Phytoecdysteroids from the Roots of Achyranthes bidentata Blume
Abstract
1. Introduction
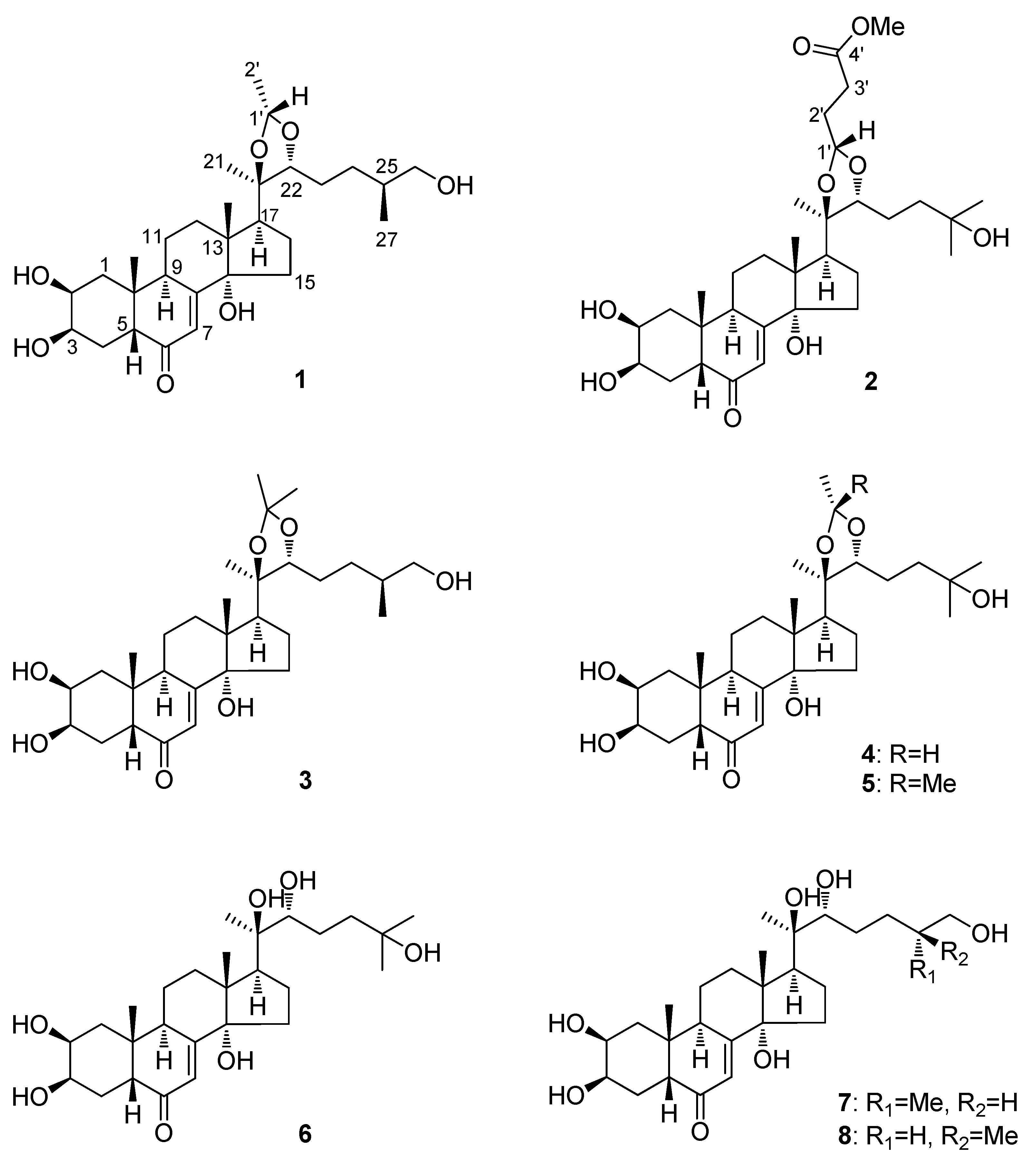
2. Results and Discussion
| No. | δH (1) a | δH (2) a | δH (3) a | δH (4) a | δH (8) b |
|---|---|---|---|---|---|
| 1 | 1.77 (m), 1.41(m) | 1.78 (m) | 1.78 (m) | 1.79(m) | 2.15 (m), 1.93 (m) |
| 2 | 3.82 (m) | 3.82 (m) | 3.82 (m) | 3.82 (m) | 4.19 (m) |
| 3 | 3.94 (m) | 3.94 (m) | 3.94 (m) | 3.94 (m) | 4.23 (m) |
| 4 | 1.73 (m), 1.69 (m) | 1.71 (m) | 1.73 (m), 1.69 (m) | 1.72 (m) | 2.04 (m), 1.82 (m) |
| 5 | 2.38 (m) | 2.36 (m) | 2.37 (m) | 2.35 (m) | 3.01 (dd, 13.2, 3.2) |
| 7 | 5.80 (d, 2.0) | 5.81 (s) | 5.80 brs | 5.80 (d, 2.0) | 6.26 (d, 1.6) |
| 9 | 3.13 (m) | 3.13 (m) | 3.13 (m) | 3.13 (m) | 3.60 (m) |
| 11 | 1.79 (m), 1.67 (m) | 1.79 (m), 1.68 (m) | 1.79 (m), 1.67 (m) | 1.78 (m), 1.68 (m) | 1.88 (m), 1.73(m) |
| 12 | 2.09 (m), 1.82 (m) | 2.09 (m), 1.83 (m) | 2.09 (m), 1.82 (m) | 2.09 (m), 1.83 (m) | 2.17 (m), 1.92 (m) |
| 15 | 1.94 (m), 1.60 (m) | 1.91 (m), 1.61 (m) | 1.94 (m), 1.60 (m) | 1.91 (m), 1.61 (m) | 2.60 (m), 2.04 (m) |
| 16 | 1.92 (m), 1.87 (m) | 1.92 (m) | 1.92 (m), 1.87 (m) | 1.93 (m) | 2.46 (m), 2.08 (m) |
| 17 | 2.32 (m) | 2.34 (m) | 2.28 (m) | 2.32 (m) | 2.95 (t, 9.2) |
| 18 | 0.85 (s) | 0.84 (s) | 0.81 (s) | 0.85 (s) | 1.22 (s) |
| 19 | 0.95 (s) | 0.95 (s) | 0.95 (s) | 0.96 (s) | 1.07 (s) |
| 21 | 1.13 (s) | 1.15 (s) | 1.15 (s) | 1.15 (s) | 1.59 (s) |
| 22 | 3.65 (dd, 8.8, 3.6) | 3.64 (m) | 3.68 (dd, 9.6, 2.8) | 3.63 (m) | 3.86 (d, 10.4) |
| 23 | 1.49 (m) | 1.54 (m) | 1.47 (m) | 1.52 (m) | 1.94 (m), 1.63 (m) |
| 24 | 1.50 (m), 1.15 (m) | 1.72 (m), 1.45 (m) | 1.68 (m), 1.15 (m) | 1.71 (m) | 2.17 (m), 1.41 (m) |
| 25 | 1.61 (m) | 1.63 (m) | 1.81 (m) | ||
| 26 | 3.34 (dd, 10.4, 6.4) | 1.19 (s) | 3.34 (dd, 10.4, 6.4) | 1.19 (s) | 3.64 (dd, 10.0, 6.4) |
| 3.42 (dd, 10.4, 5.6) | 3.42 (dd, 10.4, 5.6) | 3.76 (dd, 10.0, 5.2) | |||
| 27 | 0.93 (d, 6.8) | 1.20 (s) | 0.93 (d, 6.4) | 1.20 (s) | 1.03 (d, 6.4) |
| 1' | 5.05 (q, 4.8) | 4.97 (t, 4.0) | 5.05 (q, 4.8) | ||
| 2' | 1.29 (d, 4.8) | 1.90 (m) | 1.30 (s) | 1.29 (d, 4.8) | |
| 3' | 2.41 (t, 7.2) | 1.37 (s) | |||
| 4'-OCH3 | 3.65 (s) |
| Position | δC (1) a | δC (2) a | δC (3) a | δC (4) a | δC (8) b |
|---|---|---|---|---|---|
| 1 | 37.3 (t) | 37.3 (t) | 37.3 (t) | 37.3 (t) | 38.0 (t) |
| 2 | 68.7 (d) | 68.7 (d) | 68.7 (d) | 68.7 (d) | 68.1 |
| 3 | 68.5 (d) | 68.5 (d) | 68.5 (d) | 68.5 (d) | 68.1 |
| 4 | 32.9 (t) | 32.8 (t) | 32.8 (t) | 32.9 (t) | 32.5 (t) |
| 5 | 51.8 (d) | 51.8 (d) | 51.8 (d) | 51.8 (d) | 51.4 (d) |
| 6 | 206.4 (s) | 206.5 (s) | 206.4 (s) | 206.4 (s) | 203.5 (s) |
| 7 | 122.2 (d) | 122.2 (d) | 122.2 (d) | 122.1 (d) | 121.7 (d) |
| 8 | 167.5 (s) | 167.6 (s) | 167.6 (s) | 167.6 (s) | 166.1 (s) |
| 9 | 35.1 (d) | 35.1 (d) | 35.1 (d) | 35.1 (d) | 34.5 (d) |
| 10 | 39.2 (s) | 39.2 (s) | 39.2 (s) | 39.2 (s) | 38.7 (s) |
| 11 | 21.5 (t) | 21.5 (t) | 21.5 (t) | 21.5 (t) | 21.1 (t) |
| 12 | 32.2 (t) | 32.2 (t) | 32.3 (t) | 32.1 (t) | 31.8 (t) |
| 13 | 49.0 (s) * | 49.0 (s) * | 49.0 (s) * | 49.0 (s) * | 48.1 (s) |
| 14 | 85.2 (s) | 85.2 (s) | 85.3 (s) | 85.2 (s) | 84.2 (s) |
| 15 | 31.7 (t) | 31.7 (t) | 31.7 (t) | 31.7 (t) | 32.1 (t) |
| 16 | 22.6 (t) | 22.6 (t) | 22.4 (t) | 22.6 (t) | 21.7 (t) |
| 17 | 51.3 (d) | 51.4 (d) | 50.5 (d) | 51.3 (d) | 50.1 (d) |
| 18 | 17.6 (q) | 17.6 (q) | 17.6 (q) | 17.6 (q) | 17.9 (q) |
| 19 | 24.4 (q) | 24.4 (q) | 24.4 (q) | 24.4 (q) | 24.5 (q) |
| 20 | 85.3 (s) | 85.3 (s) | 85.7 (s) | 85.3 (s) | 77.3 (s) |
| 21 | 23.6 (q) | 23.4 (q) | 22.5 (q) | 23.7 (q) | 21.5 (q) |
| 22 | 85.5 (d) | 85.6 (d) | 83.1 (d) | 85.6 (d) | 76.8 (d) |
| 23 | 27.3 (t) | 24.6 (t) | 27.4 (t) | 24.6 (t) | 30.3 (t) |
| 24 | 32.0 (t) | 42.2 (t) | 32.0 (t) | 42.2 (t) | 32.0 (t) |
| 25 | 37.0 (d) | 71.1 (s) | 37.0 (d) | 71.1 (s) | 36.8 (d) |
| 26 | 68.2 (t) | 29.5 (q) | 68.2 (t) | 29.5 (q) | 67.4 (t) |
| 27 | 17.0 (q) | 28.9 (q) | 17.0 (q) | 28.9 (q) | 17.8 (q) |
| 1' | 102.3 (d) | 103.9 (d) | 108.0 (s) | 102.3 (d) | |
| 2' | 22.0 (q) | 31.0 (t) | 29.3 (q) | 22.0 (q) | |
| 3' | 29.2 (t) | 27.2 (q) | |||
| 4' | 175.6 (s) | ||||
| 4'-OCH3 | 52.1 (q) |
 ) correlations of 1 and 2.
) correlations of 1 and 2.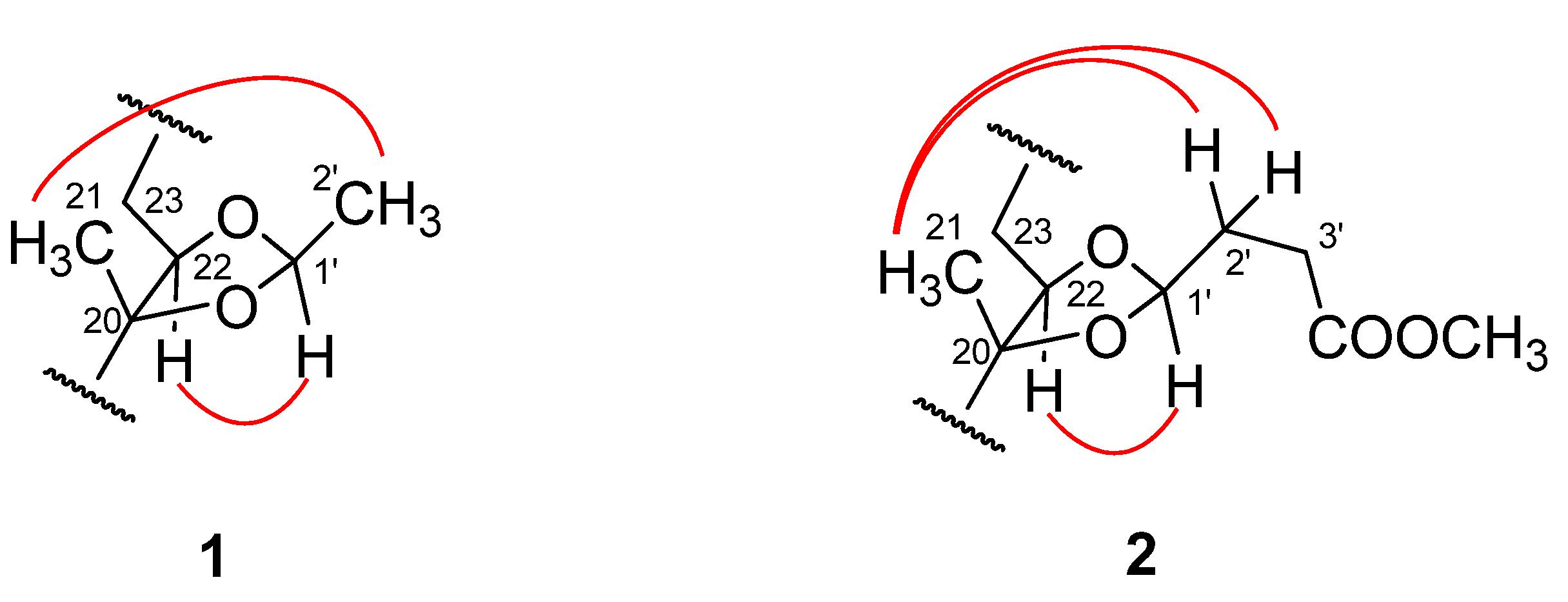
3. Experimental
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
 + 26.8 (c = 0.35, MeOH); IR (KBr) νmax 3419, 2934, 1654 ,1450, 1139, 1156 cm−1; UV (MeOH) λmax (log ε) nm: 242 (4.14); ESIMS (+) m/z 529 [M+Na]+, 507 [M+H]+; ESIMS (−) m/z 505 [M–H]–; HRESIMS (−) m/z 505.3173 [M−H]– (calcd. for C29H45O7, 505.3160); 1H-NMR (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data are shown in Table 1 and Table 2.
+ 26.8 (c = 0.35, MeOH); IR (KBr) νmax 3419, 2934, 1654 ,1450, 1139, 1156 cm−1; UV (MeOH) λmax (log ε) nm: 242 (4.14); ESIMS (+) m/z 529 [M+Na]+, 507 [M+H]+; ESIMS (−) m/z 505 [M–H]–; HRESIMS (−) m/z 505.3173 [M−H]– (calcd. for C29H45O7, 505.3160); 1H-NMR (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data are shown in Table 1 and Table 2. + 34.0 (c = 0.20, MeOH); IR (KBr) νmax 3423, 2964, 1737, 1654, 1382, 1139, 1058 cm−1; UV (MeOH) λmax (log ε) nm: 242 (3.75); ESIMS (+) m/z 601 [M+Na]+, 579 [M+H]+; ESIMS (−) m/z 577 [M−H]−; HREIMS m/z 578.3450 [M]+ (calcd. for C32H50O9, 578.3449); 1H-NMR (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data are shown in Table 1 and Table 2.
+ 34.0 (c = 0.20, MeOH); IR (KBr) νmax 3423, 2964, 1737, 1654, 1382, 1139, 1058 cm−1; UV (MeOH) λmax (log ε) nm: 242 (3.75); ESIMS (+) m/z 601 [M+Na]+, 579 [M+H]+; ESIMS (−) m/z 577 [M−H]−; HREIMS m/z 578.3450 [M]+ (calcd. for C32H50O9, 578.3449); 1H-NMR (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data are shown in Table 1 and Table 2.3.4. Acidic Hydrolysis of Compounds 1 and 3
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- He, C.C.; Hui, R.R.; Tezuka, Y.; Kadota, S.; Li, J.X. Osteoprotective effect of extract from Achyranthes bidentata in ovariectomized rats. J. Ethnopharmacol. 2010, 127, 229–234. [Google Scholar] [CrossRef]
- Ren, X.C.; Xu, X.X.; Xu, D.J.; Gao, J. Effects of Achyranthes bidentata saponins on bone metabolism of osteoporotis rats induced by retioic acid. Chin. J. Exp. Trad. Med. Form. 2011, 17, 128–130. [Google Scholar]
- Xiang, D.B.; Li, X.Y. Antitumor activity and immuno-potentiating actions of Achyranthes bidentata polysaccharides. Acta Pharmacol. Sin. 1993, 14, 556–561. [Google Scholar]
- Hu, J.; Qi, Y.X.; Li, Q.X.; Shan, B.E. The research of extract of Achyranthes bidentata Blume anti-tumor activity. Chin. J. Microbiol. Immunol. 2005, 25, 415–418. [Google Scholar]
- Wang, Y.F.; Wang, Q.D.; Liu, C.J.; Jiang, J.H.; Sun, W.X.; Xia, W.; Wu, Y. Antitumor activity of the crude saponins from Achyranthes bidentata. J. Henan Med. Univ. 1997, 32, 4–6. [Google Scholar]
- Tan, F.; Deng, J. Analysis of the constitutes and antisenile function of Achyranthes bidentata polysaccharides. Acta Bot.Sin. 2002, 44, 795–798. [Google Scholar]
- Deng, H.B.; Cui, D.P.; Jiang, J.M.; Feng, Y.C.; Cai, N.S.; Li, D.D. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on nonenzyme glycation in D-galactose induced mouse aging model. Biomed. Environ. Sci. 2003, 16, 267–275. [Google Scholar]
- Zhang, Z.Y.; Wang, S.K.; Guo, L.K. Aging changes of neurotensin-like and dynorphin-like neurons in the central nucleus of amygdala of the rat anti-aging effect of radix Achyranthis bidentatae: Immunohistochemical and image quantitative analytical study. Chin. J. Anat. 1996, 19, 123–127. [Google Scholar]
- Yi, S.; Ken, Y. New anti-inflammatory ergostane-type ecdysteroids from the sclerothium of Polyporus umbellatus. Biol. Med. Chem. Lett. 2008, 18, 3417–3420. [Google Scholar] [CrossRef]
- Chen, Q.H.; Liu, Z.Y.; He, J.H. Achyranthes bidentata polysaccharide enhances immune response in weaned piglets. Immunopharmacol. Immunotoxicol. 2009, 31, 253–260. [Google Scholar] [CrossRef]
- Xiang, D.B.; Li, X.Y. Effects of Achyranthes bidentata polysaccharides in interleukin-1 and tumor necrosis factor-alpha production from mouse peritoneal macrophages. Acta Pharmacol. Sin. 1993, 14, 332–336. [Google Scholar]
- Li, Z.K.; Li, D.D. The immunodulatory effect of Achyranthes bidentata polysaccharides. Yao Xue Xue Bao 1997, 32, 881–887. [Google Scholar]
- Li, C.C.; Hu, X.G.; Zhang, W.X.; Xie, L.W.; Zhang, H.Y.; Dong, L. Eosinophils apoptosis, fas mRNA and bcl-2 mRNA expressions in asthma model of young rat and effects of Achyranthes bidentata polysaccharides. Chin. J. Pediatr. 2003, 41, 657–660. [Google Scholar]
- Wang, Q.; Yang, L.; Jiang, H.; Wang, Z.B.; Yang, B.Y.; Kuang, H.X. Three new phytoecdysteroids containing a furan ring from the roots of Achyranthes bidentata Bl. Molecules 2011, 16, 5989–5997. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.T.; Meng, D.L.; Qiao, A.M. A new phytosterone from the roots of Achyranthes bidentata. Fitoterapia 2007, 78, 607–608. [Google Scholar] [CrossRef]
- Meng, D.L.; Li, X.; Wang, J.H.; Li, W. A new phytosterone from Achyranthes bidentata Bl. J. Asian Nat. Prod. Res. 2005, 7, 181–184. [Google Scholar] [CrossRef]
- Yu, B.; Tian, Z.Y.; Hui, Y.Z. Structural study on a bioactive fructan from the root of Achytanthes bidentata Bl. Chin. J. Chem. 1995, 13, 539–544. [Google Scholar]
- Chen, X.M.; Xu, Y.J.; Tian, G.Y. Physical-chemical properties and structure elucidation of abPS isolated from the root of Achyranthes bidentata. Yao Xue Xue Bao 2005, 40, 32–35. [Google Scholar]
- Li, J.X.; Hareyama, T.; Tezuka, Y.; Zhang, Y.; Miyahara, T.; Kadota, S. Five new oleanolic acid glycosides from Achyranthes bidentata with inhibitory activity on osteoclast formation. Planta Med. 2005, 71, 673–679. [Google Scholar] [CrossRef]
- Mitaine-Offer, A.C.; Marouf, A.; Hanquet, B.; Birlirakis, N.; Lacaille-Dubois, M.A. Two triterpene saponins from Achyranthes bidentata. Chem. Pharm. Bull. 2001, 49, 1492–1494. [Google Scholar] [CrossRef] [Green Version]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochemistry 2001, 57, 3346–3351. [Google Scholar]
- Buděšínský, M.; Vokáč, K.; Harmatha, J.; Cvǎcka, J. Additional minor ecdysteroid components of Leuzea carthamoides. Steroids 2008, 73, 502–514. [Google Scholar] [CrossRef]
- Zhu, T.T.; Liang, H.; Zhao, Y.Y.; Wang, B. Isolation and structure identification of C25 epimers of inokosterone from Achyranthes bidentata Blume. Yao Xue Xue Bao 2004, 39, 913–916. [Google Scholar]
- Odinokov, V.N.; Kumpun, S.; Galyautdinov, I.V.; Evrard-todeschi, N.; Veskina, N.A.; Khalilov, L.M.; Girault, J.P.; Dinan, L.; Maria, A.; Lafont, R. Low-polarity phytoecdysteroids from the juice of Serratulacoronata L. Collect. Czech. Chem. Commun. 2005, 70, 2038–2052. [Google Scholar] [CrossRef]
- The Ecdysone Handbook, 3rd ed. Available online: http://www.ecdybase.org/ (accessed on 8 March 2012).
- Píš, J.; Buděšínský, M.; Vokáč, K.; Laudová, V.; Hatmatha, J. Ecdysteroids from the roots of Leuzea carthamoides. Phytochemistry 1994, 37, 707–711. [Google Scholar] [CrossRef]
- Vokáč, K.; Buděšínský, M.; Harmatha, J.; Kohoutová, J. Ecdysteroid constituents of the mushroom Tapinella panuoides. Phytochemistry 1998, 49, 2109–2114. [Google Scholar] [CrossRef]
- Hikino, H.; Mohri, K.; Hikino, Y.; Arihara, S.; Takemoto, T. Inokosterone, aninsect metamorphosing substance from Achyranthes fauriei: Absolute configuration and synthesis. Tetrahedron 1976, 32, 3015–3021. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, M.; Zhou, Z.-Y.; Wang, J.; Cao, Y.; Chen, X.-X.; Zhang, W.-M.; Lin, L.-D.; Tan, J.-W. Phytoecdysteroids from the Roots of Achyranthes bidentata Blume. Molecules 2012, 17, 3324-3332. https://doi.org/10.3390/molecules17033324
Zhang M, Zhou Z-Y, Wang J, Cao Y, Chen X-X, Zhang W-M, Lin L-D, Tan J-W. Phytoecdysteroids from the Roots of Achyranthes bidentata Blume. Molecules. 2012; 17(3):3324-3332. https://doi.org/10.3390/molecules17033324
Chicago/Turabian StyleZhang, Mei, Zhong-Yu Zhou, Jing Wang, Yong Cao, Xue-Xiang Chen, Wei-Min Zhang, Li-Dong Lin, and Jian-Wen Tan. 2012. "Phytoecdysteroids from the Roots of Achyranthes bidentata Blume" Molecules 17, no. 3: 3324-3332. https://doi.org/10.3390/molecules17033324
APA StyleZhang, M., Zhou, Z.-Y., Wang, J., Cao, Y., Chen, X.-X., Zhang, W.-M., Lin, L.-D., & Tan, J.-W. (2012). Phytoecdysteroids from the Roots of Achyranthes bidentata Blume. Molecules, 17(3), 3324-3332. https://doi.org/10.3390/molecules17033324




