, 2H, H-5'), 1.34–1.23 (m, 12H, H-6'-11'), 0.87 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.0 (C-4), 141.1 (C-8a), 137.4 (C-2), 132.4 (C-7), 126.8 (C-4a), 126.7 (C-5), 123.6 (C-6), 115.6 (C-8), 115.0 (C-3), 102.2 (C-2'), 74.6 (C-1'), 36.7 (N–CH3), 31.8 (C-10'), 29.5 (C-9'), 29.4 (C-8'), 29.2 (C-7'), 29.0 (C6'), 29.0 (C5'), 28.0 (C-4'), 22.6 (C-11'), 19.6 (C-3'), 14.0 (C-12'). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-Methyl-2-(3'-tetradecynyl)-4(1H)-quinolone (8o) was prepared from 3d (1.0 g, 4.2 mmol) in THF (15 mL), LDA (2.4 mL, 4.2 mmol) and N-methylisatoic anhydride (7a) (0.57 g, 3.2 mmol) in THF (10 mL) as a light yellow solid (60%). m.p. 43–45 °C. IR (KBr, cm−1): 3422, 2922, 2854, 1632, 1598, 1469, 1444, 760. 1H-NMR δ: 8.39 (d, J = 8.0 Hz, 1H, H-5), 7.62 (t, J = 8.0 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.33 (t, J = 7.2 Hz, 1H, H-6), 6.21 (s, 1H, H-3), 3.72 (s, 3H, N–CH3), 2.88 (t, J = 7.2 Hz, 2H, H-1'), 2.50 (t, J = 7.6 Hz, 2H, H-2’), 2.10 (t, J = 7.2 Hz, 2H, H-5'), 1.41 (quint, J = 6.8 Hz, 2H, H-6'), 1.34–1.21 (m, 14H, H-7'-13'), 0.85 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 177.6 (C-4), 152.9 (C-2), 141.8 (C-8a), 132.1 (C-7), 126.5 (C-5), 126.3 (C-4a), 123.3 (C-6), 115.4 (C-8), 111.1 (C-3), 82.7 (C-4'), 77.1 (C-3'), 34.3 (N–CH3), 33.9 (C-1'), 31.8 (C-12'), 29.2 (C-11'), 29.1 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 29.0 (C-7'), 28.8 (C-6'), 22.6 (C-13'), 18.6 (C-2'), 18.4 (C-5'), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 352 (100).
1-Ethyl-2-(3'-tetradecynyl)-4(1H)-quinolone (8p) was prepared from 3d (1.0 g, 4.2 mmol) in THF (15 mL), LDA (2.4 mL, 4.2 mmol) and N-ethylisatoic anhydride (7b) (0.61 g, 3.2 mmol) in THF (10 mL) as white needles (56%). m.p. 74–76 °C. IR (KBr, cm−1): 3424, 2917, 2850, 1621, 1600, 1468, 1430, 1308, 760. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.63 (t, J = 8.0 Hz, 1H, H-7), 7.50 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.2 Hz, 1H, H-6), 6.29 (s, 1H, H-3), 4.24 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.91 (t, J = 7.2 Hz, 2H, H-1'), 2.58 (t, J = 7.2 Hz, 2H, H-2'), 2.09 (t, J = 6.4 Hz, 2H, H-5'), 1.42 (t, J = 7.2 Hz, N–CH2–CH3), 1.40 (quint, J = 7.2 Hz, 2H, H-6'), 1.31–1.21 (m, 14H, H-7'-13'), 0.85 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 177.5 (C-4), 152.7 (C-2), 140.8 (C-8a), 132.1 (C-7), 126.6 (C-5), 126.4 (C-4a), 123.3 (C-6), 115.4 (C-8), 111.1 (C-3), 82.7 (C-4'), 77.0 (C-3'), 41.3 (N–CH2–CH3), 33.1 (C-1'), 31.8 (C-12'), 29.2 (C-11'), 29.2 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 29.0 (C-7'), 28.8 (C-6'), 22.6 (C-13'), 18.8 (C-2'), 18.7 (C-5'), 14.2 (N–CH2–CH3), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-(n-Propyl)-2-(3'-tetradecynyl)-4(1H)-quinolone (8q) was prepared from 3d (1.0 g, 4.2 mmol) in THF (15 mL), LDA (2.4 mL, 4.2 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.66 g, 3.2 mmol) in THF (10 mL) as a light yellow semi-solid (55%). IR (KBr, cm−1): 3430, 2926, 2854, 1629, 1600, 1488, 1467, 1227, 759. 1H-NMR δ: 8.48 (d, J = 8.0 Hz, 1H, H-5), 7.67 (t, J = 7.6 Hz, 1H, H-7), 7.49 (d, J = 8.4 Hz, 1H, H-8), 7.38 (t, J = 7.6 Hz, 1H, H-6), 6.43 (s, 1H, H-3), 4.17 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.94 (t, J = 7.6 Hz, 2H, H-1'), 2.59 (t, J = 7.2 Hz, 2H, H-2'), 2.11 (t, J = 7.2 Hz, 2H, H-5'), 1.86 (sext, J = 7.6 Hz, 2H, N–CH2–CH2–CH3), 1.43 (quint, J = 7.2 Hz, 2H, H-6'), 1.33–1.22 (m, 14H, H-7'-13'), 1.09 (t, J = 7.2 Hz, 2H, N–CH2–CH2–CH3), 0.88 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 175.4 (C-4), 152.5 (C-2), 141.0 (C-8a), 132.1 (C-7), 126.7 (C-5), 126.5 (C-4a), 123.3 (C-6), 115.3 (C-8), 111.0 (C-3), 82.6 (C-4'), 77.0 (C-3’), 47.6 (N–CH2–CH2–CH3), 33.1 (C-1'), 31.5 (C-12'), 29.3 (C-11'), 29.2 (C-10'), 29.2 (C-9'), 29.1 (C-8'), 29.0 (C-7'), 28.4 (C-6'), 22.6 (C-13'), 22.1 (N–CH2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.0 (C-14'), 11.0 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 380 (100).
1-Methyl-2-(1′-tetradecynyl)-4(1H)-quinolone (8r) was prepared from 6c (1.5 g, 6.4 mmol) in THF (25 mL), LDA (3.5 mL, 6.4 mmol) and N-methylisatoic anhydride (7a) (0.85 g, 4.8 mmol) in THF (15 mL) as a white solid (57%). M.p. 41–43 °C. IR (KBr, cm−1): 3405, 2923, 2849, 2233, 1622, 1595, 1496, 1468, 777. 1H-NMR δ: 8.38 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.63 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.42 (d, J = 8.0 Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.51 (s, 1H, H-3), 3.92 (s, 3H, N–CH3), 2.49 (t, J = 7.2 Hz, 2H, H-3'), 1.63 (quint, J = 7.2 Hz, 2H, H-4'), 1.44 (m, 2H, H-5'), 1.34–1.21 (m, 16H, H-6'-13'), 0.85 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 177.1 (C-4), 141.1 (C-8a), 137.3 (C-2), 132.3 (C-7), 126.8 (C-4a), 126.6 (C-5), 123.6 (C-6), 115.6 (C-8), 115.0 (C-3), 102.0 (C-2'), 74.8 (C-1'), 36.7 (N–CH3), 31.8 (C-12'), 29.6 (C-11'), 29.6 (C-10'), 29.5 (C-9'), 29.4 (C-8'), 29.2 (C-7'), 29.0 (C6'), 28.9 (C5'), 27.9 (C-4'), 22.6 (C-13'), 19.6 (C-3'), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 352 (100).
1-Methyl-2-(3′-hexadecynyl)-4(1H)-quinolone (8s) was prepared from 3e (1.5 g, 5.7 mmol) in THF (25 mL), LDA (3.2 mL, 5.7 mmol) and N-methylisatoic anhydride (7a) (0.76 g, 4.3 mmol) in THF (15 mL) as a light yellow solid (59%). M.p. 58–61 °C. IR (KBr, cm−1): 3432, 2922, 2853, 1631, 1597, 1469, 1444, 761. 1H-NMR δ: 8.46 (d, J = 8.0 Hz, 1H, H-5), 7.73 (t, J = 7.6 Hz, 1H, H-7), 7.58 (d, J = 8.0 Hz, 1H, H-8), 7.44 (t, J = 7.6 Hz, 1H, H-6), 6.56 (s, 1H, H-3), 3.85 (s, 3H, N–CH3), 3.00 (t, J = 7.6 Hz, 2H, H-1'), 2.60 (t, J = 7.6 Hz, 2H, H-2’), 2.12 (t, J = 7.2 Hz, 2H, H-5'), 1.41 (m, 2H, H-6'), 1.32–1.19 (m, 18H, H-7'-15'), 0.88 (t, J = 6.4 Hz, 3H, H-16'). 13C-NMR δ: 175.6 (C-4), 155.3 (C-2), 141.7 (C-8a), 133.3 (C-7), 126.4 (C-5), 126.2 (C-4a), 124.4 (C-6), 115.9 (C-8), 110.5 (C-3), 83.4 (C-4'), 76.5 (C-3'), 35.6 (N–CH3), 34.1 (C-1'), 31.9 (C-14'), 29.7 (C-13'), 29.6 (C-12'), 29.6 (C-11'), 29.5 (C-10'), 29.3 (C-9'), 29.1 (C-8'), 28.9 (C-7'), 28.9 (C-6'), 22.7 (C-15'), 18.8 (C-2'), 18.6 (C-5'), 14.1 (C-16'). ESI-MS m/z (rel. int.): [M+H]+ 380 (100).
1-Methyl-2-(1'-pentadecynyl)-4(1H)-quinolone (8t) was prepared from 6d (1.5 g, 6.0 mmol) in THF (25 mL), LDA (3.3 mL, 6.0 mmol) and N-methylisatoic anhydride (7a) (0.80 g, 4.5 mmol) in THF (15 mL) as white needles (48%). M.p. 67–69 °C. IR (KBr, cm−1): 3401, 2915, 2851, 2239, 1618, 1596, 1472, 748. 1H-NMR δ: 8.42 (d, J = 8.4, Hz, 1H, H-5), 7.69 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.48 (d, J = 8.0 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.55 (s, 1H, H-3), 3.96 (s, 3H, N–CH3), 2.52 (t, J = 7.2 Hz, 2H, H-3'), 1.66 (quint, J = 7.6 Hz, 2H, H-4'), 1.46 (quint, J = 7.2 Hz, 2H, H-5'), 1.35–1.21 (m, 18H, H-6'-14'), 0.87 (t, J = 6.8 Hz, 3H, H-15'). 13C-NMR δ: 177.2 (C-4), 141.0 (C-8a), 136.6 (C-2), 132.2 (C-7), 126.7 (C-4a), 126.6 (C-5), 123.7 (C-6), 115.7 (C-8), 115.1 (C-3), 102.1 (C-2'), 74.9 (C-1'), 36.5 (N–CH3), 31.8 (C-13'), 29.6 (C-12'), 29.5 (C-11'), 29.5 (C-10'), 29.3 (C-9'), 29.3 (C-8'), 29.1 (C-7'), 29.1 (C6'), 28.9 (C5'), 28.0 (C-4'), 22.6 (C-14'), 19.5 (C-3'), 14.1 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-Cyclopropyl-2-[(E)-12-bromodec-1'-enyl]-4(1H)-quinolone (18a) was prepared from 17a (1.0 g, 3.5 mmol) in THF (15 mL), LDA (1.9 mL, 3.5 mmol) and N-cyclopropylisatoic anhydride (12) (0.52 g, 2.6 mmol) in THF (10 mL) as a light yellow solid (49%). M.p. 85–87 °C. IR (KBr, cm−1): 3425, 2926, 2850, 1648, 1616, 1594, 1475, 1416, 1310, 1137, 1034, 966, 888, 760. 1H-NMR δ: 8.39 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.90 (d, J = 8.4 Hz, 1H, H-8), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.67 (d, J = 16.0 Hz, 1H, H-1'), 6.55 (s, 1H, H-3), 6.45 (dt, J = 16.0, 6.8 Hz, 1H, H-2'), 3.41 (t, J = 6.8 Hz, 2H, H-12'), 3.28 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.30 (q, J = 6.8 Hz, 2H, H-3'), 1.83 (quint, J = 6.8 Hz, 2H, H-11'), 1.51 (quint, J = 6.8 Hz, 2H, H-4'), 1.41–1.23 (m, 12H, H-5'-10'), 0.90 (m, 4H, N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 153.0 (C-2), 142.2 (C-2'), 139.6 (C-8a), 131.5 (C-7), 126.4 (C-4a), 126.2 (C-5), 124.9 (C-1'), 123.4 (C-6), 117.4 (C-8), 108.2 (C-3), 34.1 (C-12'), 33.1 (C-3'), 32.7 (C-11'), 29.9 (C-8'), 29.4 (C-7'), 29.4 (C-9'), 29.3 (C-6'), 29.1 (C-5'), 28.6 (C-4'), 28.1 (C-10'), 24.7 (N–CH–(CH2)2), 2×12.4 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H+2]+ 432 (100), [M+H]+ 430 (94).
1-Cyclopropyl-2-[(E)-13-bromotridec-1'-enyl)-4(1H)-quinolone (18b) was prepared from 17b (1.0 g, 3.3 mmol) in THF (15 mL), LDA (1.8 mL, 3.3 mmol) and N-cyclopropylisatoic anhydride (12) (0.5 g, 2.5 mmol) in THF (10 mL) as a light yellow solid (55%). M.p. 61–63 °C. IR (KBr, cm−1): 3422, 3020, 2918, 2851, 1653, 1630, 1596, 1571, 1479, 1463, 1413, 1135, 1034, 973, 760. 1H-NMR δ: 8.38 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.89 (d, J = 8.4 Hz, 1H, H-8), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.66 (d, J = 16.0 Hz, 1H, H-1'), 6.50 (s, 1H, H-3), 6.44 (dt, J = 16.0, 6.8 Hz, 1H, H-2'), 3.41 (t, J = 6.8 Hz, 2H, H-13'), 3.27 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.30 (q, J = 6.8 Hz, 2H, H-3'), 1.85 (quint, J = 6.8 Hz, 2H, H-12'), 1.51 (quint, J = 6.8 Hz, 2H, H-4'), 1.43–1.23 (m, 14H, H-5'-11'), 0.90 (m, 4H, N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 152.9 (C-2), 142.1 (C-2'), 139.5 (C-8a), 131.4 (C-7), 126.4 (C-4a), 126.2 (C-5), 124.9 (C-1'), 123.3 (C-6), 117.4 (C-8), 108.2 (C-3), 34.1 (C-13'), 33.1 (C-3'), 32.7 (C-12'), 29.5 (C-9'), 29.5 (C-8'), 29.3 (C-10'), 29.3 (C-7'), 29.2 (C-6'), 28.7 (C-5'), 28.6 (C-4'), 28.1 (C-11'), 24.9 (N–CH–(CH2)2), 2 × 12.4 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H+2]+ 446 (100), [M+H]+ 444 (94).
1-Methyl-2-[(E)-12-bromodec-1'-enyl]-4(1H)-quinolone (19a) was prepared from 17a (1.5 g, 5.2 mmol) in THF (25 mL), LDA (2.9 mL, 5.2 mmol) and N-methylisatoic anhydride (7a) (0.69 g, 3.9 mmol) in THF (10 mL) as a yellow semi solid (63%). IR (KBr, cm−1): 3422, 2925, 2852, 1620, 1597, 1467, 1438, 761. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.67 (t, J = 7.2 Hz, 1H, H-7), 7.49 (t, J = 8.4 Hz, 1H, H-8), 7.38 (t, J = 7.2 Hz, 1H, H-6), 6.46 (s, 1H, H-3), 6.43 (d, J = 16.0 Hz, 1H, H-1'), 6.37 (dt, J = 16.0, 6.4 Hz, 1H, H-2'), 3.76 (s, 3H, N–CH3), 3.41 (t, J = 6.4 Hz, 2H, H-12'), 2.28 (q, J = 6.8 Hz, 2H, H-3'), 1.85 (quint, J = 6.8 Hz, 2H, H-11'), 1.50 (quint, J = 6.8 Hz, 2H, H-4'), 1.41 (quint, J = 6.8 Hz, 2H, H-10'), 1.37–1.23 (m, 10H, H-5'-9'). 13C-NMR δ: 177.9 (C-4), 152.5 (C-2), 142.0 (C-2'), 141.4 (C-8a), 132.2 (C-7), 126.6 (C-4a), 126.5 (C-5), 123.8 (C-1'), 123.5 (C-6), 115.5 (C-8), 109.4 (C-3), 35.5 (N–CH3), 34.1 (C-12'), 33.1 (C-3'), 32.7 (C-11'), 29.4 (C-8'), 29.3 (C-9'), 29.3 (C-7'), 29.1 (C-6'), 28.7 (C-5'), 28.5 (C-4'), 28.1 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 404 (100), [M+H+2]+ 406 (92).
1-Methyl-2-[(E)-13-bromotridec-1'-enyl)-4(1H)-quinolone (19b) was prepared from 17b (1.0 g, 3.3 mmol) in THF (15 mL), LDA (1.8 mL, 3.3 mmol) and N-methylisatoic anhydride (7a) (0.44 g, 2.5 mmol) in THF (10 mL) as a yellow semi solid (47%). IR (KBr, cm−1): 3420, 2925, 2852, 1622, 1597, 1496, 1468, 760. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.70 (t, J = 7.6 Hz, 1H, H-7), 7.52 (t, J = 8.0 Hz, 1H, H-8), 7.40 (t, J = 7.6 Hz, 1H, H-6), 6.48 (s, 1H, H-3), 6.44 (d, J = 16.0 Hz, 1H, H-1'), 6.37 (dt, J = 16.0, 6.4 Hz, 1H, H-2'), 3.79 (s, 3H, N–CH3), 3.41 (t, J = 6.8 Hz, 2H, H-13'), 2.29 (q, J = 7.2 Hz, 2H, H-3'), 1.86 (quint, J = 6.8 Hz, 2H, H-12'), 1.52 (quint, J = 6.8 Hz, 2H, H-4'), 1.43 (quint, J = 6.8 Hz, 2H, H-11'), 1.37–1.22 (m, 12H, H-5'-10'). 13C-NMR δ: 177.4 (C-4), 152.3 (C-2), 142.4 (C-2'), 141.4 (C-8a), 132.4 (C-7), 126.7 (C-4a), 126.6 (C-5), 123.9 (C-1'), 123.6 (C-6), 115.5 (C-8), 109.4 (C-3), 35.6 (N–CH3), 34.1 (C-13'), 33.2 (C-3'), 32.8 (C-12'), 29.5 (C-9'), 29.5 (C-8'), 29.3 (C-10'), 29.3 (C-7'), 29.1 (C-6'), 28.7 (C-5'), 28.6 (C-4'), 28.1 (C-11'). ESI-MS m/z (rel. int.): [M+H]+ 418 (100), [M+H+2]+ 420 (94).
1-Cyclopropyl-2-[(E)-1′-tridecenyl]-4(1H)-quinolone (22a) was prepared from 21a (1.5 g, 6.7 mmol) in THF (25 mL), LDA (3.7 mL, 6.7 mmol) and N-cyclopropylisatoic anhydride (12) (1.0 g, 5.0 mmol) in THF (20 mL) as white needles (56%). M.p. 86–88 °C. IR (KBr, cm−1): 3425, 2922, 2851, 1617, 1595, 1571, 1479, 1465, 1420, 1310, 1136, 1031, 966, 759. 1H-NMR δ: 8.37 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.88 (d, J = 8.4 Hz, 1H, H-8), 7.62 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.33 (t, J = 8.0 Hz, 1H, H-6), 6.65 (d, J = 16.0 Hz, 1H, H-1'), 6.48 (s, 1H, H-3), 6.42 (dt, J = 16.0, 6.8 Hz, 1H, H-2′), 3.26 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.29 (q, J = 6.8 Hz, 2H, H-3′), 1.52 (quint, J = 6.8 Hz, 2H, H-4'), 1.36–1.24 (m, 16H, H-5'-12'), 0.86–0.90 (m, 7H, H-13', N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 152.8 (C-2), 142.1 (C-8a), 139.3 (C-2'), 131.4 (C-7), 126.5 (C-4a), 126.1 (C-5), 124.9 (C-1'), 123.3 (C-6), 117.4 (C-8), 108.2 (C-3), 33.1 (C-3'), 31.8 (C-11'), 29.8 (N–CH–(CH2)2), 29.6 (C-10'), 29.5 (C-9), 29.5 (C-8'), 29.4 (C-7'), 29.3 (C6'), 29.2 (C5'), 28.6 (C-4'), 22.6 (C-12'), 14.0 (C-13'), 12.3 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-Cyclopropyl-2-[(E)-1′-tetradecenyl]-4(1H)-quinolone (22b) was prepared from 21b (1.5 g, 6.3 mmol) in THF (25 mL), LDA (3.5 mL, 6.3 mmol) and N-cyclopropylisatoic anhydride (12) (0.96 g, 4.7 mmol) in THF (20 mL) as white crystals (50%). M.p. 96–98 °C. IR (KBr, cm−1): 3422, 2919, 2848, 1620, 1598, 1572, 1482, 1465, 1419, 1306, 1132, 1037, 966, 750. 1H-NMR δ: 8.36 (d, J = 8.0 Hz, 1H, H-5), 7.87 (d, J = 8.4 Hz, 1H, H-8), 7.62 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.32 (t, J = 8.0 Hz, 1H, H-6), 6.65 (d, J = 16.0 Hz, 1H, H-1'), 6.46 (s, 1H, H-3), 6.42 (dt, J = 16.0, 6.8 Hz, 1H, H-2'), 3.25 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.28 (q, J = 6.8 Hz, 2H, H-3'), 1.51 (quint, J = 6.8 Hz, 2H, H-4'), 1.37–1.21 (m, 18H, H-5'-13'), 0.86–0.91 (m, 7H, H-14', N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 152.8 (C-2), 142.1 (C-8a), 139.3 (C-2'), 131.3 (C-7), 126.5 (C-4a), 126.1 (C-5), 124.9 (C-1'), 123.2 (C-6), 117.4 (C-8), 108.3 (C-3), 33.1 (C-3'), 31.8 (C-12'), 29.8 (N–CH–(CH2)2), 29.6 (C-11'), 29.6 (C-10'), 29.6 (C-9), 29.5 (C-8'), 29.4 (C-7'), 29.3 (C6'), 29.2 (C5'), 28.7 (C-4'), 22.6 (C-13'), 14.1 (C-14'), 12.3 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H]+ 380 (100).
1-Cyclopropyl-2-(3′-undecynyl)-4(1H)-quinolone (23) was prepared from 3b (1.5 g, 7.7 mmol) in THF (25 mL), LDA (4.3 mL, 7.7 mmol) and N-cyclopropylisatoic anhydride (12) (1.17 g, 5.8 mmol) in THF (25 mL) as a yellow oil (48%). IR (KBr, cm−1): 3424, 2928, 2855, 1628, 1601, 1553, 1481, 1466, 1420, 1311, 1132, 1044, 759. 1H-NMR δ: 8.34 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.87 (d, J = 8.4 Hz, 1H, H-8), 7.60 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.31 (t, J = 6.8 Hz, 1H, H-6), 6.25 (s, 1H, H-3), 3.27 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 3.17 (t, J = 7.2 Hz, 2H, H-1'), 2.54 (m, 2H, H-2'), 2.07 (m, 2H, H-5'), 1.41 (quint, J = 6.8 Hz, 2H, H-6'), 1.26–1.15 (m, 6H, H-7'-9'), 0.93 (m, 4H, N–CH–(CH2)2), 0.85 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 178.1 (C-4), 155.6 (C-2), 142.8 (C-8a), 131.1 (C-7), 126.3 (C-4a), 126.1 (C-5), 123.1 (C-6), 117.6 (C-8), 111.0 (C-3), 82.9 (C-4'), 77.5 (C-3'), 33.0 (C-1'), 31.6 (C-8'), 29.1 (C-7'), 28.7 (C-6'), 28.7 (C-5'), 26.8 (N–CH–(CH2)2), 22.5 (C-9'), 18.6 (C-2'), 14.0 (C-10'), 12.3 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H]+ 336 (100).
3.3. Biological Evaluation
3.3.1. In Vitro Antibacterial Activities against Fast Growing Strains of Mycobacteria and EMRSA-15 and EMRSA-16
In vitro M. smegmatis, M. fortuitum, M. phlei, EMRSA-15 and -16 inhibitory effect of the 4(1H)-quinolone derivatives was assessed in 96-well plates using the broth dilution assay according to the previously reported protocols [7,23]. The plates containing test compounds, the antibiotic drugs and growth media were incubated at 37 °C for 72 h for the rapidly-growing mycobacterial strains and 18 h for EMRSA-15 and -16. A methanolic solution of MTT (0.05%) was used to determine the MIC by a colour change from yellow to blue. Tests were carried out in triplicate and all MIC values were determined in separate duplicate experiments. Tetracycline and norfloxacin were used as a positive control for the EMRSA strains and isoniazid and ethambutol for the mycobacterial strains.
3.3.2. Spot-Culture Growth Inhibition Assay (SPOTi) against M. bovis BCG
A diluted culture (~500 viable cells) of M. bovis BCG was spotted into 10% OADC supplemented Middlebrook 7H10 agar medium in a 24-well plate having various concentrations of the 4(1H)-quinolone derivatives and isoniazid. The plates were incubated at 37 °C for two weeks and the MIC values were determined visually as the minimum concentrations where no growth was observed [24].
3.3.3. M. tuberculosis MurE Inhibition Assay
The M. tuberculosis MurE ligase activity was determined by the phosphate colorimetric detection method as previously reported [7]. The compounds were tested at concentrations of 1000, 300, 100 and 30 µM and the amount of phosphate released was determined by means of a phosphate calibration curve using the Pi ColorLock kit reagents (Innova Biosciences, Cambridge, UK). Isoniazid was used as a negative control and IC50 values were determined by extrapolation from the plot of percent inhibition vs. concentration.
3.3.4. Cytotoxicity Assay
The cytotoxic activity of the synthetic compounds was determined by means of an XTT Cell Proliferation Kit II (Roche Diagnostics, Mannheim, Germany) using the human diploid embryonic lung cell line MRC-5 [11]. The quinolone derivatives were tested at concentrations of 100, 60, 30 and 10 µM in triplicate and the percentage of viable cells at each concentration was determined by comparing with the control.
4. Conclusions
Using the SAR information gained from our previous studies, a further group of quinolone derivatives possessing a diverse set of alkynyl/(E)-alkenyl substituents at C-2 of the quinolone nucleus were synthesized. Although the antimycobacterial potencies are comparable to those of the previously reported alkenyl analoges, the new series of alkynyl bearing compounds displayed higher selectivity towards mycobacteria and MRSA strains compared to MRC-5 cells. It is reasonable to conclude that the improved cytotoxicity of this group of analoges is due to the introduction of a triple bond in the aliphatic side chain of the quinolone nucleus. The selectivity shown by this class of new quinolones make them promising leads for synthesizing further compounds with improved antibacterial activity. Compounds with a terminal bromine atom at the side chain of N-alkyl-2-alkynyl/(E)-alkenyl-4-(1H)-quinolones with improved antimycobacterial activity will be explored due their increased probability of covalent bonding to particular target proteins.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The Austrian Science Fund (FWF) project no. P21152-B18 and P24324-B21 is gratefully acknowledged for financial support. We thank Andreas Leitner, Department of Pharmaceutical Chemistry, for measurement of IR spectra.
- Sample Availability: Samples of the compounds 8a–b, 8e–f, 8h, 8j–k, 18a–b, 23 are available from the authors.
References
- Comroe, J.H. Pay Dirt: The Story of Streptomycin. Part I: From Waksman to Waksman. Am. Rev. Respir. Dis. 1978, 117, 773–781. [Google Scholar]
- Guzman, J.D.; Gupta, A.; Bucar, F.; Gibbons, S.; Bhakta, S. Antimycobacterials from Natural Sources: Ancient times, Antibiotic era and Novel Scaffolds. Front. Biosci. 2012, 17, 1861–1881. [Google Scholar] [CrossRef]
- Ruiz, J. Mechanisms of Resistance to Quinolones: Target Alterations, Decreased Accumulation and DNA Gyrase Protection. J. Antimicrob. Chemother. 2003, 51, 1109–1117. [Google Scholar] [CrossRef]
- WHO. Available online: http://www.who.int/tb/challenges/en/ (accessed on 3 March 2012).
- Saraf, L.J.; Wilson, S.E. Telavancin, a New Lipoglycopeptide Antimicrobial, in Complicated Skin and Soft Tissue Infections. Infect. Drug Resist. 2011, 4, 87–95. [Google Scholar] [Green Version]
- Gordon, E.; Flouret, B.; Chantalat, L.; van Heijenoort, J.; Mengin-Lecreulx, D.; Diseberg, O. Crystal Structure of UDP-N-Acetylmuramoyl-L-Alanyl-D-Glutamate-Meso-Diaminopimelate Ligase from Escherichia coli. J. Biol. Chem. 2001, 276, 10999–11006. [Google Scholar]
- Guzman, J.D.; Wube, A.; Evangelopoulos, D.; Gupta, A.; Hüfner, A.; Basavannacharya, C.; Raman, M.M.; Thomaschitz, C.; Bauer, R.; McHugh, T.D.; et al. Interaction of N-Methyl-2-Alkenyl-4-Quinolones with ATP-Dependent MurE Ligase of Mycobacterium tuberculosis: Antibacterial Activity, Molecular Docking and Inhibition Kinetics. J. Antimicrob. Chemother. 2011, 66, 1766–1772. [Google Scholar] [CrossRef]
- Adams, M.; Wube, A.A.; Bucar, F.; Bauer, R.; Kunert, O.; Haslinger, E. Quinolone Alkaloids from Euodia rutaecarpa: A Potent New Group of Antimycobacterial Compounds. Int. J. Antimicrob. Agents 2005, 26, 262–264. [Google Scholar] [CrossRef]
- Adams, M.; Bauer, R.; Bucar, F.; Wube, A.A. Pharmaceutical Preparation for Treating Mycobacterial Infections with 4-quinolones from Euodia rutaecarpa. WO 2006094327, 14 September 2006. [Google Scholar]
- Wube, A.A.; Hüfner, A.; Thomaschitz, C.; Blunder, M.; Kollroser, M.; Bauer, R.; Bucar, F. Design, Synthesis and Antimycobacterial Activities of 1-methyl-2-alkenyl-4(1H)-Quinolones. Bioorg. Med. Chem. 2011, 19, 567–579. [Google Scholar] [CrossRef]
- Wube, A.A.; Bucar, F.; Hochfellner, C.; Blunder, M.; Bauer, R.; Hüfner, A. Synthesis of N-Substituted 2-[(1E)-alkenyl]-4-(1H)-Quinolone Derivatives as Antimycobacterial Agents Against Non-Tubercular Mycobacteria. Eur. J. Med. Chem. 2011, 46, 2091–2101. [Google Scholar]
- Gillespie, S.H.; Kennedy, H. Fluoroquinolones: A New Treatment for Tuberculosis? Int. J. Tuberc. Lung Dis. 1998, 2, 265–271. [Google Scholar]
- Zhou, L.; Chen, L.; Skouta, R.; Jiang, H.-F.; Li, C.-J. Palladium-Catalyzed 1,4-Addition of Terminal Alkynes to Unsaturated Carbonyl Compounds Promoted by Electron-Rich Ligand. Org. Biomolec. Chem. 2008, 6, 2969–2977. [Google Scholar] [CrossRef]
- Xing, Y.; O’Doherty, G.A. De Novo Asymmetric Synthesis of Cladospolide B-D: Structural Reassignment of Cladospolide D via the Synthesis of Its Enantiomer. Org. Lett. 2009, 11, 1107–1110. [Google Scholar] [CrossRef]
- Rai, D.; Johar, M.; Manning, T.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Design and Studies of Novel 5-Substituted Alkynylpyrimidine Nucleosides as Potent Inhibitors of Mycobacteria. J. Med. Chem. 2005, 48, 7012–7017. [Google Scholar] [CrossRef]
- Kidwai, M.; Jain, R.; Bhardwaj, S. 1, 4-Addition of Terminal Alkynes to Conjugated Enones in Water Using Green Catalyst Bis[(L)Prolinato-N,O]Zn-an Environmentally Benign Protocol. Catal. Lett. 2011, 141, 183–190. [Google Scholar] [CrossRef]
- Zhang, D.; Ready, I.M. Iron-Catalyzed Carbometalation of Propargylic and Homopropargylic Alcohols. J. Am. Chem. Soc. 2006, 128, 15050–15051. [Google Scholar] [CrossRef]
- Trost, B.M.; Ball, Z.T.; Laemmerhold, K.M. An Alkyne Hydrosilylation-Oxidation Strategy for the Selective Installation of Oxygen Functionality. J. Am. Chem. Soc. 2005, 127, 10028–10038. [Google Scholar]
- Banaszak, E.; Xu, L.-W.; Bardeau, J.-F.; Castanet, A.-S.; Mortie, J. First Syntheses of Model long-Chain Trichloro[U-(Trimethylsilyl)Alkynyl]Silanes Suitable for Self-assembled Monolayers on Silicon Surfaces. Tetrahedron 2009, 65, 3961–3966. [Google Scholar]
- Novotný, J.; Pospěchová, K.; Hrabálek, A.; Čáp, R.; Vávrová, K. Synthesis of Fluorescent C24-Ceramide: Evidence for Acyl Chain Length Dependent Differences in Penetration of Exogenous NBD-Ceramides into Human Skin. Bioorg. Med. Chem. Lett. 2009, 19, 6975–6977. [Google Scholar]
- Houghton, S.A.; Furst, L.; Boddy, C.N. Biomimetic Transannular Oxa-conjugated Addition Approach to the 2,6-Disubstituted Dihydropyran of Laulimalide Yields an Unprecedented Transannular Oxetane. J. Org. Chem. 2009, 74, 1454–1463. [Google Scholar]
- Porter, N.A.; Chang, V.H.-T.; Magnin, D.R.; Wright, B.T. Free Radical Macrocyclization-Transannular Cyclization. J. Am. Chem. Soc. 1988, 110, 3554–3560. [Google Scholar]
- Wube, A.A.; Bucar, F.; Gibbons, S.; Asres, K. Sesquiterpenes from Warburgia ugandensis and their Antimycobacterial Activity. Phytochemistry 2005, 66, 2309–2315. [Google Scholar]
- Evangelopoulos, D.; Bhakta, S. Rapid Methods for Testing Inhibitors of Mycobacterial Growth. In Antibiotic Resistance Protocols; Methods in Molecular Biology Human Press: London, UK, 2010; p. 279. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).