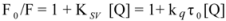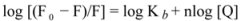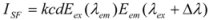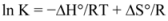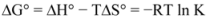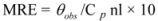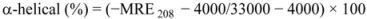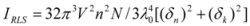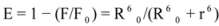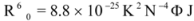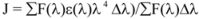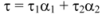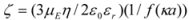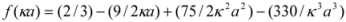2.1. Analysis of the Fluorescence Spectra
Fluorescence quenching is the decrease of the quantum yield of fluorescence from a fluorophore induced by a variety of molecular interactions with a quencher molecule, including excited-state reactions, molecular rearrangements, energy transfer, ground-state complex formation and collisional quenching processes. The fluorescence of hTf comes from the Trp, Tyr and Phe residues. As a matter of fact, the intrinsic fluorescence of hTf is almost exclusively contributed by Trp alone, since Phe has a very low quantum yield and the fluorescence of Tyr is practically totally quenched when ionized or close to an amino group, a carboxyl group, or a Trp. This viewpoint was backed by the experimental observation of Sulkowska
et al. [
22], when a small molecule was bound to hTf, changes of the intrinsic fluorescence intensity of hTf were induced by the micro-environment of the Trp and Tyr residues. The participation of Trp and Tyr groups was assessed through different excitation wavelengths. At a wavelength of 280 nm, both the Trp and Tyr residues in hTf became excited, whereas at 295 nm, this was the case only for the Trp residues.
Fluorescence spectroscopy is an extremely sensitive technique that has provided a wealth of insight into biological processes [
23]. By studying this quenching processes, one can obtain information about the binding of ROP and ASA to hTf, such as the binding mechanism, binding constant and binding site. An intrinsic fluorescence experiment was thus performed to evaluate the interaction of ROP and ASA with hTf.
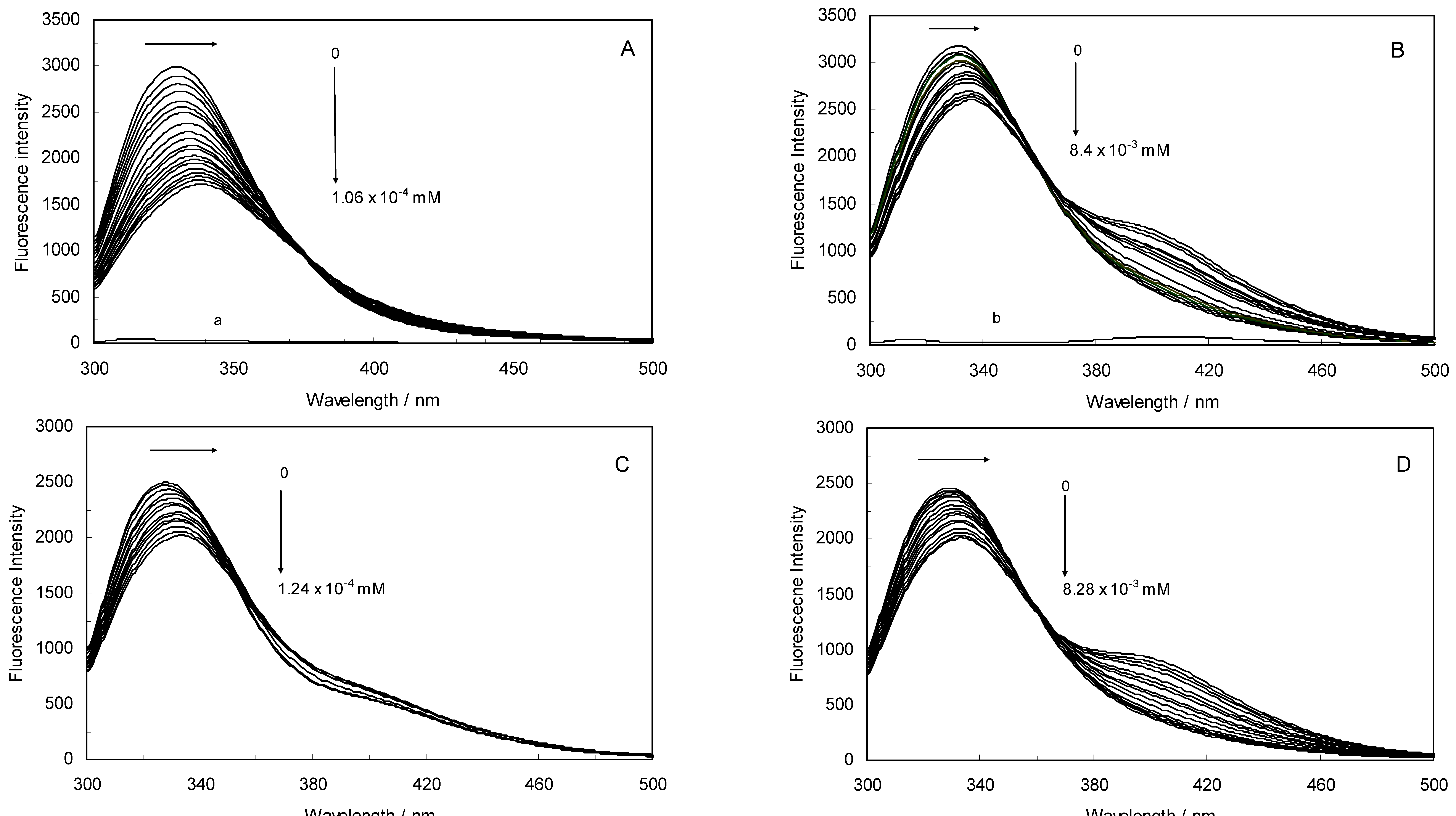
Figure 1A,B displays the fluorescence quenching of hTf induced by ROP and ASA (a single drug), excited at 280 nm. As can be seen, the fluorescence intensity of hTf gradually decreased upon increasing the concentration of ROP or ASA, indicating that the two drugs became bound to hTf. On the other hand, the emission spectra for binary systems portrayed an isobestic point, pointing at the existence of bound and free ROP and ASA in equilibrium. The quenching took place when the quencher was sufficiently close to Trp or/and Tyr residues [
24].
Figure 1.
(A) Fluorescence emission spectra of the hTf-ROP system. [hTf] = 3.8 × 10−3 mM and [ROP] was increased from 0 to 1.06 × 10−4 mM; (B) Fluorescence emission spectra of the hTf-ASA system. [hTf] = 3.8 × 10−3 mM and [ASA] was increased from 0 to 8.4 × 10−3 mM; (C) Fluorescence emission spectra of the (hTf-ASA)-ROP system. [hTf] = 3.8 × 10−3 mM and [ROP] was increased from 0 to 1.24 × 10−4 mM, [ASA] = 4.62 × 10−3 mM; (D) Fluorescence emission spectra of the (hTf-ROP)-ASA system. [hTf] = 3.8 × 10−3 mM and [ASA] was increased from 0 to 8.28 × 10−3 mM, [ROP] = 1.33 × 10−5 mM. The a and b represent the curves of ROP and ASA, respectively. All experiments were performed under identical conditions (T = 298 K; pH 7.4, λex = 280 nm). The horizontal arrow shows the red shift.
Figure 1.
(A) Fluorescence emission spectra of the hTf-ROP system. [hTf] = 3.8 × 10−3 mM and [ROP] was increased from 0 to 1.06 × 10−4 mM; (B) Fluorescence emission spectra of the hTf-ASA system. [hTf] = 3.8 × 10−3 mM and [ASA] was increased from 0 to 8.4 × 10−3 mM; (C) Fluorescence emission spectra of the (hTf-ASA)-ROP system. [hTf] = 3.8 × 10−3 mM and [ROP] was increased from 0 to 1.24 × 10−4 mM, [ASA] = 4.62 × 10−3 mM; (D) Fluorescence emission spectra of the (hTf-ROP)-ASA system. [hTf] = 3.8 × 10−3 mM and [ASA] was increased from 0 to 8.28 × 10−3 mM, [ROP] = 1.33 × 10−5 mM. The a and b represent the curves of ROP and ASA, respectively. All experiments were performed under identical conditions (T = 298 K; pH 7.4, λex = 280 nm). The horizontal arrow shows the red shift.
Figure 1C,D illustrate the fluorescence intensity of hTf by ROP and ASA (ternary systems), excited at 280 nm. The fluorescence of hTf was quenched upon interaction with ROP and ASA, and during the interactions, the isobestic point was observed. Under the experimental conditions, the ROP presented no emission spectra within the 300–500 nm and the emission from ASA was registered in the emission ranges 300–320 nm and 380–500 nm. The shift in maximum wavelength towards longer wavelengths (red shift) for the binary and ternary systems was believed to be due to the conformational changes induced by the interactions, leading to a further exposure of Trp and Tyr residues to a polar solvent and an increased polarity of the fluorophores [
25,
26].
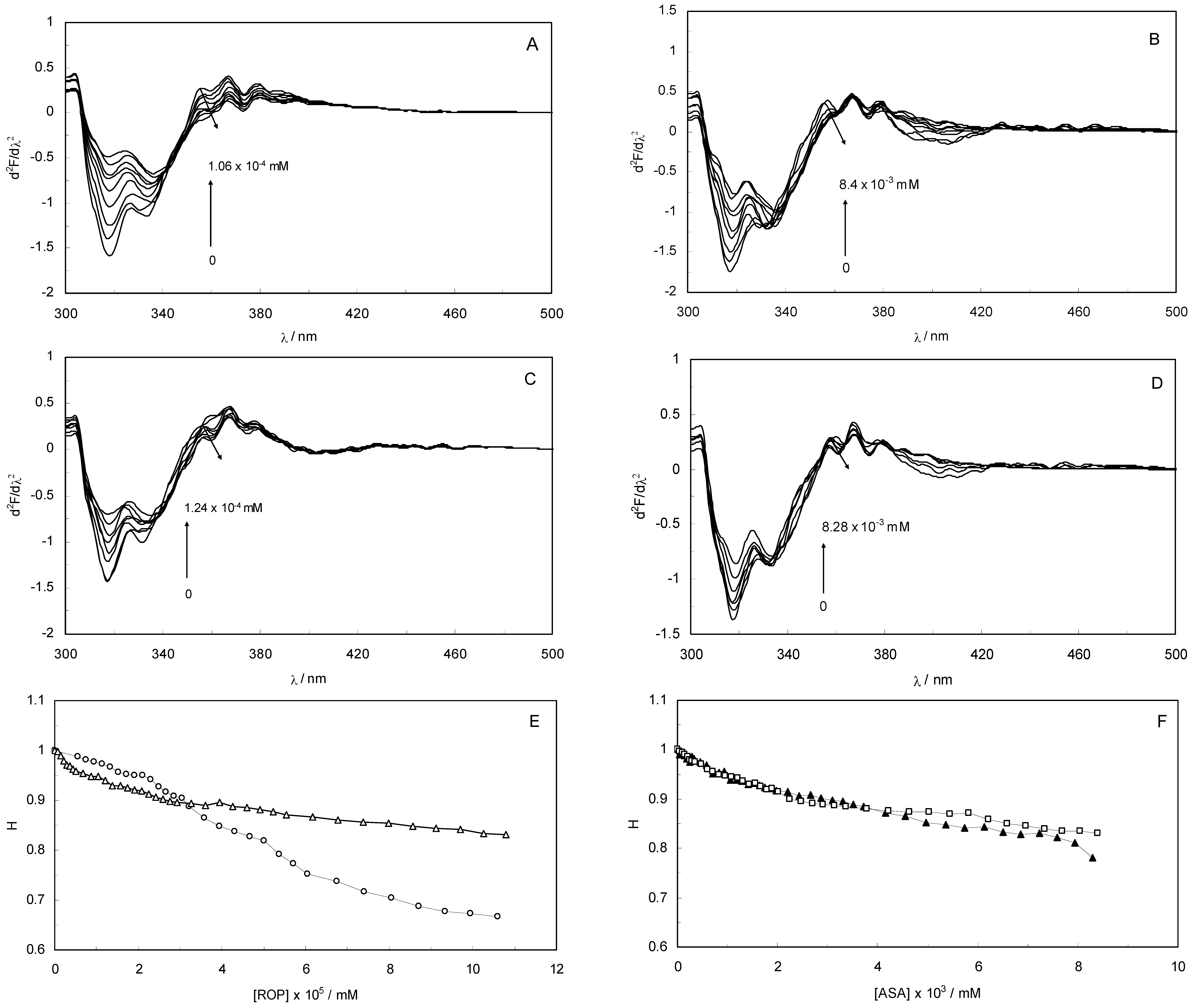
The overlapping of the quenching curves may indicate that different binding sites of ROP and ASA existed in sub-domains of hTf. In order to confirm the quenching mechanism, the fluorescence quenching data was analyzed by the Stern-Volmer equation:
where F
0 and F denote the steady-state fluorescence intensities in the absence and presence of a quencher, respectively; K
sv is the Stern-Volmer quenching constant; [Q] is the concentration of quencher; k
q is the quenching rate constant; and τ
0 is the average lifetime of the bio-molecule without quencher (
i.e., 10
−8 s) [
27,
28]. Hence, Equation (1) was applied to determine K
sv by linear regression of a plot of F
0/F against [Q]. The K
sv values for binary and ternary systems at different temperatures (298, 308 and 318 K) were calculated and are listed in
Table 1.
Table 1.
Thermodynamic parameters and Ksv values for the drugs when bound to hTf in binary and ternary systems at various temperatures (pH 7.4).
Table 1.
Thermodynamic parameters and Ksv values for the drugs when bound to hTf in binary and ternary systems at various temperatures (pH 7.4).
| System | T/K | KSV1/M−1 | KSV2/M−1 | ΔG°/kJ mol−1 | ΔH°/kJ mol−1 | ΔS°/J mol−1 | R |
|---|
| λ
ex
= 280 nm | λ
ex
= 280 nm |
|---|
| hTf-ROP | 298 | (1.16 ± 0.02) × 107 | (5.29 ± 0.02) × 106 | −36.93 ± 0.32 | | | 0.9982 |
| | 308 | (1.03 ± 0.02) × 107 | (5.05 ± 0.02) × 106 | −35.71 ± 0.26 | −79.17 ± 0.29 | −145.25 ± 0.31 | 0.9983 |
| | 318 | (9.83 ± 0.02) × 106 | (4.92 ± 0.02) × 105 | −35.01 ± 0.29 | | | 0.9981 |
| (hTf-ASA)ROP | 298 | (2.34 ± 0.03) × 105 | ------ | −36.17 ± 0.21 | | | 0.9976 |
| | 308 | (2.13 ± 0.03) × 105 | ------ | −35.83 ± 0.31 | −71.54 ± 0.32 | −120.22 ± 0.29 | 0.9983 |
| | 318 | (2.02 ± 0.03) × 105 | ------ | −35.14 ± 0.27 | | | 0.9981 |
| hTf-ASA | 298 | (2.94 ± 0.02) × 104 | ------ | −37.14 ± 0.27 | | | 0.9978 |
| | 308 | (2.47 ± 0.02) × 104 | ------ | −36.21 ± 0.27 | −69.32 ± 0.14 | −110.36 ± 0.31 | 0.9980 |
| | 318 | (2.15 ± 0.02) × 104 | ------ | −35.41 ± 0.31 | | | 0.9977 |
| (hTf-ROP)ASA | 298 | (3.35 ± 0.03) × 104 | ------ | −36.81 ± 0.33 | | | 0.9985 |
| | 308 | (3.02 ± 0.03) × 104 | ------ | −36.04 ± 0.27 | −81.21 ± 0.30 | −151.76 ± 0.28 | 0.9991 |
| | 318 | (2.77 ± 0.03) × 104 | ------ | −31.11 ± 0.30 | | | 0.9983 |
The results demonstrated that the values of Stern-Volmer quenching constants, K
sv, decreased with rising temperatures, which indicated that the possible quenching mechanism of fluorescence of hTf by ROP and ASA, were of the static quenching type. Furthermore, the maximum diffusion collision quenching rate constant, k
q, of various quenchers with the biopolymer is 2.0 × 10
10 L mol
−1 s
−1, which revealed that the probable quenching mechanism of binary and ternary systems were initiated by complexes formation rather than by dynamic collision. Since static quenching is due to ground-state complexes formation between the fluorophore and quenchers [
23,
24,
25,
29].
The Stern-Volmer quenching constant increased with an increasing affinity binding of hTf, ROP and ASA.
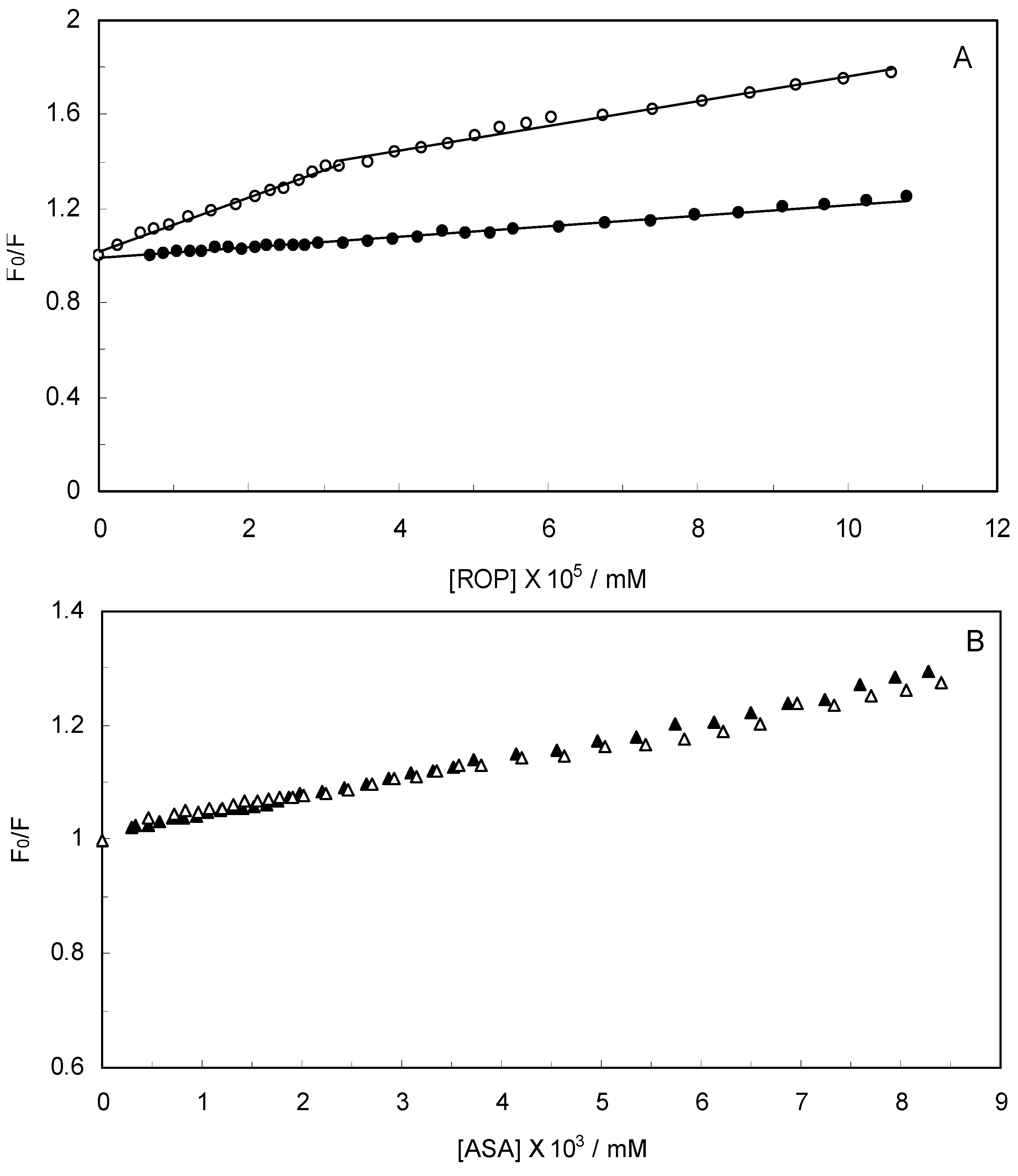
Figure 2 displays the Stern-Volmer plots of binary (hTf-ROP or ASA) and ternary [(hTf-ROP)-ASA and (hTf-ASA)-ROP] systems excited at 280 nm. The Stern-Volmer dependencies of hTf-ROP (
Figure 2A) demonstrated a deviation from the straight line when the concentration of ROP was higher than 3.22 × 10
−5 mM. Therefore, the hTf could be quenched by two binding sites with different interaction behavior. The quenching was not purely collisional but may be due to the formation of either the ground-static complex or the static quenching process. Alternatively, there might be more than one independent binding site on the hTf for ROP and they are not all accessible to the hTf-ROP complex. The Stern-Volmer linear curves for (hTf-ASA)-ROP, (hTf-ASA) and (hTf-ROP)-ASA (see
Figure 2B) revealed that static quenching occurred when these drugs approached the environment of the hTf fluorophores.
Figure 2.
(A) Stern-Volmer plots for the binding of ROP to hTf in the absence and presence of ASA. [ASA] = 4.62 × 10−3 mM, binary system (○); ternary system (●); (B) Stern-Volmer curves for the binding of ASA to hTf in the absence and presence of ROP. [ROP] = 1.33 × 10−5 mM, binary system (∆); ternary system (▲).
Figure 2.
(A) Stern-Volmer plots for the binding of ROP to hTf in the absence and presence of ASA. [ASA] = 4.62 × 10−3 mM, binary system (○); ternary system (●); (B) Stern-Volmer curves for the binding of ASA to hTf in the absence and presence of ROP. [ROP] = 1.33 × 10−5 mM, binary system (∆); ternary system (▲).
2.2. Binding Parameters
When small molecules bind independently to a set of equivalent sites on a macromolecule, the binding constant (Kb) and the number of binding sites (n) can be determined by the following equation:
where F
0 and F are the fluorescence intensity of the protein in the absence and presence of a quencher, and [Q] is the quencher concentration [
30]. The values of K
b and n for binary and ternary systems were calculated and are presented in
Table 2.
Table 2.
The binding constants (Kb) and the number of binding sites (n), for the binary and ternary systems at pH = 7.4 and T = 298 K.
Table 2.
The binding constants (Kb) and the number of binding sites (n), for the binary and ternary systems at pH = 7.4 and T = 298 K.
| System | K
b ( L mol−1) | n | R | K
b (L mol−1) | n | R |
|---|
| λ
ex
= 280 nm | λ
ex
= 280 nm | λ
ex
= 295 nm | λ
ex
= 295 nm |
|---|
| hTf-ROP | K
b1 = (1.69 ± 0.03) × 106 | N1 = 0.82 | 0.997 | (3.75 ± 0.03) × 108 | 1.52 | 0.998 |
| K
b2 = (1.86 ± 0.03) × 105 | N2 = 0.59 | 0.998 |
| hTf-ASA | (7.83 ± 0.02) × 103 | 0.74 | 0.999 | (5.51 ± 0.02) × 103 | 0.78 | 0.998 |
| (hTf-ASA)-ROP | (8.42 ± 0.02) × 105 | 1.14 | 0.998 | (5.44 ± 0.02) × 106 | 1.20 | 0.996 |
| (hTf-ROP)-ASA | (2.06 ± 0.02) × 104 | 0.89 | 0.997 | (3.58 ± 0.02) × 104 | 1.19 | 0.995 |
According to Equation (2), the Hill plot of hTf-ROP can be fitted with two lines, which would indicate the presence of two binding sites with different Kb values. Each linear function represents the ROP interaction to hTf with special behavior, whereas one saturable binding site was found for hTf-ASA in the absence and presence of ROP. The strong binding of ROP to hTf took place or that the transfer of energy from excited fluorophores to ROP molecule facilitated. This suggests that the hTf fluorescence was significantly more quenched by ROP. The decrease in binding constant revealed the availability of higher concentrations of free drugs in the plasma.
2.3. Analysis of Second Derivative Fluorescence Spectra
Second derivative fluorescence spectroscopy is a sensitive and reliable technique for monitoring and characterizing the transitions that take place in the Trp micro-environment of proteins.
Figure 3A–D display the second derivative fluorescence spectra for binary and ternary systems, featuring two negative bands at 319 nm and 334 nm. The origin of these bands was presumably the transition of the electrons back to the different vibration levels of the ground state. When the concentrations of ROP and ASA in the binary and ternary systems were increased, the intensity of the shortest wavelength band was altered. The increase in the intensity of the shortest wavelength band was observed upon increasing the concentration of ROP and ASA, signifying that the binding of ROP and ASA to hTf became affected in addition to conformational changes in hTf and the micro-environment of the Trp residues. As can be seen in
Figure 3 the negative band at 319 nm for the binary and ternary systems was the most sensitive to the changes in the tertiary structure of hTf upon addition of ROP and ASA. The structure of the C-lobe of hTf imposed extra rigidity and less flexibility as opposed to the N-lobe [
8,
9,
31], for which reason the negative band at 319 nm was related to the conformational changes of the N-lobe. Hence, the modifications of the tertiary structure of the C-lobe concerned the negative band at 334 nm. This result demonstrated the partially unfolded states of hTf.
Figure 3.
(A) The second derivative fluorescence spectra of hTf in the presence of ROP; (B) The second derivative fluorescence spectra of hTf in the presence of ASA; (C) The second derivative fluorescence spectra for the (hTf-ASA)-ROP system; (D) The second derivative fluorescence spectra for the (hTf-ROP)-ASA system; (E) A plot of the H versus [ROP] for hTf-ROP in the absence and presence ASA. Binary system (○); ternary system (∆). [ASA] = 4.62 × 10−3 mM for the ternary system; (F) A plot of the H versus [ASA] for hTf-ASA in the absence and presence ROP. Binary system (□); ternary system (▲). [ROP] = 1.33 × 10−5 mM for the ternary system.
Figure 3.
(A) The second derivative fluorescence spectra of hTf in the presence of ROP; (B) The second derivative fluorescence spectra of hTf in the presence of ASA; (C) The second derivative fluorescence spectra for the (hTf-ASA)-ROP system; (D) The second derivative fluorescence spectra for the (hTf-ROP)-ASA system; (E) A plot of the H versus [ROP] for hTf-ROP in the absence and presence ASA. Binary system (○); ternary system (∆). [ASA] = 4.62 × 10−3 mM for the ternary system; (F) A plot of the H versus [ASA] for hTf-ASA in the absence and presence ROP. Binary system (□); ternary system (▲). [ROP] = 1.33 × 10−5 mM for the ternary system.
The H parameter (H = h/h′) depended on the dielectric constant of the solvent and was used as an empirical measure of the effect of the relative hydrophobicity of solvent in the second derivative of the spectra. Here, h is the difference in intensities between the minimum, around 320–350 nm, and the shoulder, at 370 nm, and h′ represents the difference in intensities between the minimum, around 320–350 nm, and the maximum, at 400 nm. H is the only empirical parameter and is related to changes in the degree of polarity in the environment of all the Trp residues in hTf [
32].
Figure 3E,F present a plot of the H values versus the concentrations of ROP and ASA for the binary and ternary systems, and as can be seen, a decrease in H values upon addition of ROP and ASA indicated a red shift. The polarity around the Trp residues was increased and the hydrophobicity was decreased, causing a further exposure of Trp residues from the core of hTf to the polar solvent.
Figure 3E shows a higher slope for the binding of ROP to hTf, probably due to the conformational changes of the Trp residues and the further exposure to the polar solvent. As can be seen in
Figure 3F, the hTf-ASA and (hTf-ROP)-ASA spectra overlapped, revealing that ROP did not affect or only weakly participated in the changes of the tertiary structure of hTf, in the presence of ASA.
2.4. Synchronous Fluorescence
The synchronous fluorescence spectroscopy technique was introduced by Lioyd in 1971. The method provides information about the molecular environment in the vicinity of chromophore molecules with several advantages, such as sensitivity, spectral simplification, spectral bandwidth reduction and the avoidance of several perturbing effects. According to the theory of Miller, when the D-value (Δλ) between excitation and emission wavelengths becomes stabilized at 15 nm or 60 nm, the synchronous fluorescence gives the characteristic information of Trp or Tyr residues [
33,
34]. Thus, the synchronous fluorescence applied to the synchronous luminescence is:
where I
SF is the relative intensity of synchronous fluorescence; E
ex is the excitation function at the given excitation wavelength; E
em is the normal emission function at the corresponding emission wavelength; c is the analytical concentration; d is the thickness of the sample cell; and k is the characteristic constant comprising the instrumental geometry factor and related parameters. Due to the relationship of the synchronous fluorescence intensity (I
SF) and the concentration of hTf, I
SF should be in direct proportion to the concentration of hTf [
35].
The synchronous fluorescence of hTf at Δλ = 15 nm and 60 nm are characteristic of Tyr and Trp residues, respectively. It is considered that the λ
max of the Trp residues are relative to the polarity of the micro-environment. As reported, the λ
max at 330–332 nm indicates that Trp residues are located in the nonpolar region. In other words, they are buried in a hydrophobic cavity in hTf. The λ
max at 350–352 nm shows that Trp residues are exposed to polar solvent, hence, the hydrophobic cavity in hTf is disagglomerated and the structure of hTf is looser [
36].
For the first time, ROP and ASA were used to determine the hTf with a synchronous fluorescence technique.
Figure 4 shows the Trp and Tyr residues of the fluorescence spectra of hTf at various concentrations of ROP and ASA for binary systems, respectively. It is apparent from
Figure 4A,B that the addition of ROP and ASA to hTf (binary and ternary systems) led to a dramatic decrease in the intensity of fluorescence emission maximum of the Trp and Tyr residues. As seen in
Figure 4A,B, the maximum emission wavelength of the Trp and Tyr residues do not have a significant shift. As displayed in
Figure 4C,D for hTf-ASA complex and ternary systems (data not shown), the emission maximum of Trp and Tyr residues were red-shifted, which demonstrated that the polarity of the fluorophores increased, causing the hydrophobic cavities to be moved to a more hydrophilic environment.
Figure 4.
(A) Synchronous fluorescence spectra of the hTf-ROP system. [hTf] = 3.8 × 10−3 mM and [ROP] was increased from 0 to 1.06 × 10−4mM, ∆λ = 60 nm; (B) Synchronous fluorescence spectra of the hTf-ROP system. [hTf] = 3.8 × 10−3mM and [ROP] was increased from 0 to 1.06 × 10−4mM, ∆λ = 15 nm; (C) Synchronous fluorescence spectra of the hTf-ASA system. [hTf] = 3.8 × 10−3 mM and [ASA] was increased from 0 to 8.4 × 10−3 mM, ∆λ = 60 nm; (D) Synchronous fluorescence spectra of the hTf-ASA system. [hTf] = 3.8 × 10−3mM and [ASA] was increased from 0 to 8.4 × 10−3mM, ∆λ = 15 nm.
Figure 4.
(A) Synchronous fluorescence spectra of the hTf-ROP system. [hTf] = 3.8 × 10−3 mM and [ROP] was increased from 0 to 1.06 × 10−4mM, ∆λ = 60 nm; (B) Synchronous fluorescence spectra of the hTf-ROP system. [hTf] = 3.8 × 10−3mM and [ROP] was increased from 0 to 1.06 × 10−4mM, ∆λ = 15 nm; (C) Synchronous fluorescence spectra of the hTf-ASA system. [hTf] = 3.8 × 10−3 mM and [ASA] was increased from 0 to 8.4 × 10−3 mM, ∆λ = 60 nm; (D) Synchronous fluorescence spectra of the hTf-ASA system. [hTf] = 3.8 × 10−3mM and [ASA] was increased from 0 to 8.4 × 10−3mM, ∆λ = 15 nm.
Figure 5A displayed a higher slope when Δλ was 15 nm, indicating a significant contribution of Tyr residues of hTf in the absence and presence of ASA, and ROP closer to Tyr residues as compared to Trp residues. The binding of ROP with hTf when Δλ was 60 nm, revealing a contribution of Trp residues in the fluorescence of hTf (without the ASA), and ROP closer to the Trp residues. The synchronous fluorescence quenching of hTf by ROP, in the presence of ASA, differed only slightly when Δλ was 15 nm (data not shown). Consequently, this suggested that the ROP and ASA approaching the Tyr residues were equal.
Figure 5B demonstrates that the slope was similar for the hTf-ASA system whether Δλ was 15 nm or 60 nm, indicating that the opportunity of ASA to approach the Tyr and Trp residues was the same. Hence, it could be concluded that ASA bound to the central cavity of hTf thus forming an hTf-ASA complex [
37].
It is also shown, in the inset of
Figure 5B that the slope was higher when Δλ was 60 nm, demonstrating a significant contribution of Trp residues of hTf in the presence of ROP, and the fact that ASA was closer to the Trp residues as compared to the Tyr residues. Indeed, the structure of the micro-environment of the Trp residues was altered by ASA in the presence of ROP. The higher slope for (hTf-ROP)-ASA when Δλ was 60 nm, which was the result of a main contribution of Trp residues of hTf in the presence of ROP, bringing ASA closer to the Trp residues (data not shown). One can see that the slope was similar when Δλ was 15 nm, revealing that ASA and ROP in (hTf-ROP)-ASA approached the Tyr residues (data not shown).
Figure 5.
(A) Synchronous fluorescence spectra of the quenching of hTf by ROP. (○) ∆λ = 15 nm and (●) ∆λ = 60 nm. Inset (A) Synchronous fluorescence spectra of the quenching of hTf by ROP in the presence of ASA. (□) ∆λ = 15 nm and (■) ∆λ = 60 nm; (B) Synchronous fluorescence spectra of the quenching of hTf by ASA. (∆) ∆λ = 15 nm and (▲) ∆λ = 60 nm. Inset (B) Synchronous fluorescence spectra of (hTf-ROP)-ASA system. (□) ∆λ = 15 nm and (■) ∆λ = 60 nm.
Figure 5.
(A) Synchronous fluorescence spectra of the quenching of hTf by ROP. (○) ∆λ = 15 nm and (●) ∆λ = 60 nm. Inset (A) Synchronous fluorescence spectra of the quenching of hTf by ROP in the presence of ASA. (□) ∆λ = 15 nm and (■) ∆λ = 60 nm; (B) Synchronous fluorescence spectra of the quenching of hTf by ASA. (∆) ∆λ = 15 nm and (▲) ∆λ = 60 nm. Inset (B) Synchronous fluorescence spectra of (hTf-ROP)-ASA system. (□) ∆λ = 15 nm and (■) ∆λ = 60 nm.
2.5. Thermodynamic Parameters and Nature of the Binding Forces
The interaction forces a small molecule and macromolecule commonly include hydrophobic force, electrostatic interactions, van der Waals interactions and hydrogen bond,
etc. The thermodynamic parameters dependent on temperature are calculated according to the van’t Hoff equation in order to elucidate the interaction forces between hTf, ROP and ASA and are listed in
Table 1:
Thus the enthalpy change (ΔH°) and entropy (ΔS°) for the binding reaction can be determined from a plot of ln K versus 1/T (spectra are not shown). Subsequently the free energy change (ΔG°) is calculated by the following equation:
where K is the binding constant at the corresponding temperature and R is the gas constant [
28,
29,
30,
38]. The negative sign for ΔG° means that the binding process was spontaneous. The negative values of entropy indicated that the binding was mainly entropy-driven and the enthalpy values were unfavorable for binary and ternary systems. Ross and Subramanian have characterized the sign and magnitude of the thermodynamic parameter associated with various individual kinds of interaction that may take place in protein association process, which can be easily concluded as: (a) ΔH° > 0 and ΔS° > 0, hydrophobic force; (b) ΔH° < 0 and ΔS° < 0, van der Waals force and hydrogen bond; (c) ΔH° < 0 ΔS° > 0, electrostatic interactions [
39]. Thus, from the thermodynamic characteristics summarized above, the negative ΔH° and ΔS° values demonstrate that van der Waals force and hydrogen bond played major roles in the binding of ROP and ASA to hTf and contributed to the stability of the complexes.
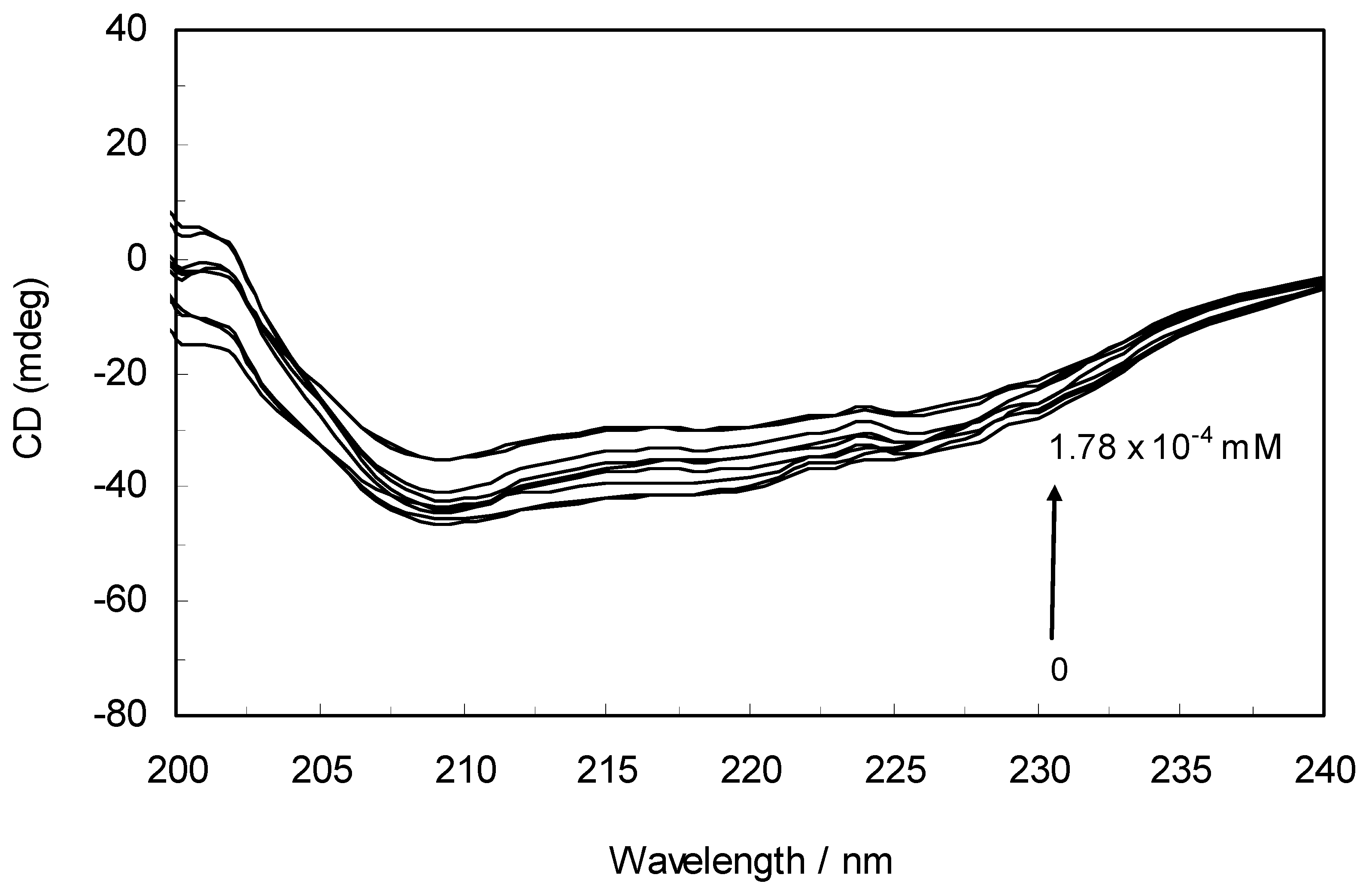
2.6. Circular Dichroism (CD) Analysis
Circular dichroism is an extremely convenient technique for detecting and monitoring the extent of conformational changes that may be associated with the activity or regulation of a protein. To further verify the possible influence of ROP and ASA (binary and ternary systems) on the secondary structure of hTf, far-UV CD studies (free and within the mixture) were performed with ROP and ASA.
The CD spectra of hTf in the absence and presence of ROP are displayed in
Figure 6. It was apparent that these spectra exhibited two negative bands in the UV region at 208 nm and 222 nm, characteristic of an α-helical structure of hTf. A reasonable explanation is that the negative peaks at 208 nm and 222 nm both resulted from n → π
* transfer for the peptide bond of the α-helix. In addition, the band intensities of hTf at 208 nm and 222 nm decreased with the negative cotton effect through the binding of ROP without causing conspicuous shifts of the peaks, clearly indicating that ROP induced a slight decrease in the α-helical structure content of hTf. From the above results, it was evident that the effect of ROP on hTf caused secondary structural changes to the hTf with loss of the helical stability [
40].
Figure 6.
Far-UV CD spectra of hTf in the absence and presence of ROP. pH 7.4 and T = 298 K. [hTf] = 3.8 × 10−3 mM. The concentrations of ROP ranged from 0 to 1.78 × 10−4 mM.
Figure 6.
Far-UV CD spectra of hTf in the absence and presence of ROP. pH 7.4 and T = 298 K. [hTf] = 3.8 × 10−3 mM. The concentrations of ROP ranged from 0 to 1.78 × 10−4 mM.
The CD results are expressed as MRE (Mean Residue Ellipticity) in deg cm2 dmol−1, which is defined as:
where θobs is the CD in milli-degree; Cp is the mole fraction; n is the number of amino acid residues (679); and l is the path length of the cell. The α-helical content could be calculated from the MRE values at 208 nm using the following equation:
where MRE208 is the observed MRE value at 208 nm; 4,000 is the MRE of the form and random coil conformation cross at 208 nm; and 33,000 is the MRE value of an α-helix at 208 nm.
Table 3 demonstrate the effect of ROP and ASA (in binary and ternary systems) on the relative amounts of α-helical, β-sheet, turn and unordered coil fractions in hTf. Since there occurred a decrease of the content of α-helix whereas there was an increase in β-sheet, turn and unordered coil contents in the hTf-ROP complex, the fraction of secondary structure was closely related to the biological activity of hTf. A decrease in α-helical structure from 37.4% to 33.7% reveals a loss of the biological activity of hTf with the highest concentration of ROP. Hence, hTf adopted a more incompact conformation state [
34,
35,
41,
42]. These results indicated an increase of the α-helix fraction and a decrease of the contents of β-sheet, turn and unordered coil in hTf-ASA in the absence and presence of ROP and (hTf-ROP)-ASA complexes. The increase of the secondary helical structure content demonstrated that ASA induced this structure in hTf.
Table 3.
Fractions of the secondary structure and binding distance (r) values of hTf with ROP and ASA complexes as binary and ternary systems, at pH = 7.4 and T = 298 K.
Table 3.
Fractions of the secondary structure and binding distance (r) values of hTf with ROP and ASA complexes as binary and ternary systems, at pH = 7.4 and T = 298 K.
| System | α-helix % | β-sheet % | Turn % | Unordered % | r/nm |
|---|
| hTf | 37.4 | 28.3 | 15.4 | 18.9 | ----- |
| hTf-ROP | 33.7 | 29.4 | 16.5 | 20.4 | 1.92 |
| (hTf-ASA)-ROP | 37.6 | 28.2 | 15.2 | 19.0 | 2.17 |
| hTf-ASA | 40.2 | 27.4 | 15.1 | 17.3 | 2.13 |
| (hTf-ROP)-ASA | 39.5 | 27.7 | 15.1 | 17.7 | 2.22 |
2.7. Characteristics of the Resonance Light Scattering Spectra
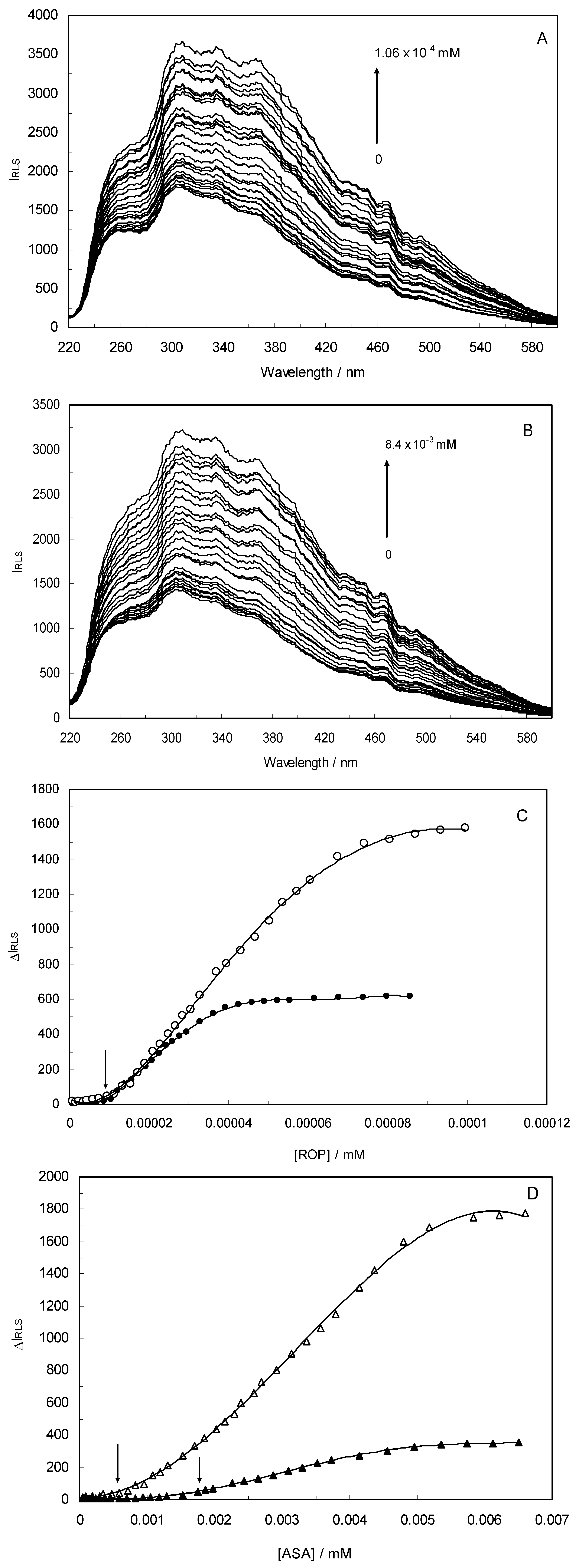
Light-scattering has been widely applied to study the aggregation, size, shape and distribution of particles in solution. When the excitation wavelength is close to the absorption bands, greatly enhanced Rayleigh light-scattering signals can be expected, known as resonance light scattering (RLS). The resonance light scattering spectra of ROP and ASA with hTf (in the binary and ternary systems) became enhanced upon increasing the concentration of drugs (see
Figure 7A,B). Under the experimental conditions, the RLS intensity of hTf was high and the coexistence of hTf and a certain concentration of ROP and ASA solutions led to a raise in RLS intensity. The results pointed at the conclusion that ROP and ASA induced aggregation of the hTf, giving rise to an enhancement of the RLS intensity. This followed the formula of RLS:
where n is the refractive index of the medium; N is the molarity of the solution; λ
0 is the wavelength of incident and scattered light; V
2 is the square of the molecular volume; and δ
n and δ
k are the fluctuations in the real and imaginary components of the refractive index of the particle, respectively [
43,
44]. When other factors are constant, I
RLS is related to the size of the formed particle and is directly proportional to the square of the molecular volume (
i.e., V
2). Apparently, with the increase of the molecular volume, I
RLS was enhanced. The values of I
RLS were plotted against varying concentrations of ROP and ASA (in the binary and ternary systems) (see
Figure 7) and it was found that the enhancement of the RLS intensity differed as a function of the concentrations of ROP and ASA. When the ROP and ASA concentrations were too low, the RLS intensity of the binary and ternary systems hardly changed. However, with increasing ROP and ASA concentrations, the RLS intensity of these systems gradually increased, and precipitation was even observed to occur in the solutions containing high concentrations of drugs.
The relationship between the RLS intensity and these drug concentrations was nonlinear. The vertical arrow shows the critical induced aggregation concentrations (C
CIAC) of ROP and ASA inducing drug aggregation on hTf [
45,
46].
Under identical experimental conditions, smaller C
CIAC values for the ternary systems signified a lower concentration of ROP and ASA for the induction of hTf aggregation. The C
CIAC values of the hTf-ROP and (hTf-ASA)-ROP complexes were 9.49 × 10
−6 mM and 1.04 × 10
−5 mM, respectively (see
Figure 7C,D), and the values for the hTf-ASA and (hTf-ROP)-ASA complexes were 5.92 × 10
−4 mM and 1.75 × 10
−3 mM, respectively. The phenomenon indicated that ROP and ASA could precipitate on the hTf in binary and ternary systems.
Figure 7.
(A) The RLS spectra of hTf at varying concentrations of ROP. [hTf] = 3.8 × 10−3 mM; (B) The RLS spectra of hTf at different concentrations of ASA. [hTf] = 3.8 × 10−3 mM, pH 7.4; (C) A plot of the ∆IRLS intensity of hTf at various concentrations of ROP in the absence and presence of ASA. [hTf] = 3.8 × 10−3 mM, and [ASA] = 4.62 × 10−3 mM for the ternary system. (○) binary system, (●) ternary system; (D) A plot of the ∆IRLS intensity of hTf at several concentrations of ASA in the absence and presence of ROP. [hTf] = 3.8 × 10−3 mM, and [ROP] = 1.33 × 10−5 mM for the ternary system. (∆) binary system, (▲) ternary system. All experiments were performed under identical conditions, T = 298 K, pH = 7.4.
Figure 7.
(A) The RLS spectra of hTf at varying concentrations of ROP. [hTf] = 3.8 × 10−3 mM; (B) The RLS spectra of hTf at different concentrations of ASA. [hTf] = 3.8 × 10−3 mM, pH 7.4; (C) A plot of the ∆IRLS intensity of hTf at various concentrations of ROP in the absence and presence of ASA. [hTf] = 3.8 × 10−3 mM, and [ASA] = 4.62 × 10−3 mM for the ternary system. (○) binary system, (●) ternary system; (D) A plot of the ∆IRLS intensity of hTf at several concentrations of ASA in the absence and presence of ROP. [hTf] = 3.8 × 10−3 mM, and [ROP] = 1.33 × 10−5 mM for the ternary system. (∆) binary system, (▲) ternary system. All experiments were performed under identical conditions, T = 298 K, pH = 7.4.
2.8. Binding Distance and Energy Transfer for Binary and Ternary Systems
In order to determine the precise location of ROP and ASA for binary and ternary systems in hTf, the efficiency of energy transfer were studied according to the forster resonance energy transfer theory (FRET), if the emitted fluorescence from a donor could be absorbed by an acceptor, energy may transfer from the donor to the acceptor. Energy transfer will happen under the following conditions: (a) the donor can produce fluorescence light; (b) the absorption spectrum of the receptor overlaps enough with the donor’s fluorescence emission spectrum; (c) the distance between the donor and the acceptor is less than 7 nm. The fluorescence quenching of hTf after binding with ROP and ASA indicated that the transfer of energy have occurred [
47]. The following equation can be used to calculate the efficiency (E) of energy transfer between the donor and acceptor:
where F and F0 are the fluorescence intensities of the biomolecule in the presence and absence of a quencher, r is the donor- acceptor distance and R0 is the critical energy transfer distance, at which 50% of the excitation energy is transferred to the acceptor. It is given by the following equation:
where K2 is the orientation factor of the dipole of the donor and acceptor, N is the refractive index of the medium, Φ is the fluorescence quantum yield of the donor in the absence of the acceptor and J expresses the degree of spectral overlap between the donor emission spectrum and the acceptor absorption spectrum. The value of J is given by:
where F(λ) is the fluorescence intensity of the fluorescent donor at wavelength λ, and ε(λ) is the molar absorption coefficient of the acceptor at wavelength λ. From the above relationships, J and E can be easily obtained, from which follows that R
0 and r can be further calculated [
48]. As seen in
Figure 8, there were a considerable overlap between the absorption spectrum of ROP with the fluorescence emission spectrum of hTf in the presence of ASA, which formed the basic of FRET. The average distance r < 7 nm and 0.5 R
0< r < 1.5 R
0 indicated that the energy transfer for binary and ternary systems occurred with a high probability.
The parameters regarding the FRET are presented in
Table 3. This results suggested that the bound ROP and ASA had a different distance to the tryptophan residues for binary and ternery complexes. The reasons for this, was that the binding sites for ROP and ASA in hTf, depending the chemical structure, molecule’s size and electro negativity of drugs [
49]. Moreover, The r values decreased with increasing values of the binding constant and binding affinity for binary and ternary systems.
2.9. Time-Resolved Fluorescence
Fluorescence lifetime decay measurements supply one of the best parameters that help us to distinguish between static and dynamic processes. In order to further substantiate the quenching mechanism of ROP and ASA to hTf, fluorescence lifetime of hTf were ascertained in the absence and presence of ROP and ASA as binary and ternary systems. Average fluorescence lifetime (τ) for biexponential iterative fittings was calculated from the decay times and the relative amplitude (α) using the following equation:
Time-resolved fluorescence decay of hTf in the absence and presence of ROP and ASA are summarized in
Table 4 (the concentrations of hTf, ROP and ASA for binary systems are 3.8 × 10
−3 mM, 8 × 10
−4 mM and 5 × 10
−2 mM, respectively, although for ternary systems the concentrations of ROP and ASA are 7.27 × 10
−5 mM and 4.6 × 10
−3 mM, respectively). The average fluorescence lifetime reduces from 4.20 to 3.59 ns, without and with the ROP and ASA for binary and ternary systems, attesting that the fluorescence quenching is essentially static mechanism. Hence, both steady-state and time-resolved measurements hint to the occurrence of static type fluorescence quenching caused by specific interaction, mainly by ground-state complex formations [
50,
51,
52].
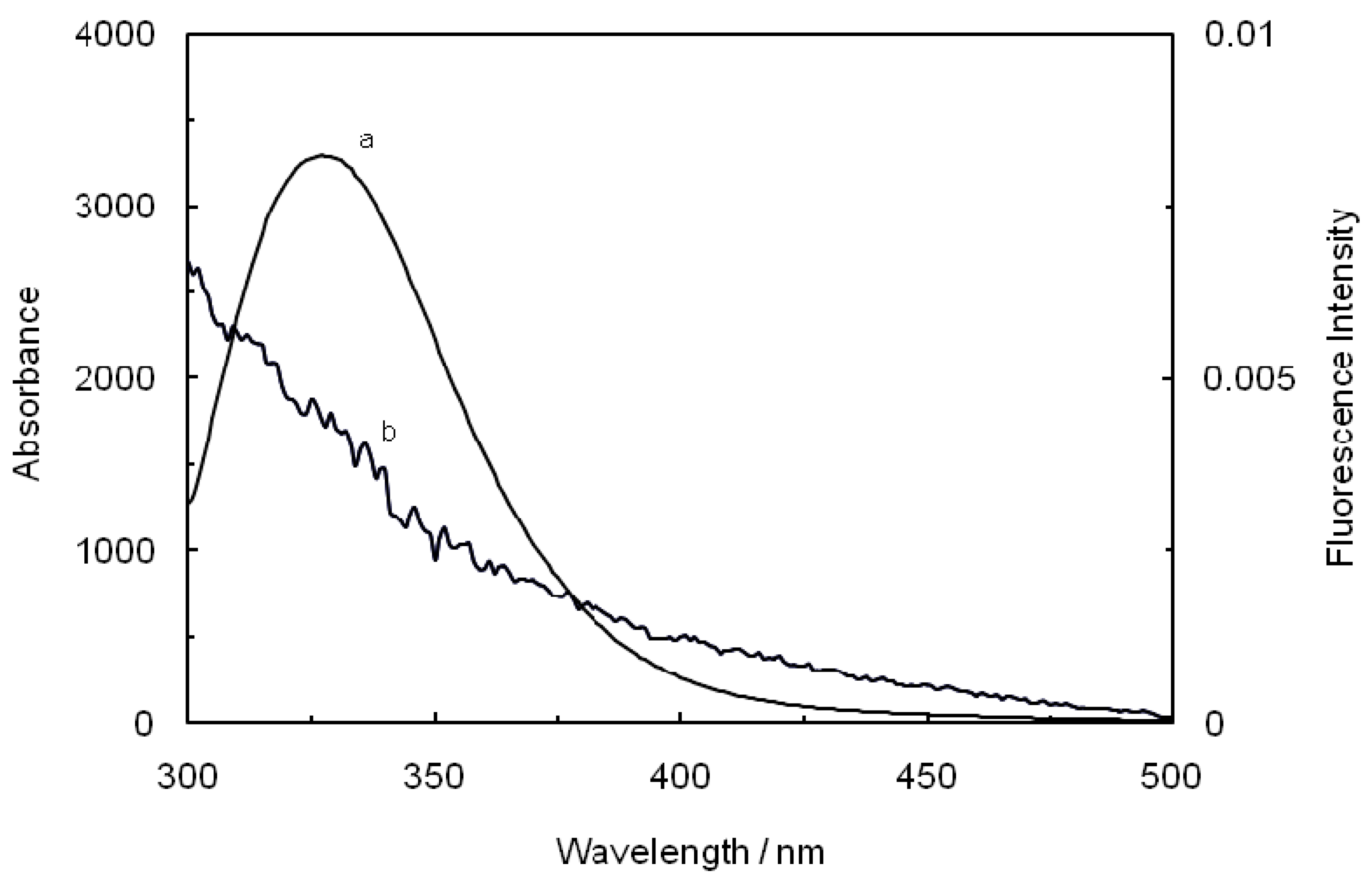
Figure 8.
Spectral overlap of the fluorescence spectra of hTf in the presence of ASA (a) with the absorption spectra of ROP (b) at pH 7.4 and T = 298 K. [hTf] = 3.8 × 10−3 mM, [ASA] = 4.6 × 10−3 mM and [ROP] = 7.27 × 10−5 mM.
Figure 8.
Spectral overlap of the fluorescence spectra of hTf in the presence of ASA (a) with the absorption spectra of ROP (b) at pH 7.4 and T = 298 K. [hTf] = 3.8 × 10−3 mM, [ASA] = 4.6 × 10−3 mM and [ROP] = 7.27 × 10−5 mM.
Table 4.
Lifetime of hTf in the presence of ROP and ASA as binary and ternary systems, (α: the relative amplitude and χ2: the error in the calculated fluorescence) at pH 7.4, T = 298 K and λex = 295 nm.
Table 4.
Lifetime of hTf in the presence of ROP and ASA as binary and ternary systems, (α: the relative amplitude and χ2: the error in the calculated fluorescence) at pH 7.4, T = 298 K and λex = 295 nm.
| System | τ1/ns | α1 | τ2/ns | α2 | τ/ns | χ2 |
|---|
| hTf | 1.957 | 0.5947 | 6.125 | 0.4972 | 4.209 | 0.9519 |
| hTf-ROP | 1.938 | 0.7634 | 6.033 | 0.3719 | 3.723 | 0.9611 |
| (hTf-ASA) ROP | 1.894 | 0.7954 | 5.772 | 0.3627 | 3.599 | 0.9520 |
| hTf-ASA | 1.942 | 0.7724 | 6.086 | 0.3771 | 3.795 | 0.9519 |
| (hTf-ROP) ASA | 1.907 | 0.7517 | 5.921 | 0.3661 | 3.601 | 0.9521 |
2.10. Three-Dimensional Fluorescence Spectra
Three-dimensional fluorescence spectroscopy is a vigorous method for providing conformational and structural information of proteins. The outstanding advantage of three-dimensional fluorescence spectra is that information regarding the fluorescence characteristics can be entirely acquired by simultaneously changing the excitation and emission wavelengths. The maximum emission wavelength and the fluorescence intensity of the residues have a close relation to the polarity of their micro-environment [
53].
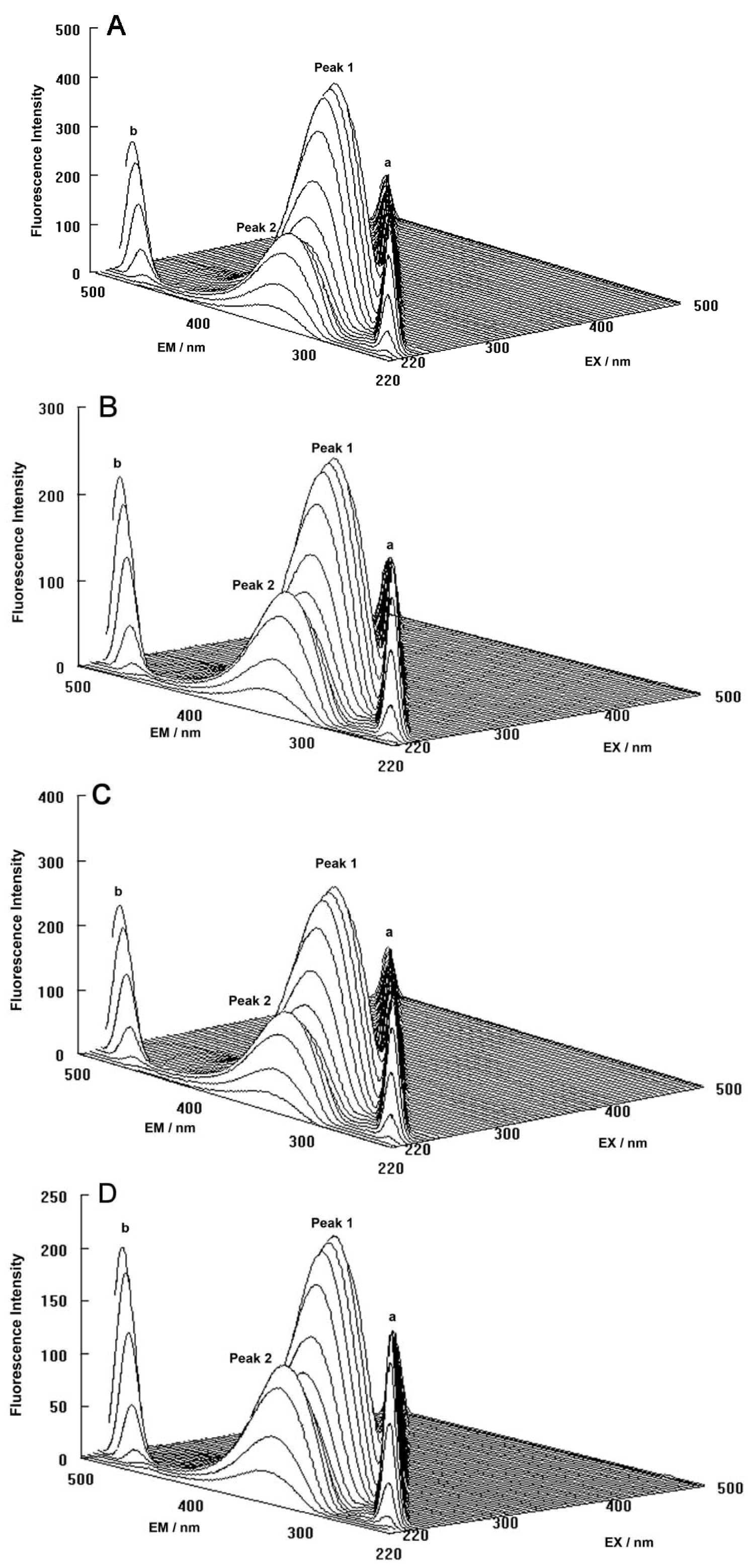
The three-dimensional fluorescence spectra and the contour maps for both the binary and ternary systems are shown in
Figure 9A–D. As can be seen, peak a is the Rayleigh scattering peak (λ
ex = λ
em) and peak b is the second-ordered scattering peak (λ
em = 2λ
ex). The fluorescence intensity of peak a and peak b increased with the addition of ROP and ASA (in the binary and ternary systems). The possible reason for this was that complexes came into being after addition of the drugs, increasing the diameter of the hTf which in turn resulted in enhanced scattering spectra.
At the same time, there were two “humps” in the three-dimensional spectra for both binary and ternary complexes marked peaks 1 and 2. Peak 1 revealed the spectral behavior of the Trp and Tyr residues. The reason for this was that when the hTf was excited at 280 nm, it mainly demonstrated the intrinsic fluorescence of Trp and Tyr residues, and the fluorescence of Phe residues could be neglected. The fluorescence spectral behavior of the polypeptide backbone structure of hTf was also reflected by peak 2, which was caused by the transition of π → π
* of hTf’s characteristic polypeptide backbone structure C=O. The fluorescence intensity of peak 2 decreased after the addition of ROP and ASA, signifying that the peptide strands structure of hTf had been modified [
54,
55].
Figure 9.
(A) Three-dimensional fluorescence spectra of hTf; (B) Three-dimensional fluorescence spectra of the hTf-ROP system; (C) Three-dimensional fluorescence spectra of the hTf-ASA system; (D) Three-dimensional fluorescence spectra of the hTf-ROP-ASA system. All experiments were performed under identical conditions, [hTf] = 3.8 × 10−3 mM, [ROP] = 7.27 × 10−5 mM, [ASA] = 4.54 × 10−3 mM, pH = 7.4 and T = 298 K.
Figure 9.
(A) Three-dimensional fluorescence spectra of hTf; (B) Three-dimensional fluorescence spectra of the hTf-ROP system; (C) Three-dimensional fluorescence spectra of the hTf-ASA system; (D) Three-dimensional fluorescence spectra of the hTf-ROP-ASA system. All experiments were performed under identical conditions, [hTf] = 3.8 × 10−3 mM, [ROP] = 7.27 × 10−5 mM, [ASA] = 4.54 × 10−3 mM, pH = 7.4 and T = 298 K.
The decrease of the intensity of peaks 1 and 2 in combination with the results from the synchronous fluorescence spectroscopy displayed that the binding of ROP and ASA to hTf induced the slight unfolding of the polypeptides of hTf, which led to a conformational change of hTf that increased the exposure of some hydrophobic regions that were previously buried. All these phenomena obtained from analyzing the two peaks indicated that the binding of ROP and ASA to hTf and the formation of binary and ternary complexes induced certain changes in the micro-environment and conformation of hTf.
2.11. Zeta-Potential Measurements
By measuring the zeta-potential, it is possible to probe a characteristic colloidal property in a complex mixture of particles, making it a useful technique to explain their behavior. The zeta-potential is generated when a liquid is forced to flow directly through a small gap, formed by two sample surfaces, under pressure. The charge carrier bound in the double layer will thus be removed, after which the potential can be measured between two electrodes. Consequently, the biomaterial’s zeta-potential demonstrates the electric surface properties. A surface charge in protein particles is due to the partial ionization of various amino acid residues. The effective charge on a protein particle is affected by pH, ionic strength and the accumulation of ligands or surfactant at the interface [
56,
57]. The zeta potential can be calculated from the electrophoretic mobility’s,
µE, using the Henry equation [
58]:
where ε0 is the permittivity of vacuum; εr and η are the relative permittivity and viscosity of water, respectively; α is the particle radius; and κ is the Debye length. The function f(κα) depends on the particle shape and was determined for the systems by:
This expression is valid for κα > 1. The interaction between the adsorbed molecules may be either attractive or repulsive, depending on the kind and magnitude of electric charge of the residues. These complicated processes involving protein adsorption are also reflected in the zeta-potential changes.
Figure 10 shows the effect of ROP and ASA concentrations on the zeta-potential of hTf (in the binary and ternary systems). At first, the adsorption of ROP and ASA on the hTf surface increased with an increasing zeta-potential after which an abrupt change occurred, decreasing the values of the zeta-potential. Higher zeta-potential values confirmed that the electrostatic forces were the primary interaction of ROP and ASA with hTf in the present study. The negative values of the zeta-potential conformed with hydrophobic interactions between ROP, ASA and hTf. Hence, the stability of the binary and ternary systems decreased when decreasing the zeta-potential values. The raise in surface charge on the colloidal particles augmented the magnitude of inter-particle electrostatic repulsion, which tended to disrupt existing protein aggregates and discourage further aggregate formation [
59]. Consequently, the ROP and ASA molecules were able to bind to hTf through a combination of electrostatic and hydrophobic interactions, forming micelle-like clusters.
2.12. Molecular Modeling
Any of the ligands, when docked in hTf, becomes attached to the N-lobe of the hTf and shows the most affinity to approximately the same active site. The best docking results for binary and ternary systems are shown in
Figure 11 and
Figure 12, respectively.
Figure 10.
(A) Zeta-potential spectra at varying concentrations of ROP in the absence and presence of ASA. [hTf] = 3.8 × 10−3 mM, [ASA] = 4.62 × 10−3 mM, for the ternary system; (B) Zeta-potential spectra at various concentrations of ASA in the absence and presence of ROP. [hTf] = 3.8 × 10−3 mM, [ROP] = 1.33 × 10−5 mM, for the ternary system. pH 7.4 and T = 298 K.
Figure 10.
(A) Zeta-potential spectra at varying concentrations of ROP in the absence and presence of ASA. [hTf] = 3.8 × 10−3 mM, [ASA] = 4.62 × 10−3 mM, for the ternary system; (B) Zeta-potential spectra at various concentrations of ASA in the absence and presence of ROP. [hTf] = 3.8 × 10−3 mM, [ROP] = 1.33 × 10−5 mM, for the ternary system. pH 7.4 and T = 298 K.
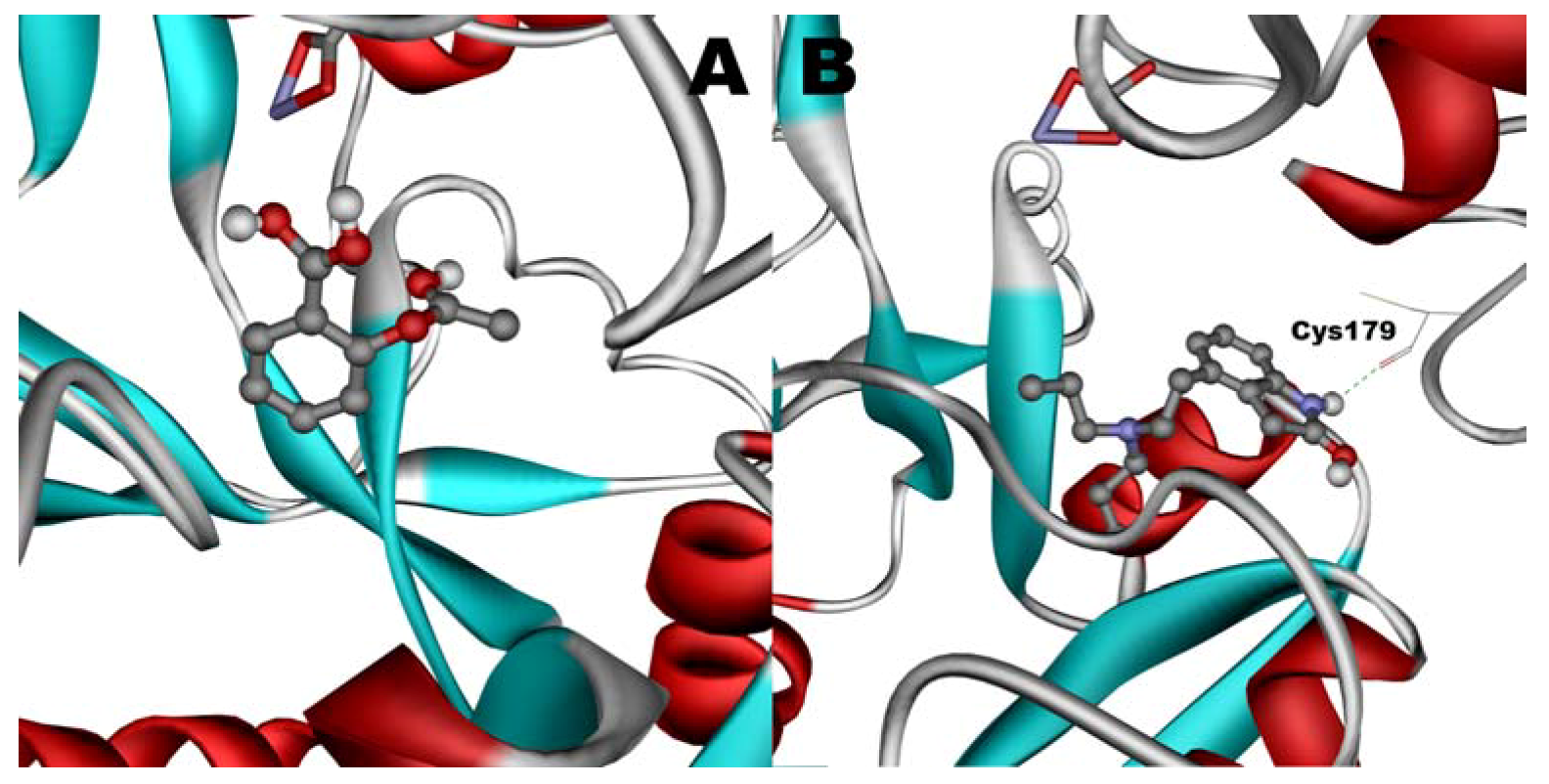
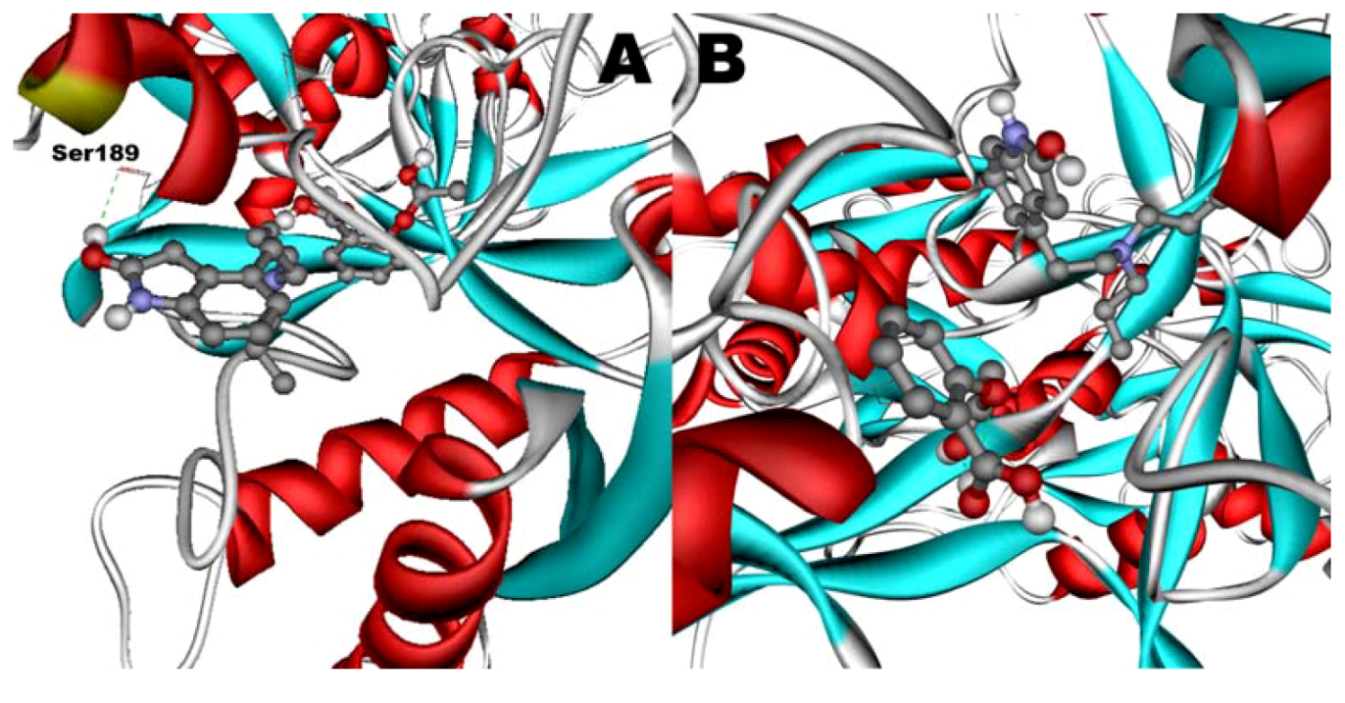
When docked alone, ROP formed a hydrogen bond to Cys179 and properly fits the binding pocket (
Figure 11B). However, when introduced to the hTf-ASA complex, it could not attach to pocket in the same orientation; and created instead a hydrogen bond to Ser189 (
Figure 12B). The inhibition constant declined to about one fourth of the previous value due to poor orientation and improper van der Waals interactions. ASA, although unable to form hydrogen bonds, had a good placement in terms of electrostatic energy and yielded a decent inhibitory constant (
Figure 11A). The presence of ROP in the complex forced ASA to dock in a different pocket and stabilize by formation of two intramolecular hydrogen bonds [
60,
61,
62]. It is predicted that presence of ROP when docking ASA shouldn’t have a major effect on affinity (
Figure 12A). The molecular dynamic results confirmed the obtained experimental data that mentioned in
Table 2.
Figure 11.
Docking of ASA (A) and ROP (B) onto hTf. ASA and ROP are shown as balls and sticks. FeCO3, if visible, is shown as sticks. Hydrogen bonds are shown as green dotted lines.
Figure 11.
Docking of ASA (A) and ROP (B) onto hTf. ASA and ROP are shown as balls and sticks. FeCO3, if visible, is shown as sticks. Hydrogen bonds are shown as green dotted lines.
Figure 12.
Docking of ASA (A) as a third ligand onto the ROP-hTf complex. ROP (B) docked onto the ASA-hTf complex. ASA and ROP are shown as balls and sticks. FeCO3, if visible, is shown as sticks. Hydrogen bonds are shown as green dotted lines.
Figure 12.
Docking of ASA (A) as a third ligand onto the ROP-hTf complex. ROP (B) docked onto the ASA-hTf complex. ASA and ROP are shown as balls and sticks. FeCO3, if visible, is shown as sticks. Hydrogen bonds are shown as green dotted lines.