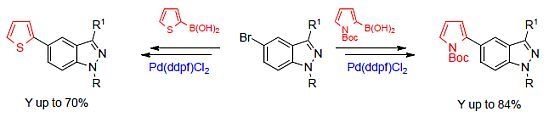
The Suzuki Reaction Applied to the Synthesis of Novel Pyrrolyl and Thiophenyl Indazoles
Abstract
:1. Introduction
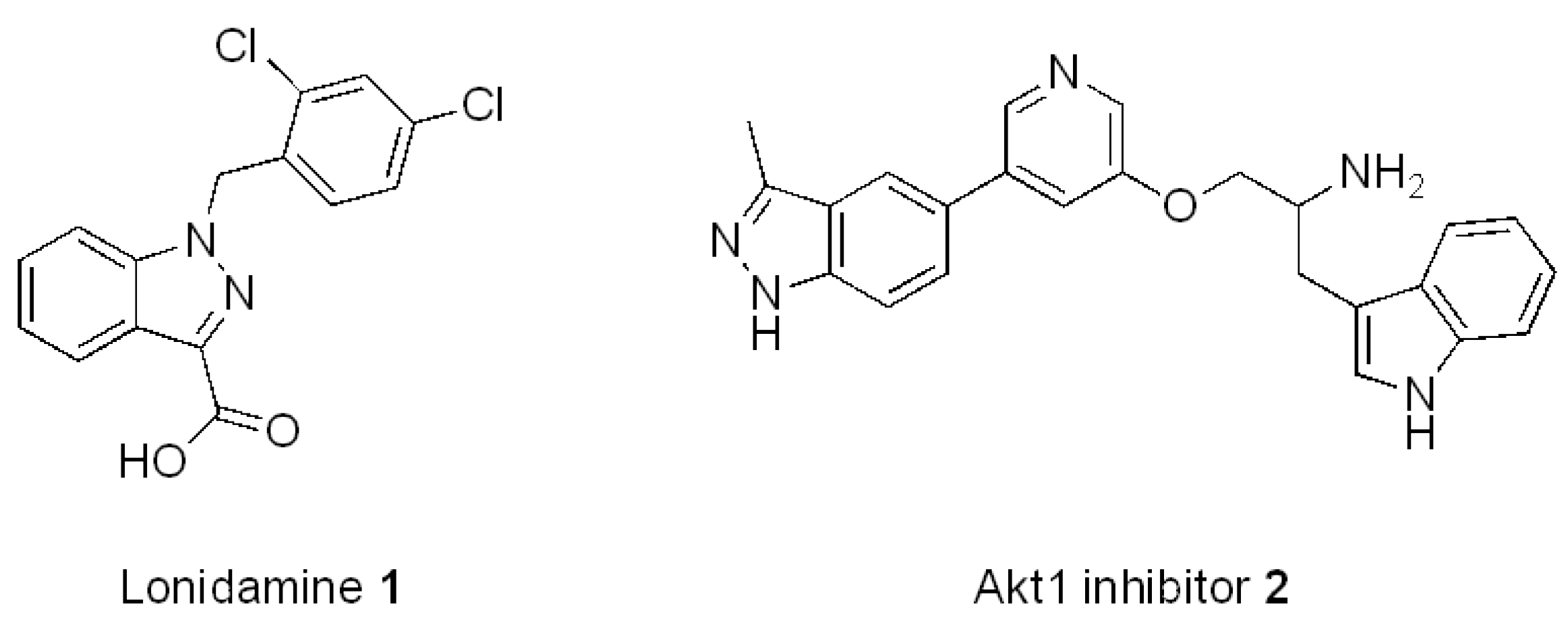
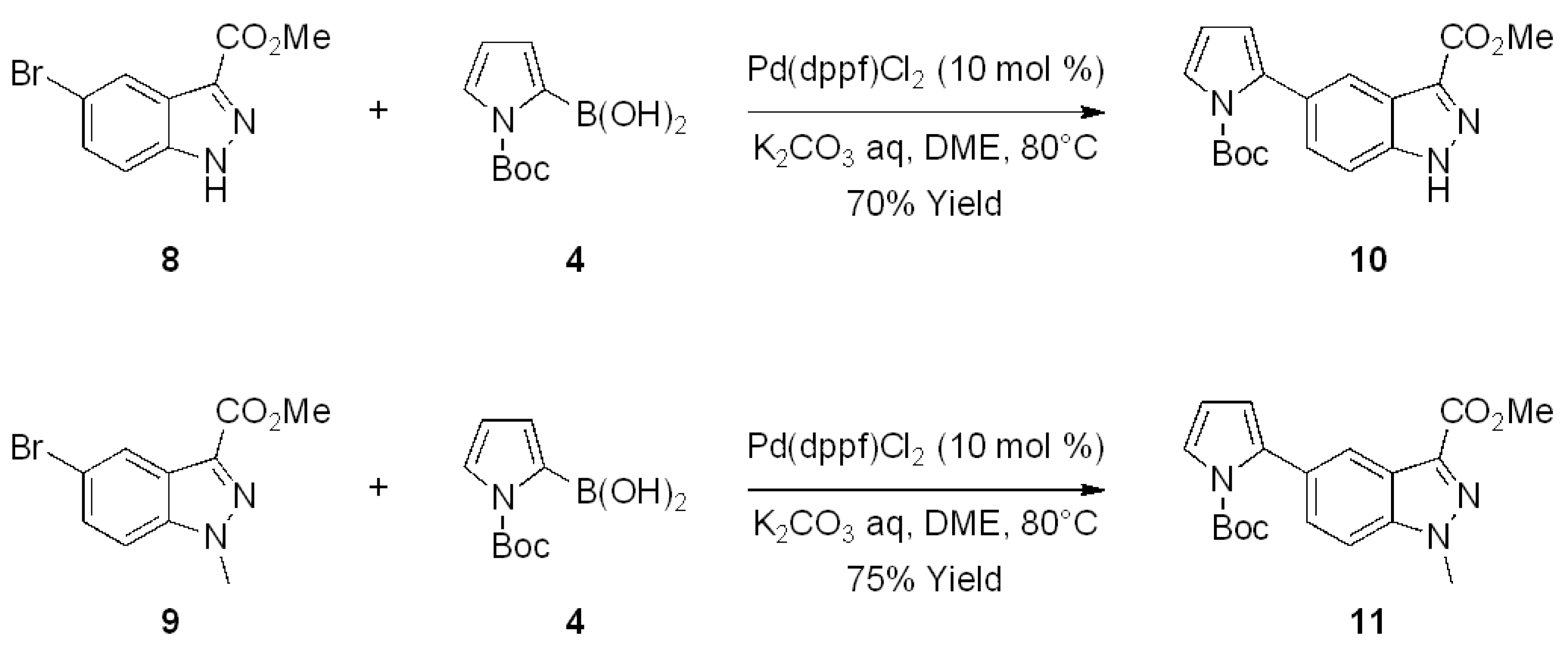
2. Results and Discussion
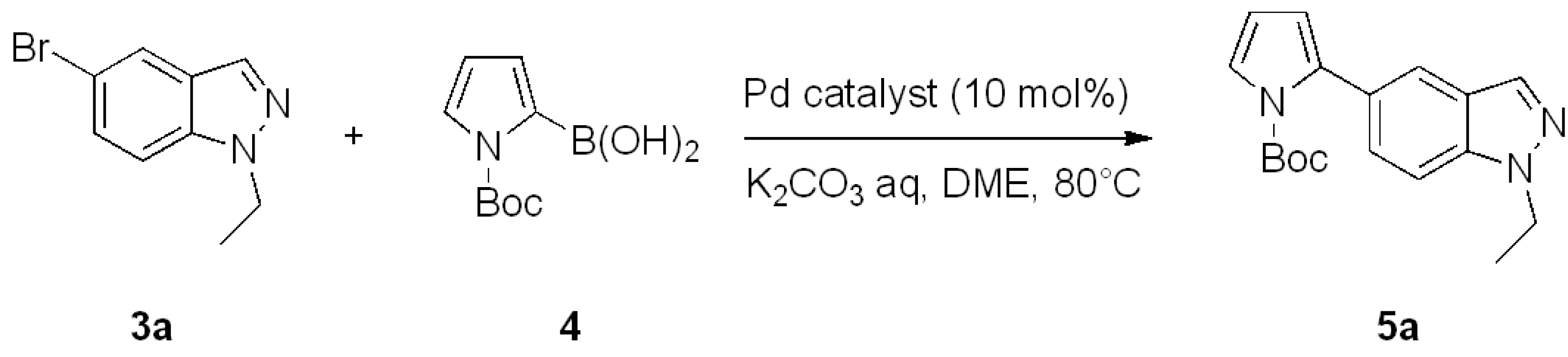
| Entry | Pd catalyst | Reaction Time | 5a Yield |
|---|---|---|---|
| 1 | Pd(PPh3)4 | 4 h | 22% |
| 2 | Pd(PPh3)2Cl2 | 4 h | 75% |
| 3 | Pd(PCy3)2 | 2 h | 57% |
| 4 | Pd(dppf)Cl2 | 2 h | 84% |
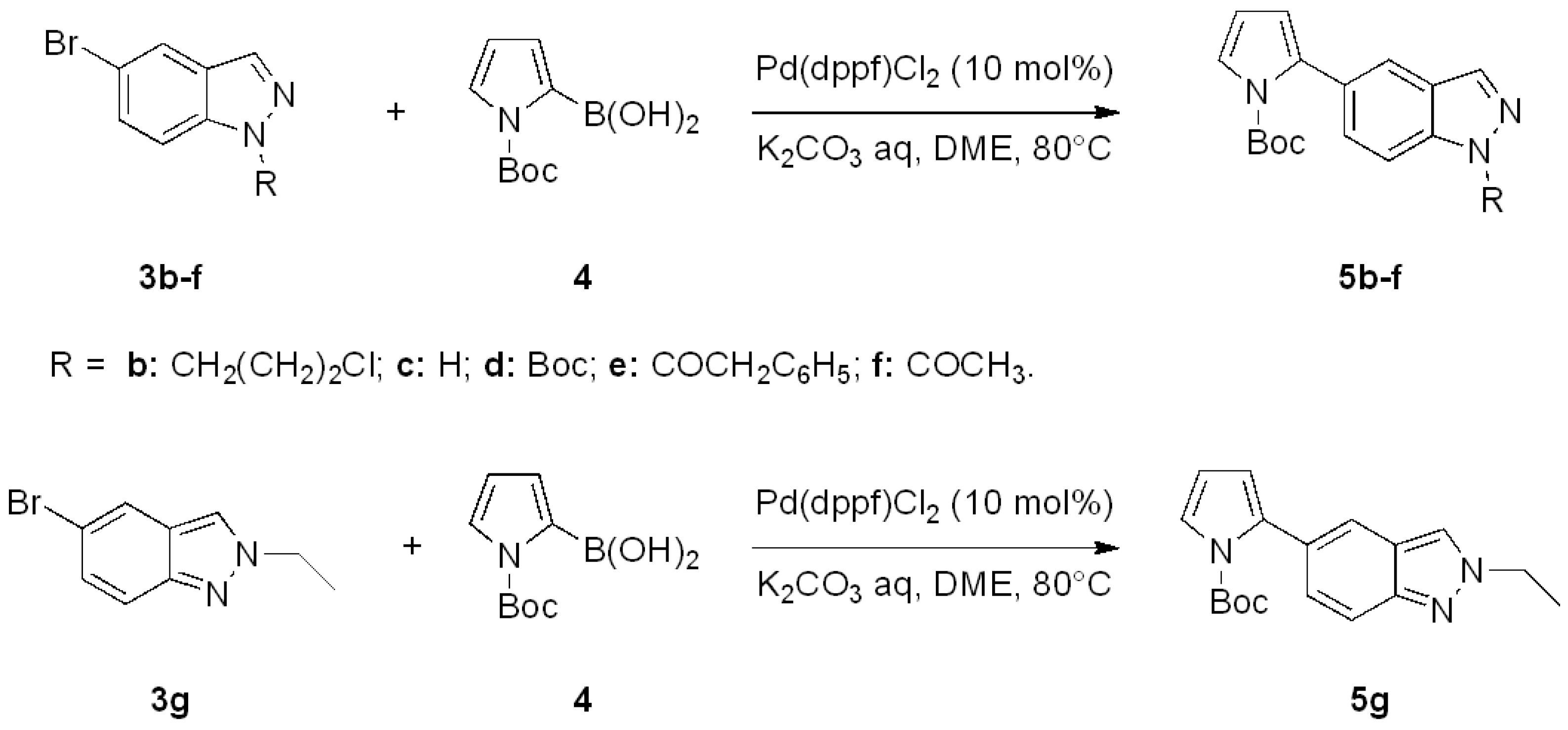
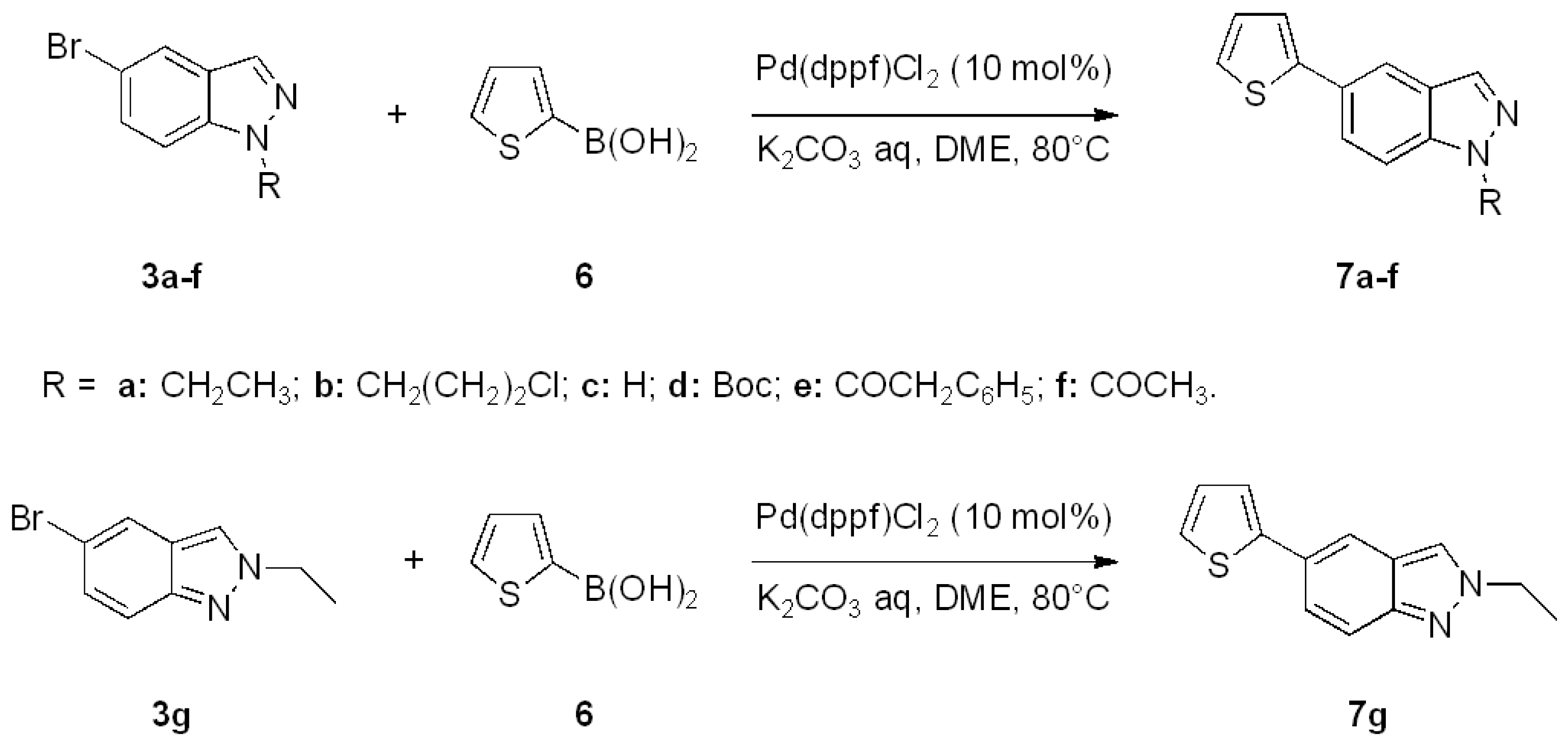
| Entry | Products 5 | Yield 5 [a] | Products 7 | Yield 7 [a] |
|---|---|---|---|---|
| a |  | 84% | 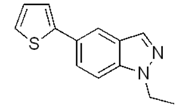 | 60% |
| b | 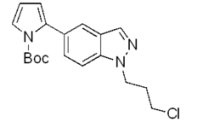 | 74% |  | 62% |
| c | 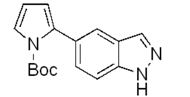 | 50% | 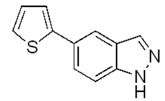 | traces |
| d | 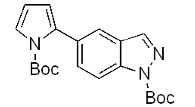 | 81% | 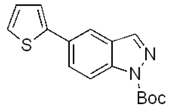 | 70% |
| e |  | 45% | 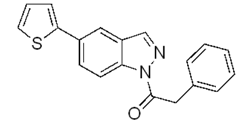 | 30% |
| f | 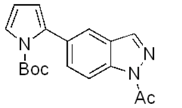 | 30% |  | 35% |
| g | 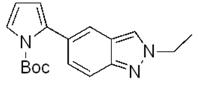 | 92% | 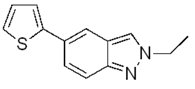 | 87% |
3. Experimental
3.1. General Experimental Methods
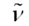 = 1728 cm−1. HRMS: calcd. for C15H11BrN2NaO 336.9952; found 336.9949.
= 1728 cm−1. HRMS: calcd. for C15H11BrN2NaO 336.9952; found 336.9949.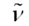 = 1726 cm−1. HRMS: calcd. for C9H7BrN2NaO 260.9639; found 260.9644.
= 1726 cm−1. HRMS: calcd. for C9H7BrN2NaO 260.9639; found 260.9644.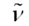 = 1733 cm−1. HRMS: calcd. for C18H21N3NaO2 334.1531; found 334.1526.
= 1733 cm−1. HRMS: calcd. for C18H21N3NaO2 334.1531; found 334.1526.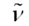 = 1725 cm−1. HRMS: calcd. for C18H21N3NaO2 334.1531; found 334.1536.
= 1725 cm−1. HRMS: calcd. for C18H21N3NaO2 334.1531; found 334.1536.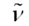 = 1725 cm−1. HRMS: calcd. for C19H22ClN3NaO2 382.1298; found 382.1299.
= 1725 cm−1. HRMS: calcd. for C19H22ClN3NaO2 382.1298; found 382.1299.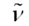 = 1734, 3469 cm−1. HRMS: calcd. for C16H17N3NaO2 306,1218; found 306.1223.
= 1734, 3469 cm−1. HRMS: calcd. for C16H17N3NaO2 306,1218; found 306.1223.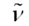 = 1736, 1737 cm−1. HRMS: calcd. for C21H25N3NaO4 406.1743; found 406.1740.
= 1736, 1737 cm−1. HRMS: calcd. for C21H25N3NaO4 406.1743; found 406.1740.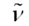 = 1731, 1739 cm−1. HRMS: calcd. for C24H23N3NaO3 424.1637; found 413.1632.
= 1731, 1739 cm−1. HRMS: calcd. for C24H23N3NaO3 424.1637; found 413.1632.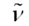 = 1719, 1778 cm−1. HRMS: calcd. for C18H19N3NaO3 348.1324; found 348.1320.
= 1719, 1778 cm−1. HRMS: calcd. for C18H19N3NaO3 348.1324; found 348.1320.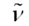 = 2988, 3002 cm−1. HRMS: calcd. for C13H13N2S 229.0799; found 229.0803.
= 2988, 3002 cm−1. HRMS: calcd. for C13H13N2S 229.0799; found 229.0803.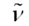 = 2978, 3037 cm−1. HRMS: calcd. for C13H13N2S 229.0799; found 229.0803.
= 2978, 3037 cm−1. HRMS: calcd. for C13H13N2S 229.0799; found 229.0803.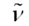 = 2966, 3001 cm−1. HRMS: calcd. for C14H14ClN2S 277.0566; found 277.0567.
= 2966, 3001 cm−1. HRMS: calcd. for C14H14ClN2S 277.0566; found 277.0567.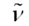 = 1743, 3001 cm−1. HRMS: calcd. for C16H16N2NaO2S 323.0830; found 323.0827.
= 1743, 3001 cm−1. HRMS: calcd. for C16H16N2NaO2S 323.0830; found 323.0827.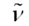 = 1713, 3001 cm−1. HRMS: calcd. for C19H14N2NaOS 341.0724; found 341.0727.
= 1713, 3001 cm−1. HRMS: calcd. for C19H14N2NaOS 341.0724; found 341.0727.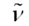 = 1713 cm−1. HRMS: calcd. for C13H10N2NaOS 265.0411; found 265.0412.
= 1713 cm−1. HRMS: calcd. for C13H10N2NaOS 265.0411; found 265.0412.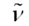 = 1728, 1774 cm−1. HRMS: calcd. for C18H19N3NaO4 364.1273; found 364.1270.
= 1728, 1774 cm−1. HRMS: calcd. for C18H19N3NaO4 364.1273; found 364.1270.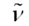 = 1716, 1774 cm−1. HRMS: calcd. for C19H21N3NaO4 378.1430; found 378.1434.
= 1716, 1774 cm−1. HRMS: calcd. for C19H21N3NaO4 378.1430; found 378.1434.4. Conclusions
Acknowledgements
References and Notes
- Cerecetto, H.; Gerpe, A.; González, M.; Arán, V.J.; de Ocáriz, C.O. Pharmacological properties of indazole derivatives: Recent developments. Mini Rev. Med. Chem. 2005, 5, 869–878. [Google Scholar]
- de Lena, M.; Lorusso, V.; Latorre, A.; Fanizza, G.; Gargano, G.; Caporusso, L.; Guida, M.; Catino, A.; Crucitta, E.; Sambiasi, D.; et al. Paclitaxel, cisplatin and lonidamide in advanced ovarian cancer. A phase II study. Eur. J. Cancer 2001, 37, 364–368. [Google Scholar]
- Woods, K.W.; Fischer, J.P.; Claiborne, A.; Li, T.; Thomas, S.A.; Zhu, G.; Diebold, R.B.; Liu, X.; Shi, Y.; Klinghofer, V.; et al. Synthesis and SAR of indazole-pyridine based protein kinase B/Akt inhibitors. Bioorg. Med. Chem. 2006, 14, 6832–6846. [Google Scholar]
- Zhao, D.; You, J.; Hu, C. Recent progress in coupling of two heteroarenes. Chem. Eur. J. 2011, 17, 5466–5492. [Google Scholar]
- Fraile, A.; Martín, M.R.; Ruano, J.L.G.; Díaz, J.A.; Arranz, E. Efficient synthesis of new 3-heteroaryl-1-functionalized 1H-indazoles. Tetrahedron 2011, 67, 100–105. [Google Scholar]
- Salovich, J.M.; Lindsley, C.W.; Hopkins, C.R. Synthesis of 1,3-diarylsubstituted indazoles utilizing a Suzuki cross-coupling/deprotection/N-arylation sequence. Tetrahedron Lett. 2010, 51, 3796–3799. [Google Scholar]
- Schmidt, A.; Beutler, A.; Snovydovych, B. Recent advances in the chemistry of indazoles. Eur. J. Org. Chem. 2008, 4073–4095. [Google Scholar]
- Schnürch, M.; Flasik, R.; Khan, A.F.; Spina, M.; Mihovilovic, M.D.; Stanetty, P. Cross-coupling reactions on azoles with two and more heteroatoms. Eur. J. Org. Chem. 2006, 3283–3307. [Google Scholar]
- El Kazzouli, S.; Bouissane, L.; Khouili, M.; Guillaumet, G. Synthesis of 4-substituted and 3,4-disubstituted indazole derivatives by palladium-mediated cross-coupling reactions. Tetrahedron Lett. 2005, 46, 6163–6167. [Google Scholar]
- Witulski, B.; Azcon, J.R.; Alayrac, C.; Arnautu, A.; Collot, V.; Rault, S. Sequential sonogashira and suzuki cross-coupling reactions in the indole and indazole series. Synthesis 2005, 5, 771–780. [Google Scholar]
- Bräse, S.; Gil, C.; Knepper, K. The recent impact of solid-phase synthesis on medicinally relevant benzoannelated nitrogen heterocycles. Bioorg. Med. Chem. 2002, 10, 2415–2437. [Google Scholar]
- Collot, V.; Dallemagne, P.; Bovy, P.R.; Rault, S. Suzuki-type cross-coupling reaction of 3-iodoindazoles with aryl boronic acids: a general and flexible route to 3-arylindazoles. Tetrahedron 1999, 55, 6917–6922. [Google Scholar]
- Yates, C.M.; Brown, P.J.; Stewart, E.L.; Patten, C.; Austin, R.J.H.; Holt, J.A.; Maglich, J.M.; Angell, D.C.; Sasse, R.Z.; Taylor, S.J.; et al. Structure guided design of 5-arylindazole glucocorticoid receptor agonists and antagonists. J. Med. Chem. 2010, 53, 4531–4544. [Google Scholar]
- Trujillo, J.I.; Kiefer, J.R.; Huang, W.; Thorarensen, A.; Xing, L.; Caspers, N.L.; Day, J.E.; Mathis, K.J.; Kretzmer, K.K.; Reitz, B.A.; et al. 2-(6-Phenyl-1H-indazol-3-yl)-1H-benzo[d]imidazoles: Design and synthesis of a potent and isoform selective PKC-ζ inhibitor. Bioorg. Med. Chem. Lett. 2009, 19, 908–911. [Google Scholar]
- Xie, W.; Herbert, B.; Ma, J.; Nguyen, T.M.; Schumacher, R.A.; Gauss, C.; Theim, A. Indoles, 1H-indazoles, 1,2-benzisoxazoles, and 1,2-benzisothiazoles, and preparation and uses thereof. WO2005/063767, 14 July 2005. [Google Scholar]
- Yasuma, T.; Sasaki, S.; Ujikawa, O.; Miyamoto, Y.; Gwaltney, S.L.; Cao, S.X.; Jennings, A. Preparation of indazole compounds as glucokinase activators for treating diabetes and obesity. WO2008/156757, 24 December 2008. [Google Scholar]
- Kerns, J.K.; Edwards, C. Preparation of indazole carboxamides as IKKβ kinase inhibitors for a treatment of a variety of disorders. WO2006/002434, 5 January 2006. [Google Scholar]
- Witherington, J.; Bordas, V.; Gaiba, A.; Naylor, A.; Rawlings, A.D.; Slingsby, B.P.; Smith, D.G.; Takle, A.K.; Ward, R.W. 6-Heteroaryl-pyrazolo[3,4-b]pyridines:potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorg. Med. Chem. Lett. 2003, 13, 3059–3062. [Google Scholar]
- Gasperi, T.; Loreto, M.A.; Norcia, G.; Tardella, P.A.; Furlotti, G. Regioselective synthesis of N-2 substituted indazoles as precursors of new pharmacophores. In Proceedings of XXIII Congresso Nazionale della Società Chimica Italiana, Sorrento, Na, Italy, July 2009.
- Prieto, M.; Zurita, E.; Rosa, E.; Muñoz, L.; Lloyd-Williams, P.; Giralt, E. Arylboronic acid and arylpinacolboronate esters in Suzuki coupling reactions involving indoles. Partner role swapping and heterocycle protection. J. Org. Chem. 2004, 69, 6812–6820. [Google Scholar]
- Tyrrell, E.; Brookes, P. The synthesis and applications of hetherocyclic boronic acids. Synthesis 2003, 469–483. [Google Scholar]
- Billingsley, K.; Buchwald, S.L. High efficient monophosphine-based catalyst for the palladium-catalyzed Suzuki–Miyaura reaction of heteroaryl halides and heteroaryl boronic acids and esters. J. Am. Chem. Soc. 2007, 129, 3358–3366. [Google Scholar]
- Barder, T.E.; Buchwald, S.L. Efficient catalyst fort he Suzuki-Miyaura coupling of potassium aryl trifluoroborates with aryl chlorides. Org. Lett. 2004, 6, 2649–2652. [Google Scholar]
- Kondolff, I.; Doucet, H.; Santelli, M. Suzuki coupling reactions of heteroarylboronic acids with aryl halides and arylboronic acids with heteroaryl bromides using a tetraphosphine/palladium catalyst. Synlett 2005, 13, 2057–2061. [Google Scholar]
- Thompson, A.E.; Hughes, G.; Batsanov, A.S.; Bryce, M.R.; Parry, P.R.; Tarbit, B. Palladium-catalyzed cross-coupling reactions of pyridylboronic acids with heteroaryl halides bearing a primary amine group: Synthesis of highly substituted bipyridines and pyrazinopyridines. J. Org. Chem. 2005, 70, 388–390. [Google Scholar]
- Kudo, N.; Perseghini, M.; Fu, G.C. A versatile method for suzuki cross-coupling reactions of nitrogen heterocycles. Angew Chem. Weinheim Bergstr. Ger. 2006, 45, 1282–1284. [Google Scholar]
- Guram, A.S.; King, A.O.; Allen, J.G.; Wang, X.; Schenkel, L.B.; Chan, J.; Bunel, E.E.; Faul, M.M.; Larsen, R.D.; Martinelli, M.J.; et al. New Air-stable catalysts for general and efficient Suzuki-Miyaura cross-coupling reactions of heteroaryl chlorides. Org. Lett. 2006, 8, 1787–1789. [Google Scholar]
- Kitamura, Y.; Sako, S.; Tsutsui, A.; Monguchi, Y.; Maegawa, T.; Kitade, Y.; Sajikia, H. Ligand-free and heterogeneous palladium on carbon-catalyzed hetero-Suzuki–Miyaura cross-coupling. Adv. Synth. Catal. 2010, 352, 718–730. [Google Scholar] [CrossRef]
- Kelly, T.A.; Fuchs, V.U.; Perry, C.W.; Snow, R.J. The efficient synthesis and simple resolution of a prolineboronate ester suitable for enzyme-inhibition studies. Tetrahedron 1993, 49, 1009–1016. [Google Scholar]
- Scott, J.D.; Stamford, A.W.; Gilbert, E.J.; Cumming, J.N.; Iserloh, U.; Wang, L.; Li, W. Iminothiadiazine dioxide compounds as BACE inhibitors and their preparation, compositions and use in the treatment of pathologies associated with beta-amyloid protein. WO/2011044187, 24 April 2011. [Google Scholar]
- Teixeira, F.C.; Ramos, H.; Antunes, I.F.; Curto, M.J.M.; Duarte, M.T.; Bento, I. Synthesis and structural characterization of 1- and 2-substituted indazoles: ester and carboxylic acid derivatives. Molecules 2006, 11, 867–889. [Google Scholar]
- Gusev, O.V.; Peganova, T.A.; Kalsin, A.M.; Vologdin, N.V.; Petrovskii, P.V.; Lyssenko, K.A.; Tsvetkov, A.V.; Beletskaya, I.P. Palladium complexes with metallocene-bridged bidentate diphosphine ligands: synthesis, structure, and catalytic activity in amination and cross-coupling reactions. Organometallics 2006, 25, 2750–2760. [Google Scholar]
- Shen, W. Palladium catalyzed coupling of aryl chlorides with arylboronic acids. Tetrahedron Lett. 1997, 38, 5575–5578. [Google Scholar]
- Gillespie, P.; Goodnow, R.A.J.; Tilley, J.W. Preparation of biaryloxymethylarenecarboxylic acids as antidiabetics. WO2006/058648, 8 June 2006. [Google Scholar]
- Yang, J.; Gharagozloo, P. Reaction of indazole with terminal di-bromoalkanes and their subsequent thermal cyclization: A facile entry into fused indazole ring system. Synth. Commun. 2005, 35, 409–415. [Google Scholar]
- Slade, D.J.; Pelz, N.F.; Bodnar, W.; Lampe, J.W.; Watson, P.S. Indazoles: Regioselective protection and subsequent amine coupling reaction. J. Org. Chem. 2009, 74, 6331–6334. [Google Scholar]
- Clutterbuck, L.A.; Posada, C.G.; Visintin, C.; Riddall, D.R.; Lancaster, B.; Gane, P.J.; Garthwaite, J.; Selwood, D.L. Oxadiazolylindazole sodium channel modulators are neuroprotective toward hippocampal neurones. J. Med. Chem. 2009, 52, 2694–2707. [Google Scholar]
- Crestey, F.; Lohou, E.; Stiebing, S.; Collot, V.; Rault, S. Protected indazole boronic acid pinacolyl esters: facile syntheses and studies of reactivities in Suzuki-Miyaura cross-coupling and hydroxydeboronation reactions. Synlett 2009, 615–619. [Google Scholar]
- de Rouville, H.J.; Villenave, D.; Rapenne, G. Synthesis of a photoswitchable azobenzene-functionalized tris(indazol-1-yl) borate ligand and its ruthenium(II) cyclopentadienide complex. Tetrahedron 2010, 66, 1885–1891. [Google Scholar]
- Mishra, R.; Jha, K.K.; Kumar, S.; Tomer, I. Synthesis, properties and biological activity of thiophene: A review. DerPharma Chemica 2011, 3, 38–54. [Google Scholar]
- Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. Development of catalyst-transfer condensation polymerization. Synthesis of p-conjugated polymers with controlled molecular weight and low polydispersity. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 753–765. [Google Scholar]
- Schmidt, A.; Snovydovych, B.; Habeck, T.; Dröttboom, P.; Gjikaj, M.; Adam, A. N-heterocyclic carbenes of 5-haloindazoles generated by decarboxylation of 5-haloindazolium-3-carboxylates. Eur. J. Org. Chem. 2007, 4909–4916. [Google Scholar]
- Britton, T.C.; Dehlinger, V.; Fivush, A.M.; Hollinshead, S.P.; Vokits, B.P. 3-Indazolyl-4-pyridinylisothiazoles as mGluR receptor inhibitors and their preparation, pharmaceutical compositions and use in the treatment of anxiety. WO2009/123855, 8 October 2009. [Google Scholar]
- Guzzo, P. Preparation of 5-pyridinone substituted indazoles as melanin-concentrating hormone (MCH1) receptor selective antagonists. WO2009/015037, 29 January 2009. [Google Scholar]
- Sample Availability: Samples of the compounds 7a, 7b and 7g are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Migliorini, A.; Oliviero, C.; Gasperi, T.; Loreto, M.A. The Suzuki Reaction Applied to the Synthesis of Novel Pyrrolyl and Thiophenyl Indazoles. Molecules 2012, 17, 4508-4521. https://doi.org/10.3390/molecules17044508
Migliorini A, Oliviero C, Gasperi T, Loreto MA. The Suzuki Reaction Applied to the Synthesis of Novel Pyrrolyl and Thiophenyl Indazoles. Molecules. 2012; 17(4):4508-4521. https://doi.org/10.3390/molecules17044508
Chicago/Turabian StyleMigliorini, Antonella, Chiara Oliviero, Tecla Gasperi, and Maria Antonietta Loreto. 2012. "The Suzuki Reaction Applied to the Synthesis of Novel Pyrrolyl and Thiophenyl Indazoles" Molecules 17, no. 4: 4508-4521. https://doi.org/10.3390/molecules17044508