MALDI-TOF MS Analysis of Native and Permethylated or Benzimidazole-Derivatized Polysaccharides
Abstract
:1. Introduction
2. Results and Discussion
2.1. MALDI-TOF MS of Native Polysaccharides
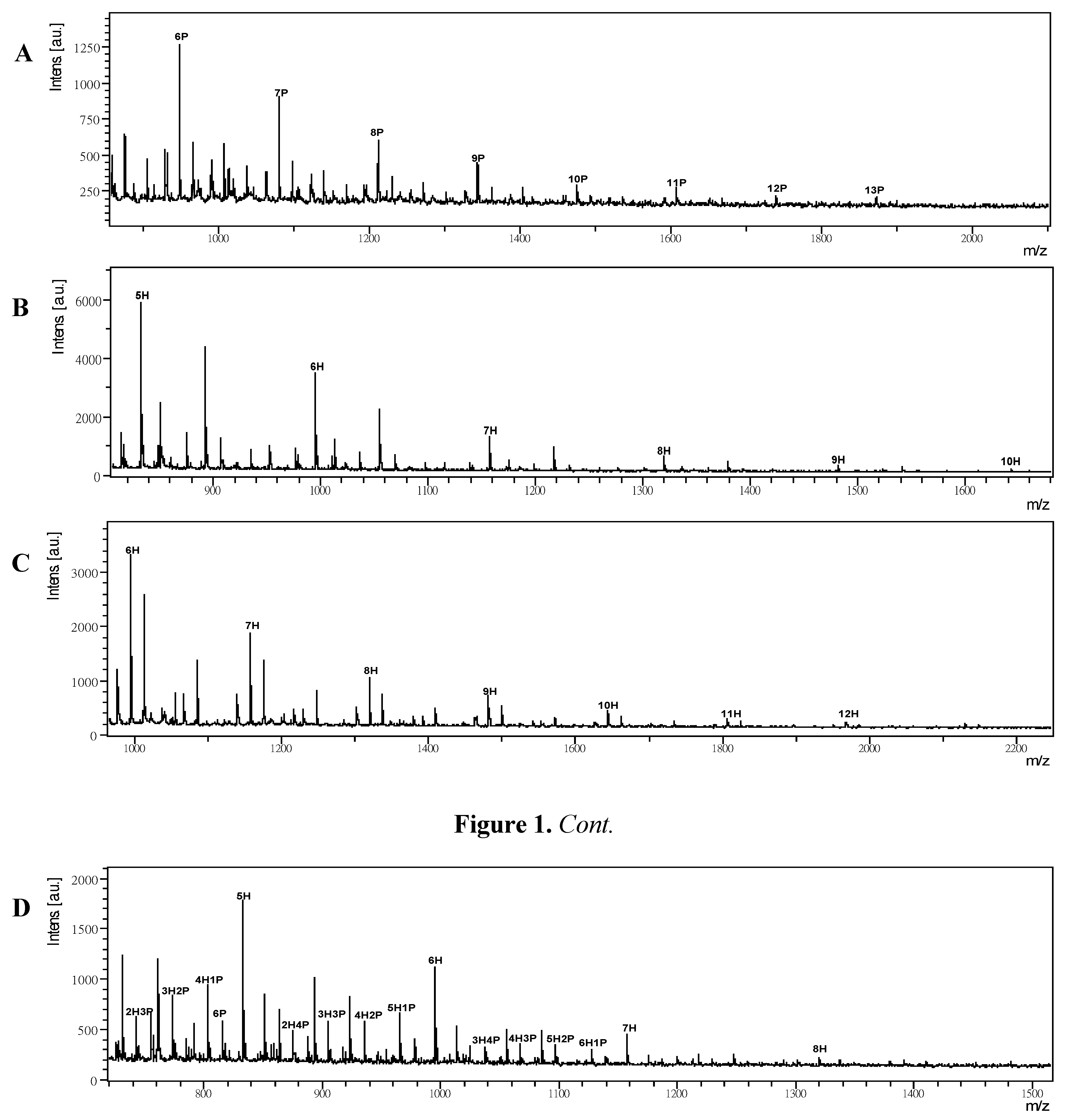
2.2. MALDI-TOF MS of Permethylated Polysaccharides
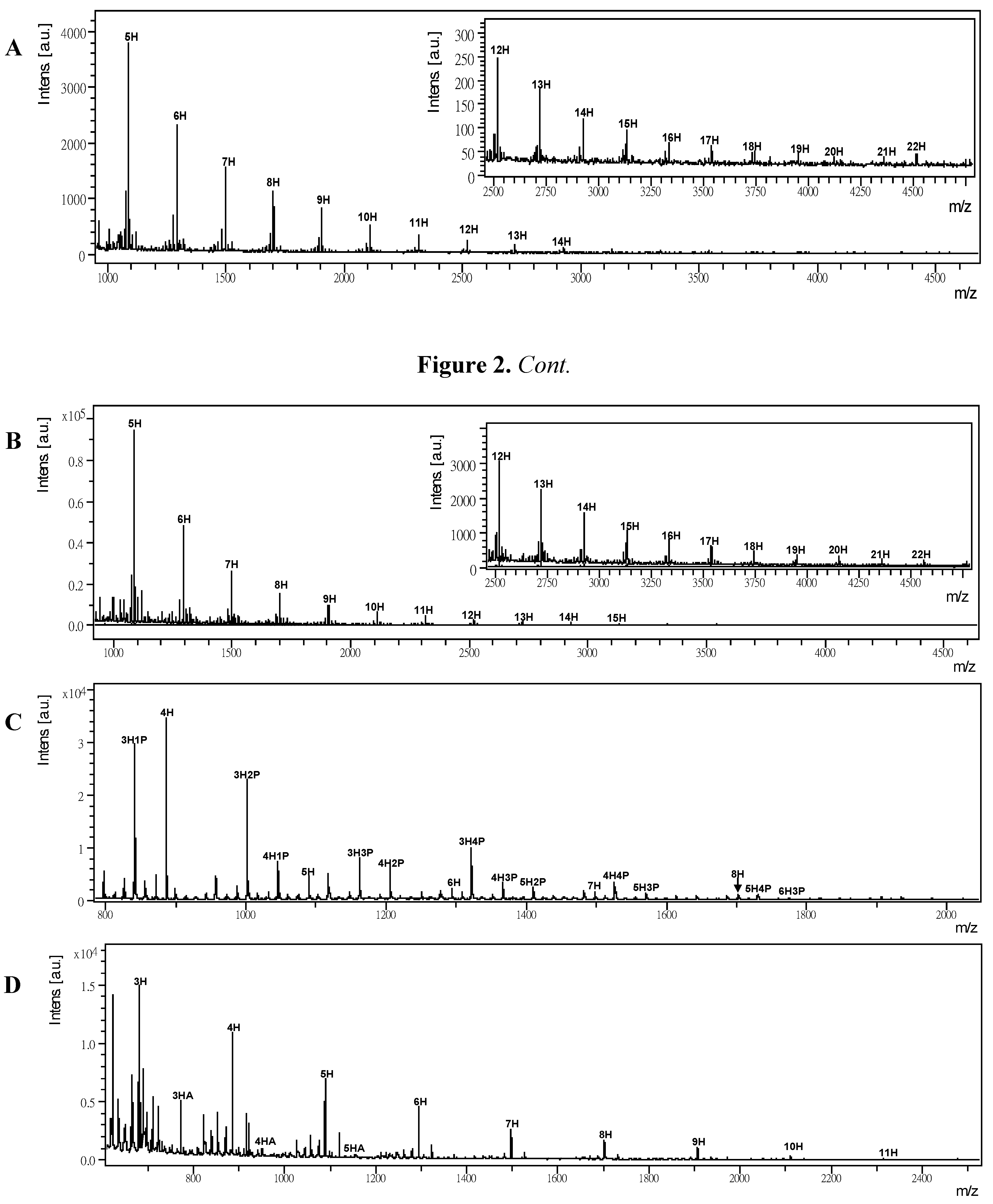
2.3. MALDI-TOF MS of Polysaccharide-BIMs
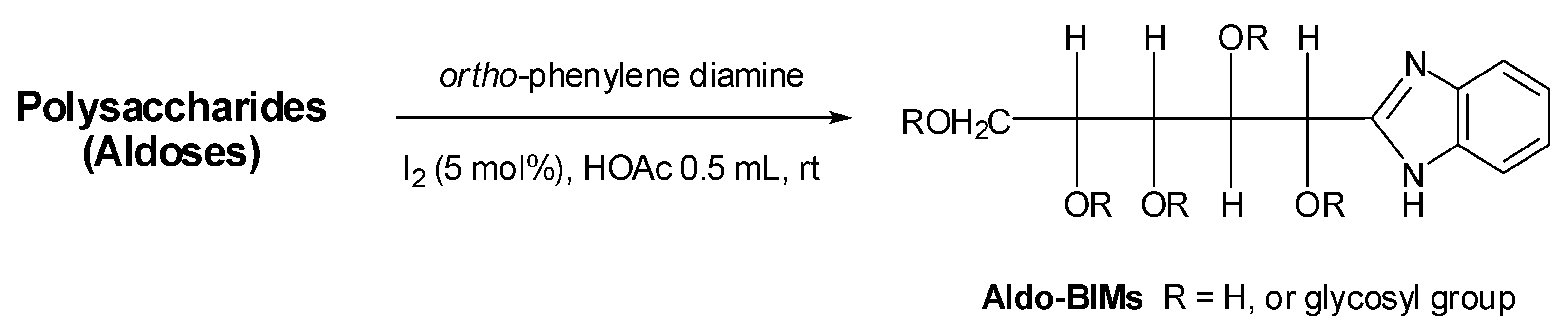
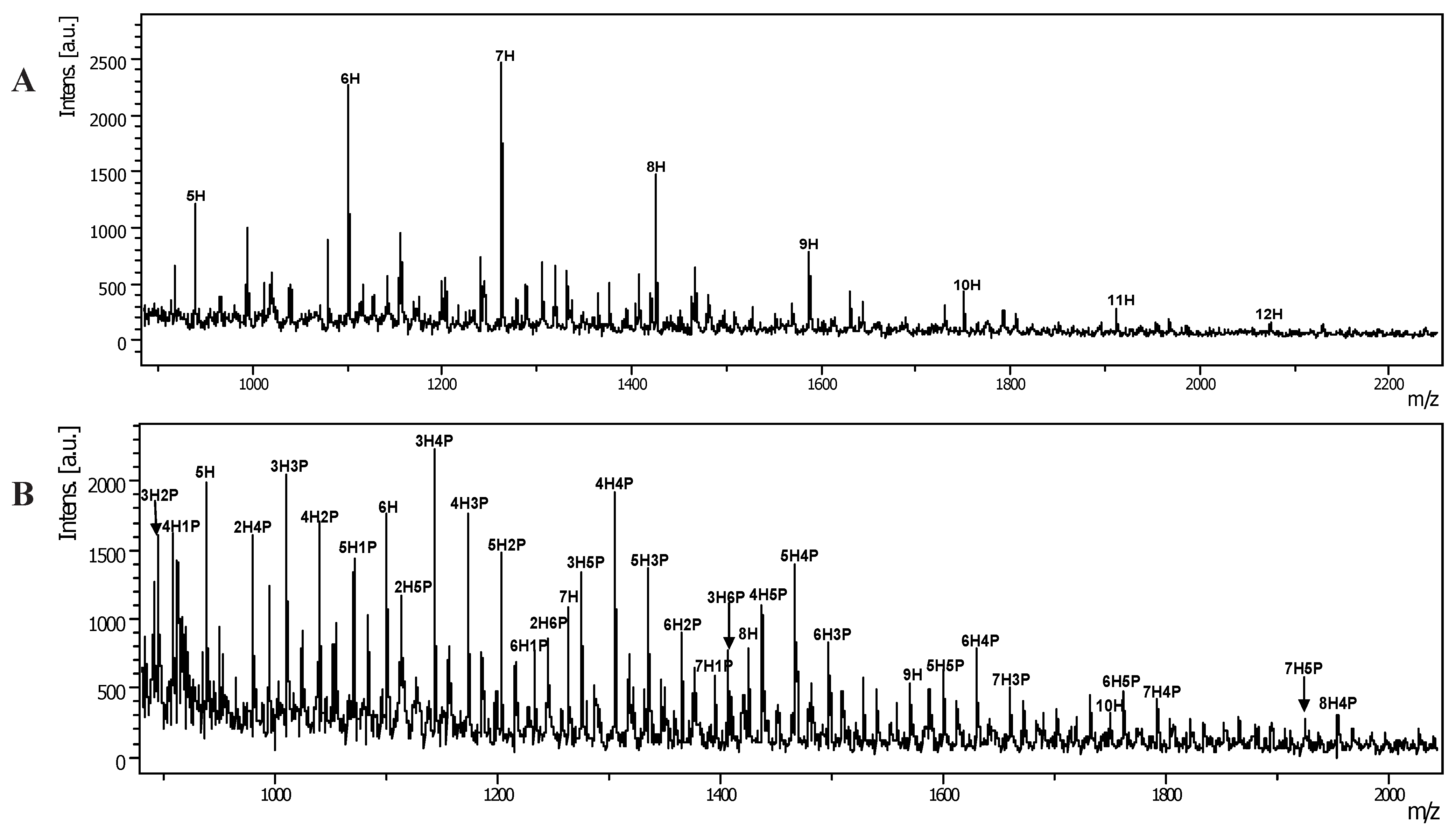
3. Experimental
3.1. Chemicals and Reagents
3.2. MALDI-TOF MS Measurement
3.3. Preparation of Permethylated Polysaccharide
3.4. Preparation of Polysaccharide-BIMs
4. Conclusions
Acknowledgements
References and Notes
- Marvin, L.F.; Roberts, M.A.; Fay, L.B. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in clinical chemistry. Clin. Chim. Acta 2003, 337, 11–21. [Google Scholar] [CrossRef]
- Chang, Y.L.; Liao, K.S.; Chen, Y.C.; Hung, W.T.; Yu, H.M.; Yang, W.B.; Fang, J.M.; Chen, C.H.; Lee, Y.C. Tagging saccharides for signal enhancement in mass spectrometric analysis. J. Mass Spectrom. 2011, 46, 247–255. [Google Scholar] [CrossRef]
- Zaia, J. Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef]
- Mechref, Y.; Novotny, M.V. Structural characterization of oligosaccharides using maldi-TOF/TOF tandem mass spectrometry. Anal. Chem. 2003, 75, 4895–4903. [Google Scholar] [CrossRef]
- Mock, K.K.; Davey, M.; Cottrell, J.S. The analysis of underivatised oligosaccharides by matrix-assisted laser desorption mass spectrometry. Biochem. Biophys. Res. Commun. 1991, 177, 644–651. [Google Scholar] [CrossRef]
- Stahl, B.; Steup, M.; Karas, M.; Hillenkamp, F. Analysis of neutral oligosaccharides by matrix-assisted laser desorption ionization mass spectrometry. Anal. Chem. 1991, 63, 1463–1466. [Google Scholar] [CrossRef]
- Hsu, N.Y.; Yang, W.B.; Wong, C.H.; Lee, Y.C.; Lee, R.T.; Wang, Y.S.; Chen, C.H. Matrix-assisted laser desorption/ionization mass spectrometry of polysaccharides with 2',4',6'-trihydroxyacetophenone as matrix. Rapid Commun. Mass Spectrom. 2007, 21, 2137–2146. [Google Scholar]
- Hakomori, S. A rapid permethylation of glycolipid andpolysaccharide catalyze by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 1964, 55, 205–208. [Google Scholar]
- Yoo, E.; Yoon, L. Applications of tandem mass spectrometry in the structure determination of permethylated sialic acid-containing oligosaccharides. Bull. Korean Chem. Soc. 2005, 26, 1347–1353. [Google Scholar] [CrossRef]
- Ariga, T.; Kohriyama, T.; Freddo, L.; Latov, N.; Saito, M.; Kon, K.; Ando, S.; Suzuk, M.; Hemling, M.E.; Rinehart, K.L.; et al. Characterization of sulfated glucuronic acid containing glycolipids reacting with IgM M-proteins in patients with neuropathy. J. Biol. Chem. 1987, 262, 848–853. [Google Scholar]
- Zaia, J. Mass spectrometry and the emerging field of glycomics. Chem. Biol. 2008, 15, 881–892. [Google Scholar] [CrossRef]
- Harvey, D.J. Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J. Chromatogr. B 2011, 879, 1196–1225. [Google Scholar] [CrossRef]
- Honda, S.; Suzuki, S.; Taga, A. Analysis of carbohydrates as 1-phenyl-3-methyl-5-pyrazolone derivatives by capaillary/microchip electrophoresis and capillary electrochromatography. J. Pharm. Biomed. Anal. 2003, 30, 1689–1714. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Liao, K.S.; Liu, Y.C.; Yang, W.B. Bis-indole derivatives for polysaccharides composition analysis and chiral resolution of D-, L-monosaccharide by ligand exchange CE using borate-CD as a chiral selector. Molecules 2011, 16, 1682–1694. [Google Scholar] [CrossRef]
- Lin, C.; Hung, W.T.; Kuo, C.Y.; Liao, K.S.; Liu, Y.C.; Yang, W.B. I2-catalyzed oxidative condensation of aldoses with diamines: Synthesis of aldo-naphthimidazoles for carbohydrate analysis. Molecules 2010, 15, 1340–1353. [Google Scholar] [CrossRef]
- Lin, C.; Lai, P.T.; Liao, S.K.S.; Hung, W.T.; Yang, W.B.; Fang, J.M. Using molecular iodine in direct oxidative condensation of aldoses with diamines: An improved synthesis of aldo-benzimidazoles and aldo-naphthimidazoles for carbohydrate analysis. J. Org. Chem. 2008, 73, 3848–3853. [Google Scholar] [CrossRef]
- Lin, C.; Hung, W.T.; Chen, C.H.; Fang, J.M.; Yang, W.B. A new naphthimidazole derivative for saccharide labeling with enhanced sensitivity in mass spectrometry detection. Rapid Commun. Mass Spectrom. 2010, 24, 85–94. [Google Scholar]
- Weskamp, T.; Böhm, V.P.W.; Herrmann, W.A. N-Heterocyclic carbenes: State of the art in transition-metal-complex synthesis. J. Organomet. Chem. 2000, 600, 12–22. [Google Scholar]
- Mohr, M.D.; Börnsen, K.O.; Widmer, H.M. Matrix-assisted laser desorption/ionization mass spectrometry: Improved matrix for oligosaccharides. Rapid Commun. Mass Spectrom. 1995, 9, 809–814. [Google Scholar] [CrossRef]
- Hao, C.; Ma, X.; Fang, S.; Liu, Z.; Liu, S.; Song, F.; Liu, J. Positive and negative-ion matrix-assisted laser desorption/ionization mass spectrometry of saccharides. Rapid Commun. Mass Spectrom. 1998, 12, 345–348. [Google Scholar]
- Bahr, U.; Pfenninger, A.; Karas, M.; Stahl, B. High-sensitivity analysis of neutral underivatized oligosaccharides by nanoelectrospray mass spectrometry. Anal. Chem. 1997, 69, 4530–4535. [Google Scholar] [CrossRef]
- Hung, W.T.; Wang, S.H.; Chen, C.H.; Yang, W.B. Structure determination of β-glucans from Ganoderma lucidum with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Molecules 2008, 13, 1538–1550. [Google Scholar] [CrossRef]
- Kao, P.F.; Wang, S.H.; Hung, W.T.; Liao, Y.H.; Lin, C.M.; Yang, W.B. Structural characterization and antioxidative activity of low-molecular-weight beta-1,3-glucan from the residue of extracted Ganoderma lucidum fruiting bodies. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Chang, W.C.; Huang, L.C.L.; Wang, Y.S.; Peng, W.P.; Chang, H.C.; Hsu, N.Y.; Yang, W.B.; Chen, C.H. Matrix-assisted laser desorption/ionization (MALDI) mechanism revisited. Anal. Chim. Acta 2007, 582, 1–9. [Google Scholar] [CrossRef]
- Shevchenko, N.M.; Anastyuk, S.D.; Gerasimenko, N.I.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae. Russ. J. Bioorgan. Chem. 2007, 33, 88–98. [Google Scholar] [CrossRef]
- Harvey, D.J. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates. Mass Spectrom. Rev. 1999, 18, 349–450. [Google Scholar] [CrossRef]
- Zaia, J. Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef]
- Sturiale, L.; Garozzo1, D.; Silipo, A.; Lanzetta, R.; Parrilli, M.; Molinaro, A. New conditions for matrix-assisted laser desorption/ionization mass spectrometry of native bacterial R-type lipopolysaccharides. Rapid Commun. Mass Spectrom. 2005, 19, 1829–1834. [Google Scholar] [CrossRef]
- Kiyohara, H.; Yamada, H. Structure of an anti-complementary arabinogalactan from the root of Angelica acutiloba kitagawa. Carbohydr. Res. 1989, 193, 173–192. [Google Scholar] [CrossRef]
- Zhang, H.; Singh, S.; Reinhold, V.N. Congruent strategies for carbohydrate sequencing. Anal. Chem. 2005, 77, 6263–6270. [Google Scholar] [CrossRef]
- Sample Availability: Samples of aldo–BIMs are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hung, W.-T.; Wang, S.-H.; Chen, Y.-T.; Yu, H.-M.; Chen, C.-H.; Yang, W.-B. MALDI-TOF MS Analysis of Native and Permethylated or Benzimidazole-Derivatized Polysaccharides. Molecules 2012, 17, 4950-4961. https://doi.org/10.3390/molecules17054950
Hung W-T, Wang S-H, Chen Y-T, Yu H-M, Chen C-H, Yang W-B. MALDI-TOF MS Analysis of Native and Permethylated or Benzimidazole-Derivatized Polysaccharides. Molecules. 2012; 17(5):4950-4961. https://doi.org/10.3390/molecules17054950
Chicago/Turabian StyleHung, Wei-Ting, Shwu-Huey Wang, Yi-Ting Chen, Hui-Ming Yu, Chung-Hsuan Chen, and Wen-Bin Yang. 2012. "MALDI-TOF MS Analysis of Native and Permethylated or Benzimidazole-Derivatized Polysaccharides" Molecules 17, no. 5: 4950-4961. https://doi.org/10.3390/molecules17054950
APA StyleHung, W.-T., Wang, S.-H., Chen, Y.-T., Yu, H.-M., Chen, C.-H., & Yang, W.-B. (2012). MALDI-TOF MS Analysis of Native and Permethylated or Benzimidazole-Derivatized Polysaccharides. Molecules, 17(5), 4950-4961. https://doi.org/10.3390/molecules17054950



