Antifungal Activities of New Coumarins
Abstract
:1. Introduction
2. Results and Discussion
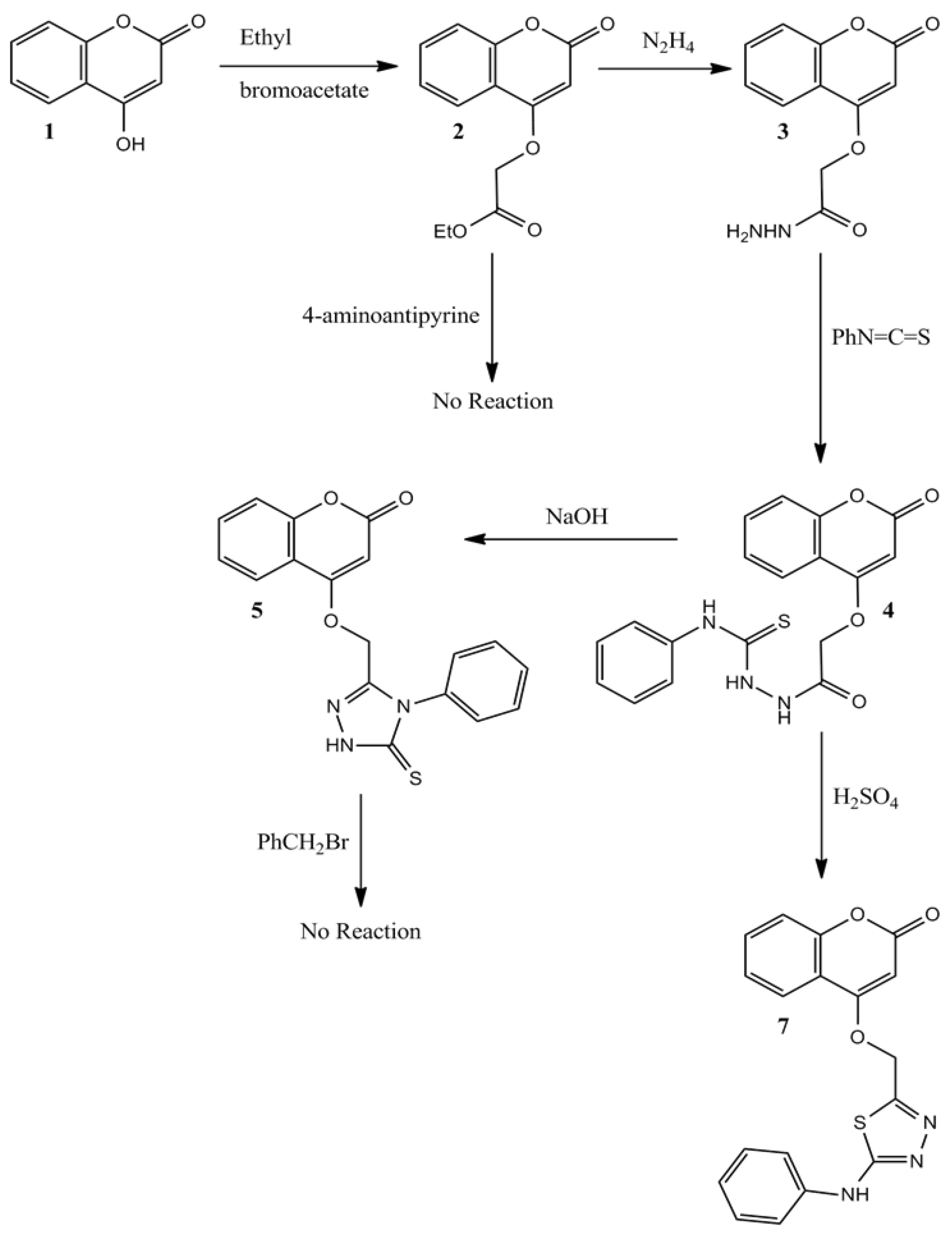
2.1. Chemistry
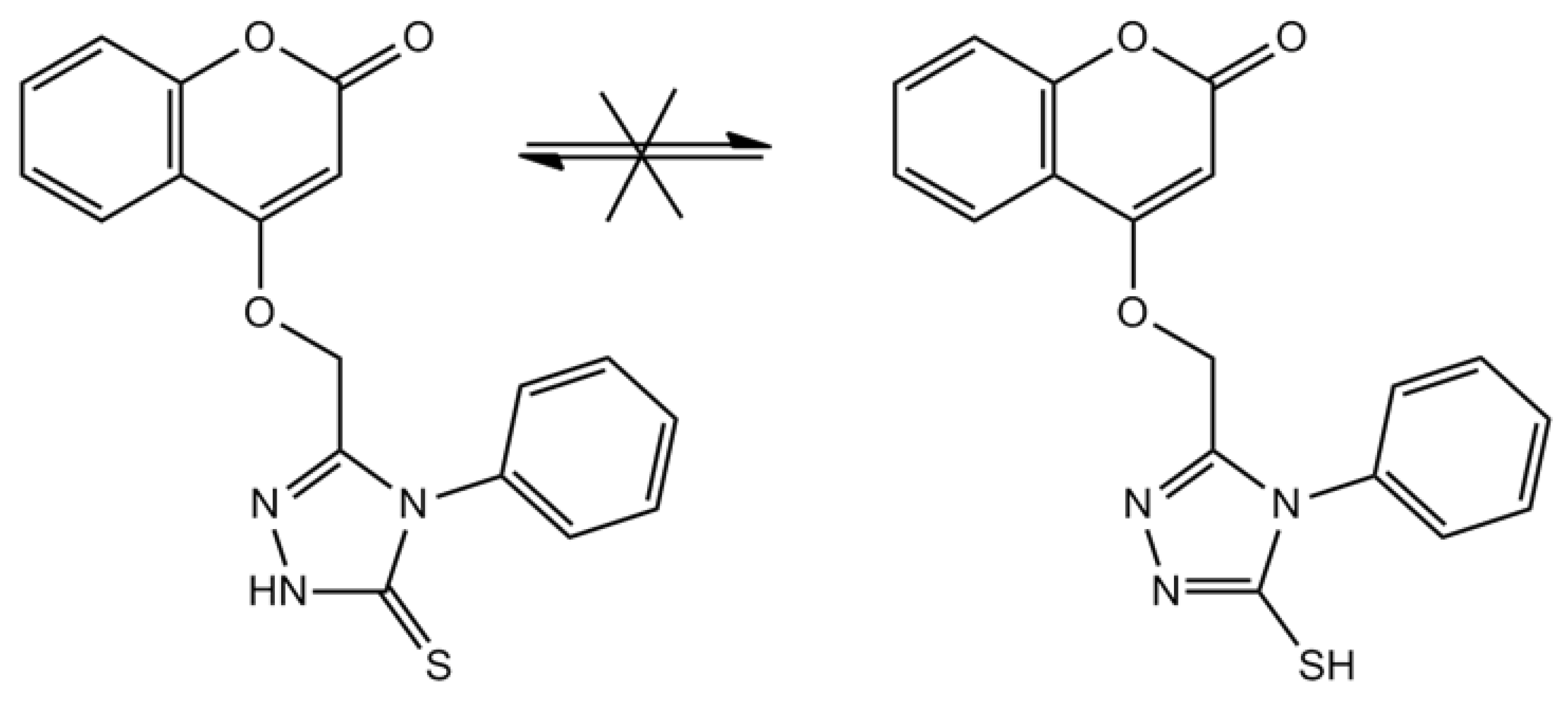
2.2. Computational Studies
2.2.1. Atomic Charges and Stabilities
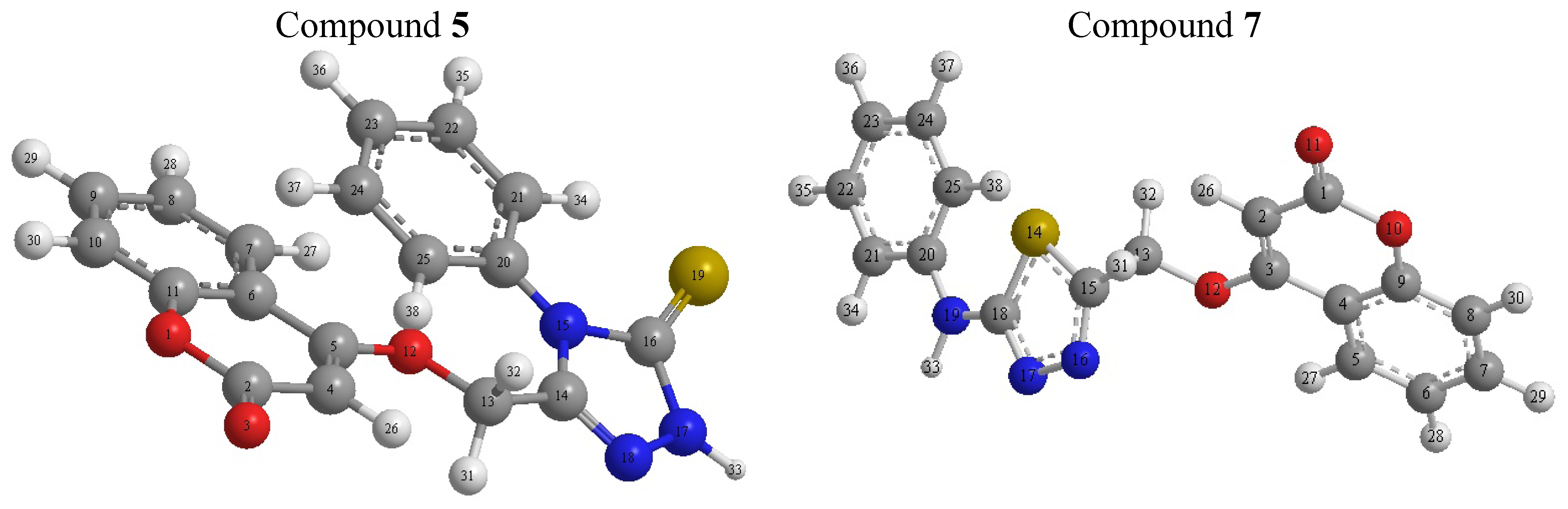
2.2.2. Density Function Theory (DFT)


| HOMO | LUMO | ∆E | HOMO −1 | LUMO +1 | ∆E |
|---|---|---|---|---|---|
| −2.840 | −0.599 | −2.241 | −6.415 | −0.205 | −6.210 |
| −3.625 | −1.055 | 2.570 | −8.830 | −0.067 | 8.763 |
2.3. Antifungal Activity
- • Interference with the synthesis of cellular walls, causing damage that can lead to altered cell permeability characteristics or disorganized lipoprotein arrangements, ultimately resulting in cell death.
- • Deactivation of various cellular enzymes that play a vital role in the metabolic pathways of these microorganisms.
- • Denaturation of one or more cellular proteins, causing the normal cellular processes to be impaired.


3. Experimental
3.1. General
3.1.1. Ethyl 2-((2-oxo-2H-chromen-4-yl)oxy)acetate (2)
3.1.2. 2-(2-Oxo-2H-chromen-4-yloxy)acetohydrazide (3)
3.1.3. 2-(2-(2-Oxo-2H-chromen-4-yloxy)acetyl)-N-phenylhydrazinecarbothioamide (4)
3.1.4. 4-((5-Mercapto-4-phenyl-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (5)
3.1.5. 4-((5-(Phenylamino)-1,3,4-thiadiazol-2-yl)methoxy)-2H-chromen-2-one (7)
3.2. DFT
3.3. Evaluation of Antifungal Assay

4. Conclusions
References and Notes
- Rice, S.A.; Givskov, M.; Steinberg, P.; Kjelleberg, S. Bacterial signals and antagonists: The interaction between bacteria and higher organisms. J. Mol. Microbiol. Biotechnol. 1999, 1, 23–31. [Google Scholar]
- Ironmonger, A.; Whittaker, B.; Andrew, J.; Baron, B.; Chris, J.; Alison, E.; Ashcroft, G.; Nelson, A. Scanning conformational space with a library of stereo- and regiochemically diverse aminoglycoside derivatives: The discovery of new ligands for RNA hairpin sequences. Org. Biomol. Chem. 2007, 5, 1081–1086. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Mohammed, A.; Ibrahim, H.; Abbas, A. Study the biological activities of tribulus terrestris extracts. World Acad. Sci. Eng. Technol. 2009, 57, 433–435. [Google Scholar]
- Kumar, G.; Kumar, D.; Devi, S.; Verma, R.; Johari, R. Synthesis, spectral characterization of biologically active compounds derived from oxalyldihydrazide and 5-tert-butyl-2-hydroxy-3-(3-phenylpent-3-yl)benzaldehyde and their Cu(II), Ni(II) and Co(II) Complexes. Int. J. Eng. Sci. Technol. 2011, 3, 1630–1635. [Google Scholar]
- Kadhum, A.H.; Mohamad, A.; Al-Amiery, A.A. Antimicrobial and anti-oxidant activities of new metal complexes derived from 3-aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar] [CrossRef]
- El-Agrody, A.; Abd El-Latif1, M.; El-Hady, N.; Fakery, A.; Bedair, A. Hetero aromatization with 4-ydroxycoumarin Part II: Synthesis of some new pyrano[2,3-d]pyrimidines, [1,2,4]triazolo [1,5-c]pyrimidines and Pyrimido[1,6-b] [1,2,4]triazine derivatives. Molecules 2001, 6, 519–527. [Google Scholar] [CrossRef]
- Rositca, D.N.; Vayssilov, G.N.; Rodios, N.; Bojilova, A. Regio- and Stereoselective [2 + 2] Photodimerization of 3-Substituted 2-Alkoxy-2-oxo-2H-1,2-benzoxaphosphorines. Molecules 2002, 7, 420–432. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Bayati, R.; Saour, K.; Radi, M. Cytotoxicity, Antioxidant and Antimicrobial activities of novel 2-quinolone derivatives derived from coumarins. Res. Chem. Intermed. 2011, 38, 559–569. [Google Scholar]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.H.; Mohamad, A. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.H.; Mohamad, A. The Antioxidant Activity of New Coumarin Derivatives. Int. J. Mol. Sci. 2011, 12, 5757–5761. [Google Scholar]
- Kadhum, A.H.; Al-Amiery, A.A.; Sikara, M.; Mohamad, A. Synthesis, Structure elucidation and DFT studies of new thiadiazoles. Int. J. Phys. Sci. 2011, 6, 6692–6697. [Google Scholar]
- Al-Amiery, A.A.; Kadhum, A.H.; Aday, H.; Al-Majedy, Y.; Al-Temimi, A.; Mohamad, A.; Al-Bayati, R. Co-crystal structure of mixed molecules. Int. J. Phys. Sci. 2012, 7, 1564–1570. [Google Scholar]
- Jones, G. Organic Reactions; John Wiley & Sons: New York, NY, USA, 1967; Volume 15, pp. 204–599. [Google Scholar]
- Tuyen, N.; Van, S.; Norbert, K. Synthesis of coumarins by ring-closing metathesis using Grubbs’ catalyst. TetrahedronLett. 2003, 44, 4199–4201. [Google Scholar]
- Su-Jin, P.; Jong-Cheol, L.; Kee-In, L. A Facile Synthesis of 4-Hydroxycoumarin and 4-Hydroxy-2-quinolone Derivatives. Bull. Korean Chem. Soc. 2007, 28, 1203–1205. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, K.; Abdulreazak, H.; Abood, H. Synthesis, characterization, theoretical crystal structure and antibacterial activities of some transition metal complexes of the thiosemicarbazone (Z)-2-(pyrrolidin-2-ylidene)hydrazinecarbothioamide. Bioinorg. Chem. Appl. 2011, 2011, 1–6. [Google Scholar]
- Kadhum, A.; Wasmi, B.; Mohamad, A.; Al-Amiery, A.A. Takriff MS Preparation, characterization, and theoretical studies of azelaic acid derived from oleic acid by use of a novel ozonolysis method. Res. Chem. Inter. Med. 2012, 38, 659–668. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Ibrahim, H.H.; Temimi, A.A. Antioxidant, antimicrobial, and theoretical studies of the thiosemicarbazone derivative Schiff base 2-(2-imino-1-methylimidazolidin-4-ylidene) hydrazinecarbothioamide (IMHC). Org. Med. Chem. Lett. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.; Mohamad, A. Antifungal and Antioxidant Activities of Pyrrolidonethiosemicarbazone Complexes. Bioinorg. Chem. Appl. 2012, 2012, 1–5. [Google Scholar]
- Al-Amiery, A.A. Synthesis and antioxidant, antimicrobial evaluation, DFT studies of novel metal complexes derivate from Schiff base. Res. Chem. Intermed. 2012, 38, 745–759. [Google Scholar] [CrossRef]
- Al-Amiery, A.A. Antimicrobial and Antioxidant Activities of New Metal Complexes Derived from (E)-3-((5-phenyl-1,3,4-oxadiazol-2-ylimino)methyl)naphthalen-2-ol. Med. Chem. Res. 2012, in press. [Google Scholar]
- Prasad, K.; Kumar, L.; Shekar, S.; Prasad, M.; Revanasiddappa, H. Oxovanadium complexes with bidentate N, O ligands: Synthesis, characterization, DNA binding, nuclease activity and antimicrobial studies. Chem. Sci. J. 2011, 12, 1–10. [Google Scholar]
- Thangadurai, T.; Natarajan, K. Mixed ligand complexes of ruthenium(II) containing α,β-unsaturated-β-ketoamines and their antibacterial activity. Transit. Met. Chem. 2001, 26, 500–504. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Viswanathamurthi, P.; Natarajan, K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transit. Met. Chem. 2001, 26, 105–109. [Google Scholar] [CrossRef]
- Daw, Z.Y.; EL-Baroty, G.S.; Mahmoud, A.E. Inhibition of Aspergillus parasiticus growth and aflatoxin production by some essential oils. Chem. Mikrobiol. Technol. Lebensm. 1994, 16, 129–135. [Google Scholar]
- Myiut, S.; Daud, W.R.W.; Mohamed, A.B.; Kadhum, A.A.H. Gas chromatographic determination of eugenol in ethanol extract of cloves. J. Chromatogr. Biomed. Appl. 1996, 676, 193–195. [Google Scholar]
- Sample Availability: Samples of the compounds . Ethyl 2-((2-oxo-2H-chromen-4-yl)oxy)acetate (2), 2-(2-Oxo-2H-chromen-4-yloxy)acetohydrazide (3), 2-(2-(2-Oxo-2H-chromen-4-yloxy)acetyl)-N-phenylhydrazinecarbothioamide (4), 4-((5-Mercapto-4-phenyl-4H-1,2,4-triazol-3-yl)methoxy)-2H-chromen-2-one (5) and 4-((5-(Phenylamino)-1,3,4-thiadiazol-2-yl)methoxy)-2H-chromen-2-one (7), are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Antifungal Activities of New Coumarins. Molecules 2012, 17, 5713-5723. https://doi.org/10.3390/molecules17055713
Al-Amiery AA, Kadhum AAH, Mohamad AB. Antifungal Activities of New Coumarins. Molecules. 2012; 17(5):5713-5723. https://doi.org/10.3390/molecules17055713
Chicago/Turabian StyleAl-Amiery, Ahmed A., Abdul Amir Hassan Kadhum, and Abu Bakar Mohamad. 2012. "Antifungal Activities of New Coumarins" Molecules 17, no. 5: 5713-5723. https://doi.org/10.3390/molecules17055713
APA StyleAl-Amiery, A. A., Kadhum, A. A. H., & Mohamad, A. B. (2012). Antifungal Activities of New Coumarins. Molecules, 17(5), 5713-5723. https://doi.org/10.3390/molecules17055713





