Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
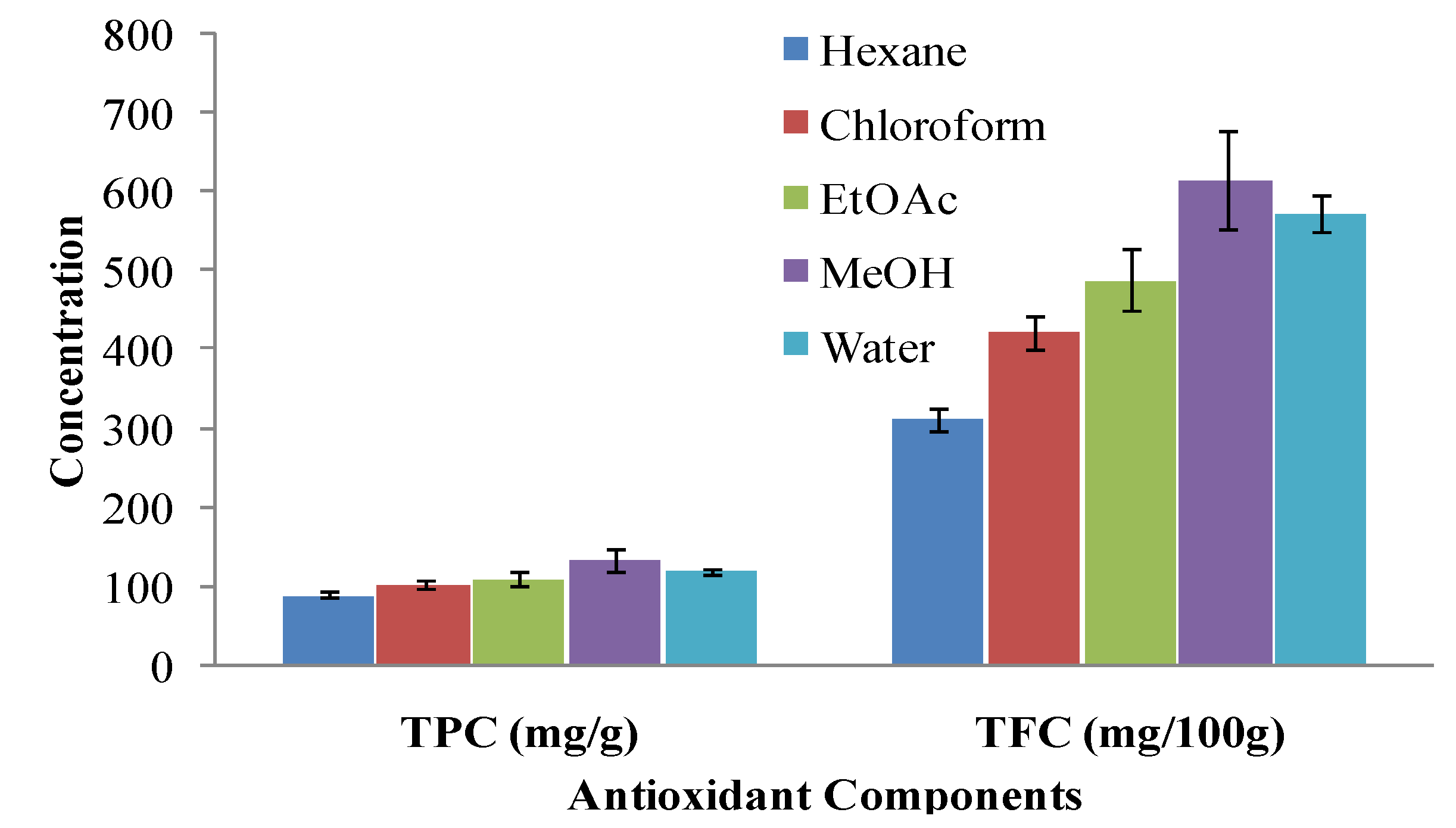
2.2. Total Phenolic Content (TPC)
2.3. Total Flavonoid Content (TFC)
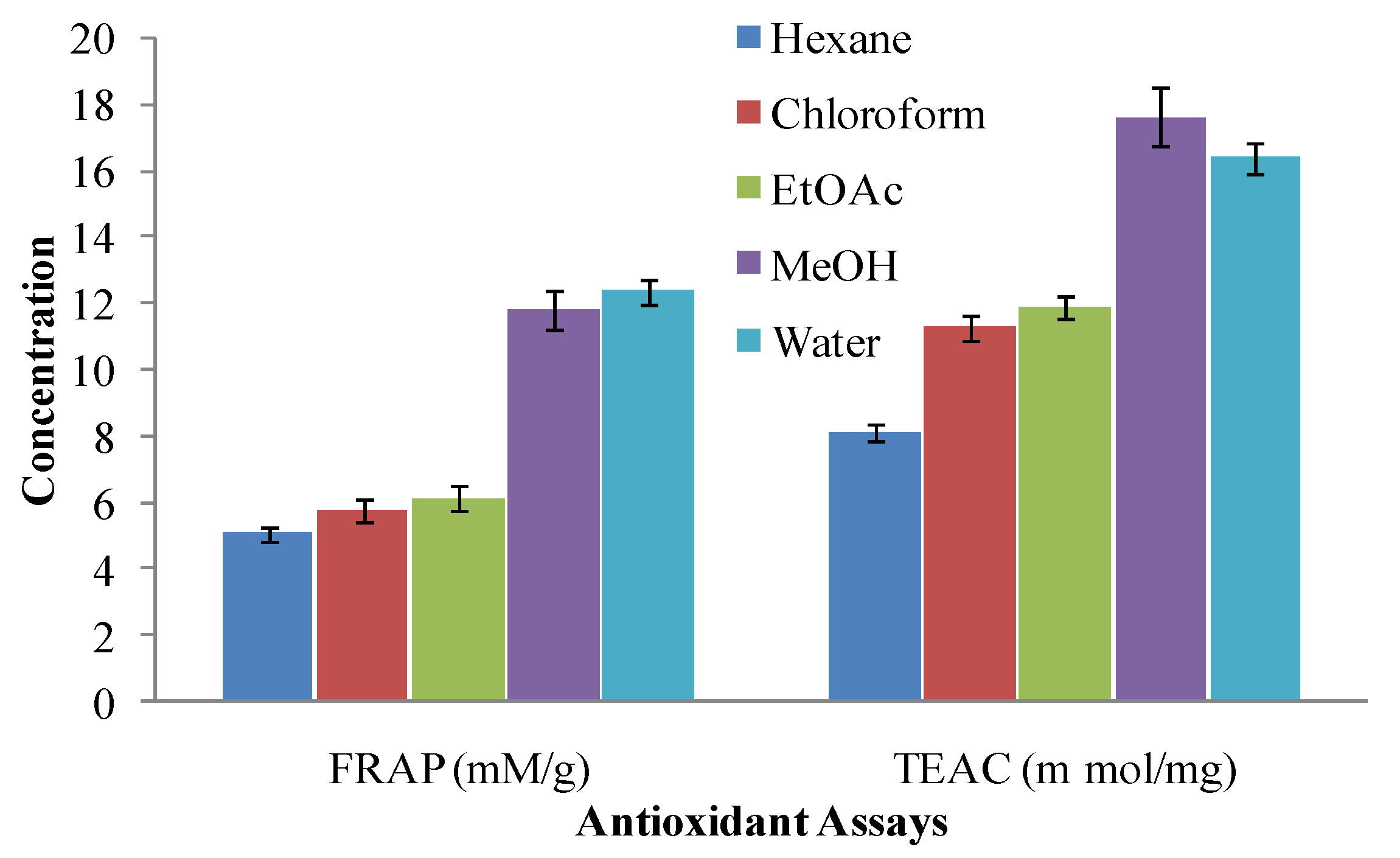
2.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
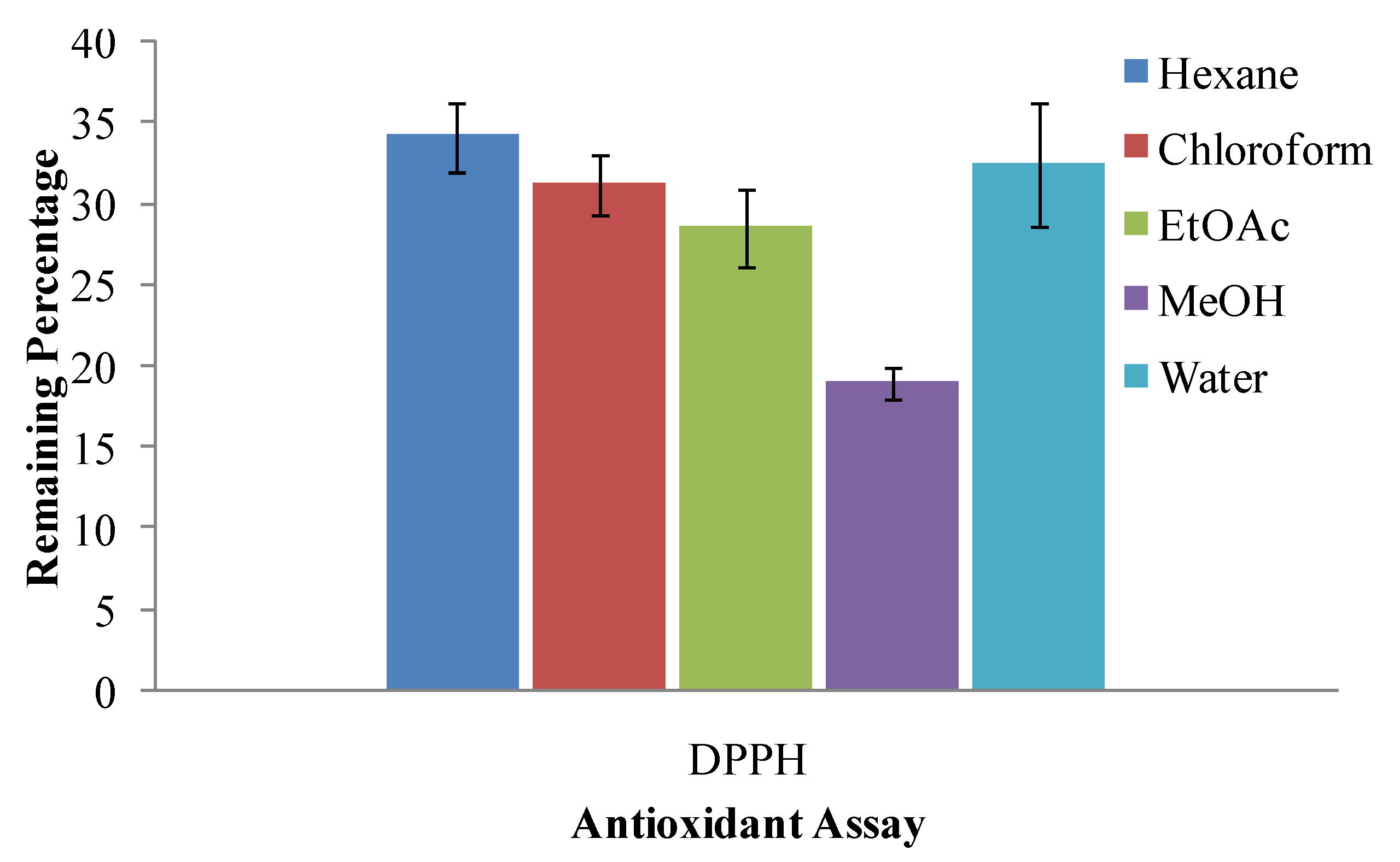
2.6. 2,2-Diphenyl-2-picrylhydrazyl Hydrate (DPPH) Radical Scavenging Assay
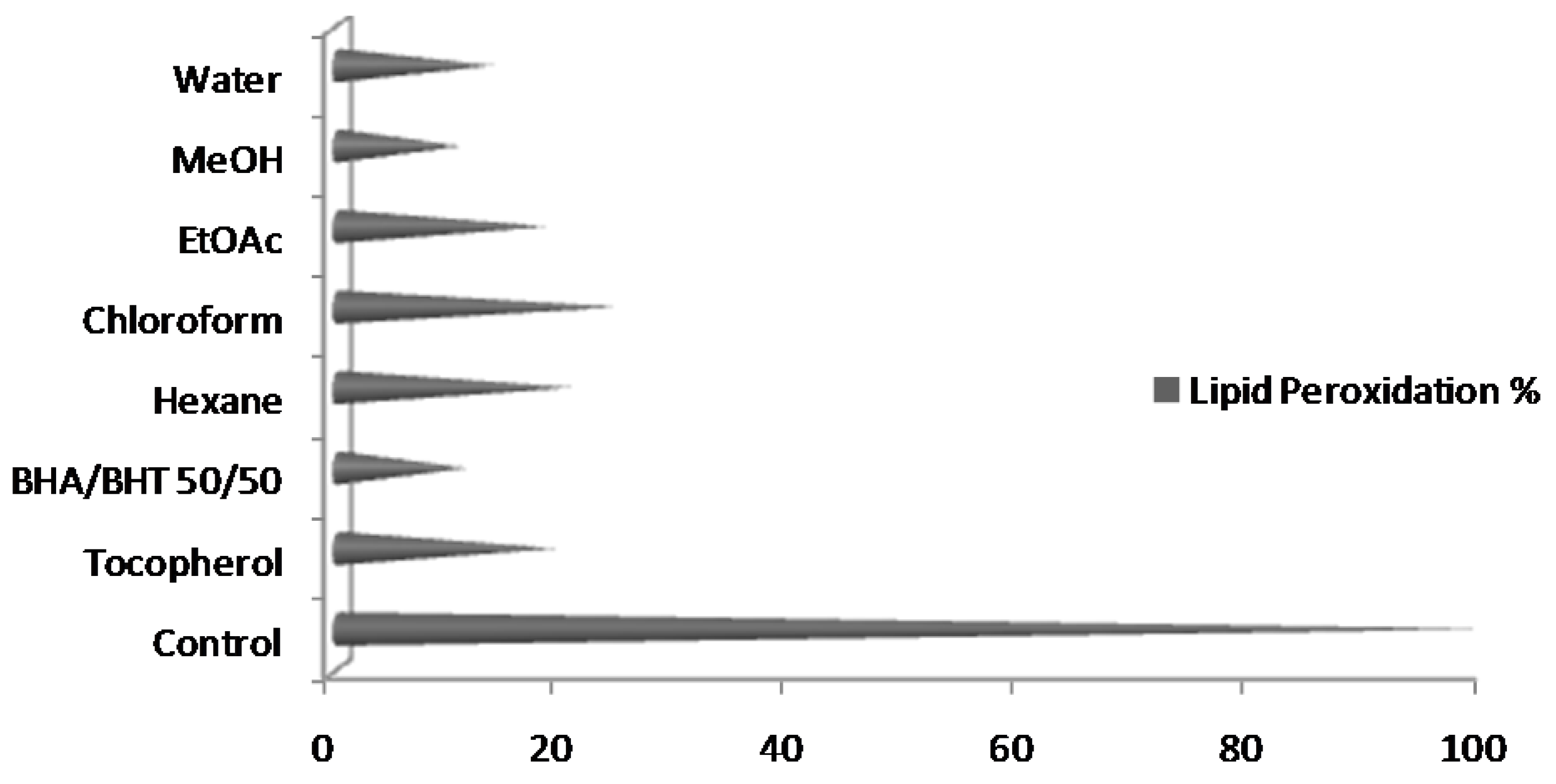
2.7. Lipid Peroxidation
2.8. Statistical Analysis
3. Experimental
3.1. Chemicals and Reagents
3.2. Collection of Samples and Extraction of Antioxidants
3.3. Chemical Composition
3.4. Estimation of Total Phenolic Contents (TPC)
3.5. Estimation of Total Flavonoid Contents (TFC)
3.6. Estimation of Antioxidant Activity
3.6.1. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.6.3. DPPH• Scavenging Assay
3.6.4. Lipid Peroxidation
3.7. Statistical Analysis
4. Conclusions
References and Notes
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and [beta]-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Wen, Q.; Li, L.; Wu, H.; Li, X. Antioxidant activities of water-soluble polysaccharide extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohydr. Polym. 2010, 81, 323–329. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Yingming, P.; Ying, L.; Hengshan, W.; Min, L. Antioxidant activities of several Chinese medicine herbs. Food Chem. 2004, 88, 347–350. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Girennavar, B.; Patil, B.S. Antioxidant capacity of pummelo and navel oranges: Extraction efficiency of solvents in sequence. LWT Food Sci. Technol. 2008, 41, 376–384. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Teresa, C.; Rossana, P.; Maria, P.A.; Paride, P.; Francesco, P.F.; Luciano, V.; Pinarosa, A. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar]
- Sanja, C.; Milka, M.; Danijiela, V.; Adisa, P. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annual L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar]
- Hayat, M.Q.; Khan, M.A.; Ashraf, M.; Jabeen, S. Ethnobotany of the genus Artemisia L. (Asteraceae) in Pakistan. Ethnobotany Res. App. 2009, 7, 147–162. [Google Scholar] [CrossRef]
- Abdul, M.; Ibrar, A.; Waheed, A.; Muhammad, A.; Rizwana, Q.; Izhar, H.; Bushra, M. Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malar. J. 2010, 9, 310–319. [Google Scholar]
- Brinch-Pedersen, H.; Borg, S.; Tauris, B.; Holm, P.B. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007, 46, 308–326. [Google Scholar] [CrossRef]
- Odhav, B.; Beekrum, S.; Akula, U.; Baijnath, H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Compost. Anal. 2007, 20, 430–435. [Google Scholar] [CrossRef]
- Gupta, S.; Jyothi Lakshmi, A.; Manjunath, M.; Prakash, J. Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT Food Sci. Technol. 2005, 38, 339–345. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pasuk, S.; Ritthiruangdej, P. Relationship between antioxidant properties and chemical composition of some Thai plants. J. Food Compost. Anal. 2008, 21, 229–240. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Natarajan, S.S. A rapid method for depletion of Rubisco from soybean (Glycine max) leaf for proteomic analysis of lower abundance proteins. Phytochemistry 2009, 70, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.; Marfo, E.; Plahar, W. Nutritional quality and antinutritional composition of four non-conventional leafy vegetables. Food Chem. 1998, 61, 287–291. [Google Scholar] [CrossRef]
- Jimoh, F.; Adedapo, A.; Afolayan, A. Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food Chem. Toxicol. 2010, 48, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Sathaye, S.; Bagul, Y.; Gupta, S.; Kaur, H.; Redkar, R. Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp. Toxicol. Pathol. 2011, 63, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem. 2010, 122, 1205–1211. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Jung, C.H.; Seog, H.M.; Choi, I.W.; Park, M.W.; Cho, H.Y. Antioxidant properties of various solvent extracts from wild ginseng leaves. LWT Food Sci. Technol. 2006, 39, 266–274. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; de Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Mohsen, S.M.; Ammar, A.S.M. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem. 2009, 112, 595–598. [Google Scholar] [CrossRef]
- Hayes, J.; Allen, P.; Brunton, N.; O’Grady, M.; Kerry, J. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kwak, J.H.; Kim, J.H.; Choi, G.N.; Kim, D.O.; Heo, H.J. Neuronal cell protective and antioxidant effects of phenolics obtained from Zanthoxylum piperitum leaf using in vitro model system. Food Chem. 2010, 125, 417–422. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Berker, K.I.; Guclu, K.; Tor, I.; Apak, R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Tyug, T.S.; Prasad, K.N.; Ismail, A. Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chem. 2010, 123, 583–589. [Google Scholar] [CrossRef]
- Santas, J.; Carbo, R.; Gordon, M.; Almajano, M. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Akowuah, G.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Michielin, E.M.Z.; Wiese, L.P.L.; Ferreira, E.A.; Pedrosa, R.C.; Ferreira, S.R.S. Radical-scavenging activity of extracts from Cordia verbenacea DC obtained by different methods. J. Supercrit. Fluids 2011, 86, 89–96. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Compost. Anal. 2006, 19, 544–551. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Makkar, H.P.S.; Goodchild, A.V. Quantification of Tannins: A Laboratory Manual; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syrian Arab Republic, 1996. [Google Scholar]
- Wheeler, E.; Ferrel, R. A method for phytic acid determination in wheat and wheat fractions. Cereal Chem. 1971, 48, 312–320. [Google Scholar]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. from Portugal. Food Chem. Toxicol. 2009, 47, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Bhanger, M.; Anwar, F. Antioxidant properties and components of bran extracts from selected wheat varieties commercially available in Pakistan. LWT Food Sci. Technol. 2007, 40, 361–367. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.; Anwar, F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005, 93, 265–272. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the first and second authors. |
| Sr. No | Contents | Amount (% dry weight basis) |
|---|---|---|
| 1 | Ash | 7.5 |
| 2 | Carbohydrate | 8.3 |
| 3 | Fat | 6.07 |
| 4 | Fibre | 14.2 |
| 5 | Moisture | 11.4 |
| 6 | Protein | 24.37 mg/100 g |
| 7 | Phytate | 140.4 |
| 8 | Total Tannins | 0.61 |
| 9 | Tocopherol | 2.74 |
| TPC | TFC | FRAP | TEAC | DPPH | L.P | |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 0.972 | 1 | ||||
| FRAP | 0.866 | 0.887 | 1 | |||
| TEAC | 0.971 | 0.980 | 0.949 | 1 | ||
| DPPH | 0.825 | 0.698 | 0.493 | 0.671 | 1 | |
| L.P | −0.832 | −0.824 | −0.884 | −0.845 | −0.643 | 1 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules 2012, 17, 6020-6032. https://doi.org/10.3390/molecules17056020
Iqbal S, Younas U, Chan KW, Zia-Ul-Haq M, Ismail M. Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules. 2012; 17(5):6020-6032. https://doi.org/10.3390/molecules17056020
Chicago/Turabian StyleIqbal, Shahid, Umer Younas, Kim Wei Chan, Muhammad Zia-Ul-Haq, and Maznah Ismail. 2012. "Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents" Molecules 17, no. 5: 6020-6032. https://doi.org/10.3390/molecules17056020
APA StyleIqbal, S., Younas, U., Chan, K. W., Zia-Ul-Haq, M., & Ismail, M. (2012). Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules, 17(5), 6020-6032. https://doi.org/10.3390/molecules17056020




