Effect of Electrospun Non-Woven Mats of Dibutyryl Chitin/Poly(Lactic Acid) Blends on Wound Healing in Hairless Mice
Abstract
:1. Introduction
2. Results
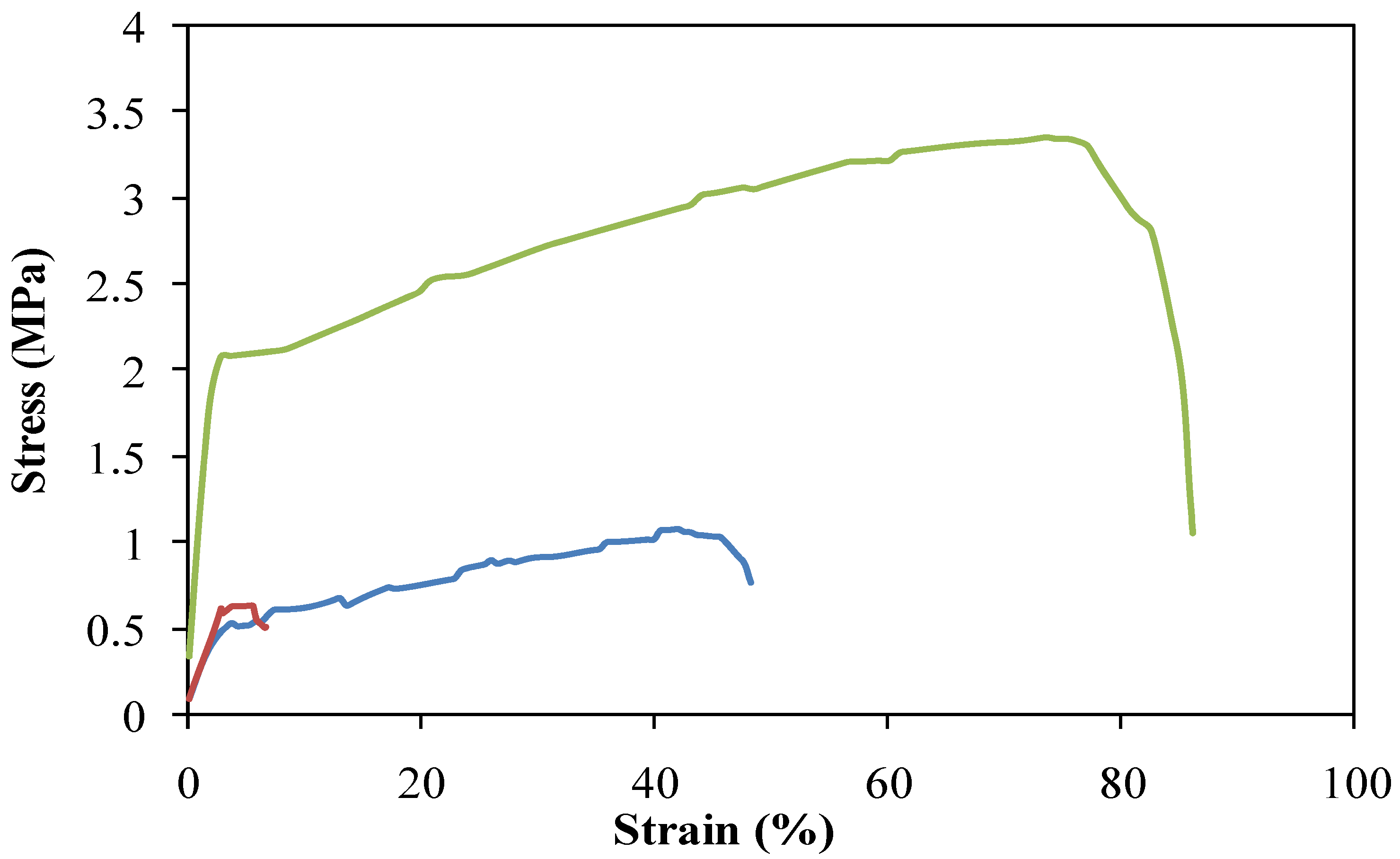
2.1. Morphology of Electrospun DBCNFM Composed of DBC/PLA Blend Nanofibers
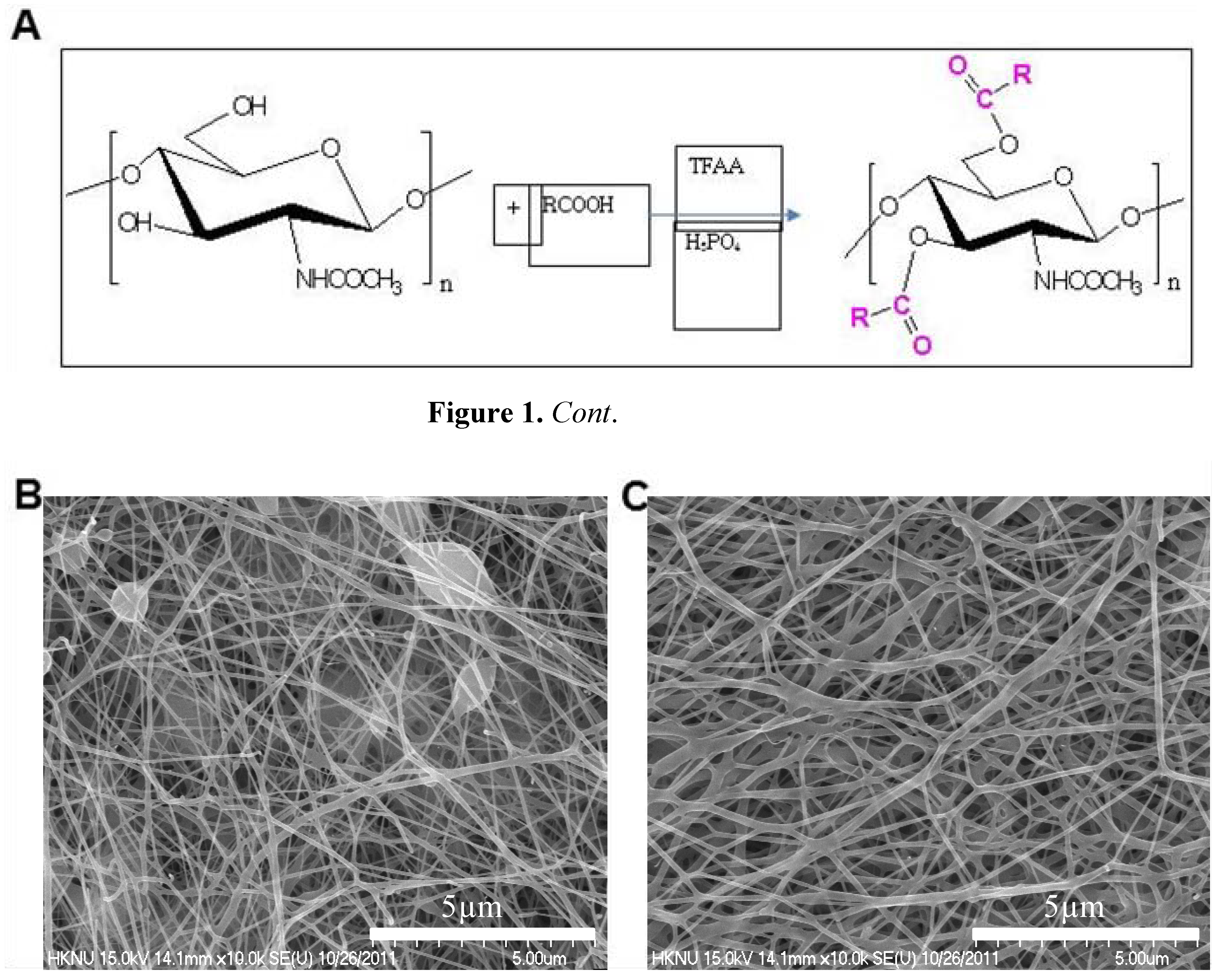
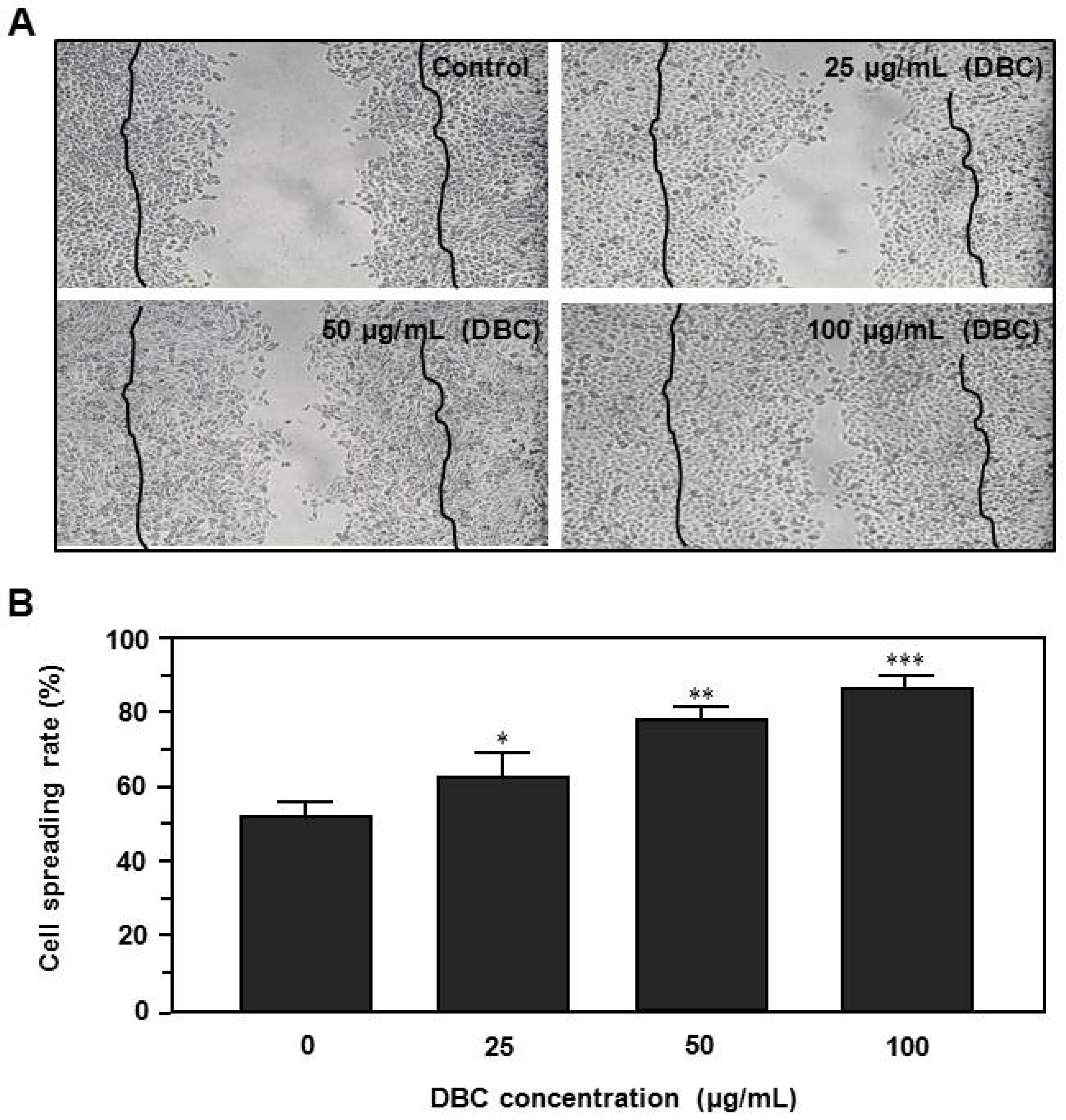
2.2. Effects of DBC on Spreading Rate of HaCaT Keratinocytes
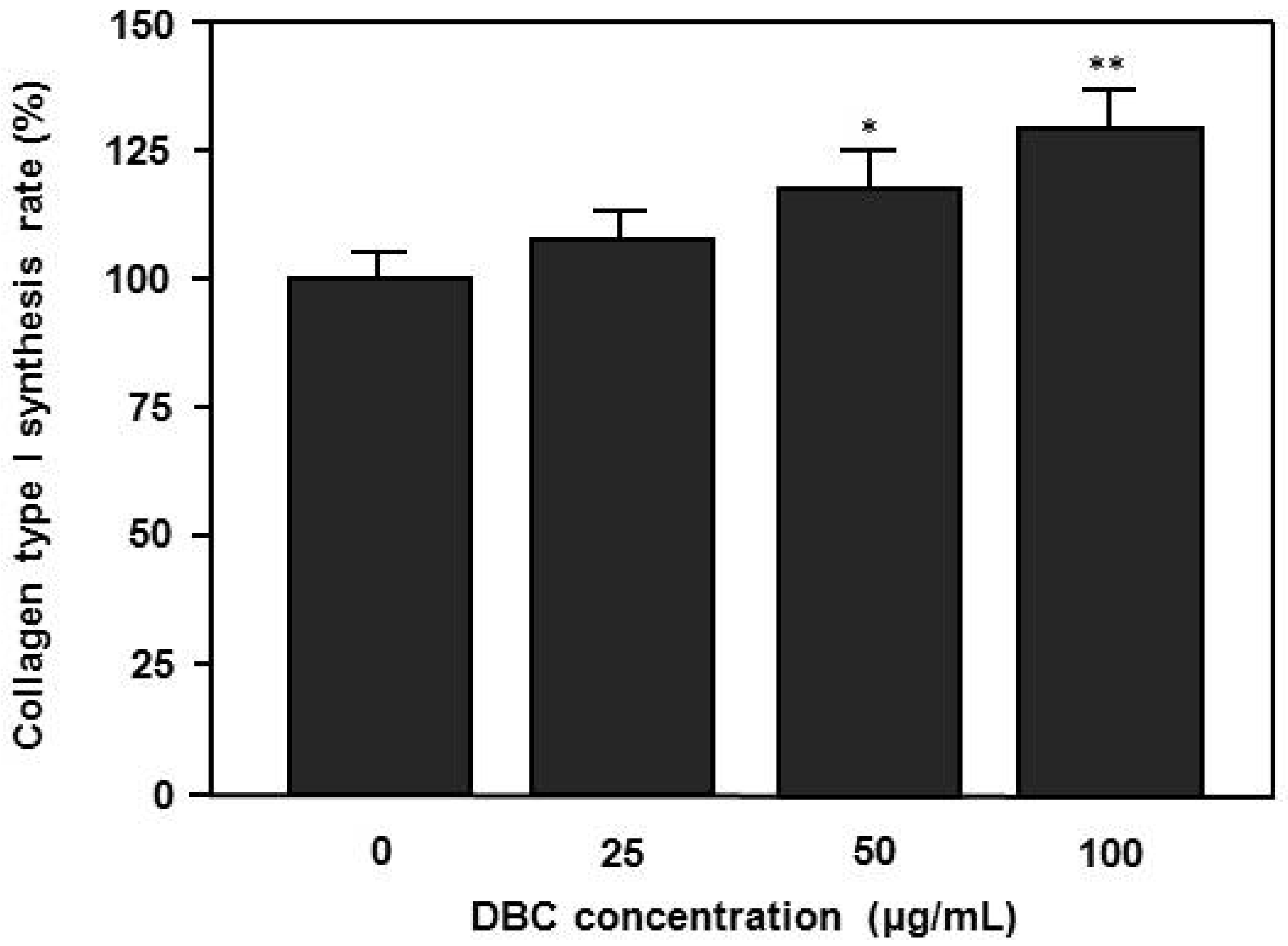
2.3. Effects of DBC on Collagen Synthesis of HaCaT Keratinocytes
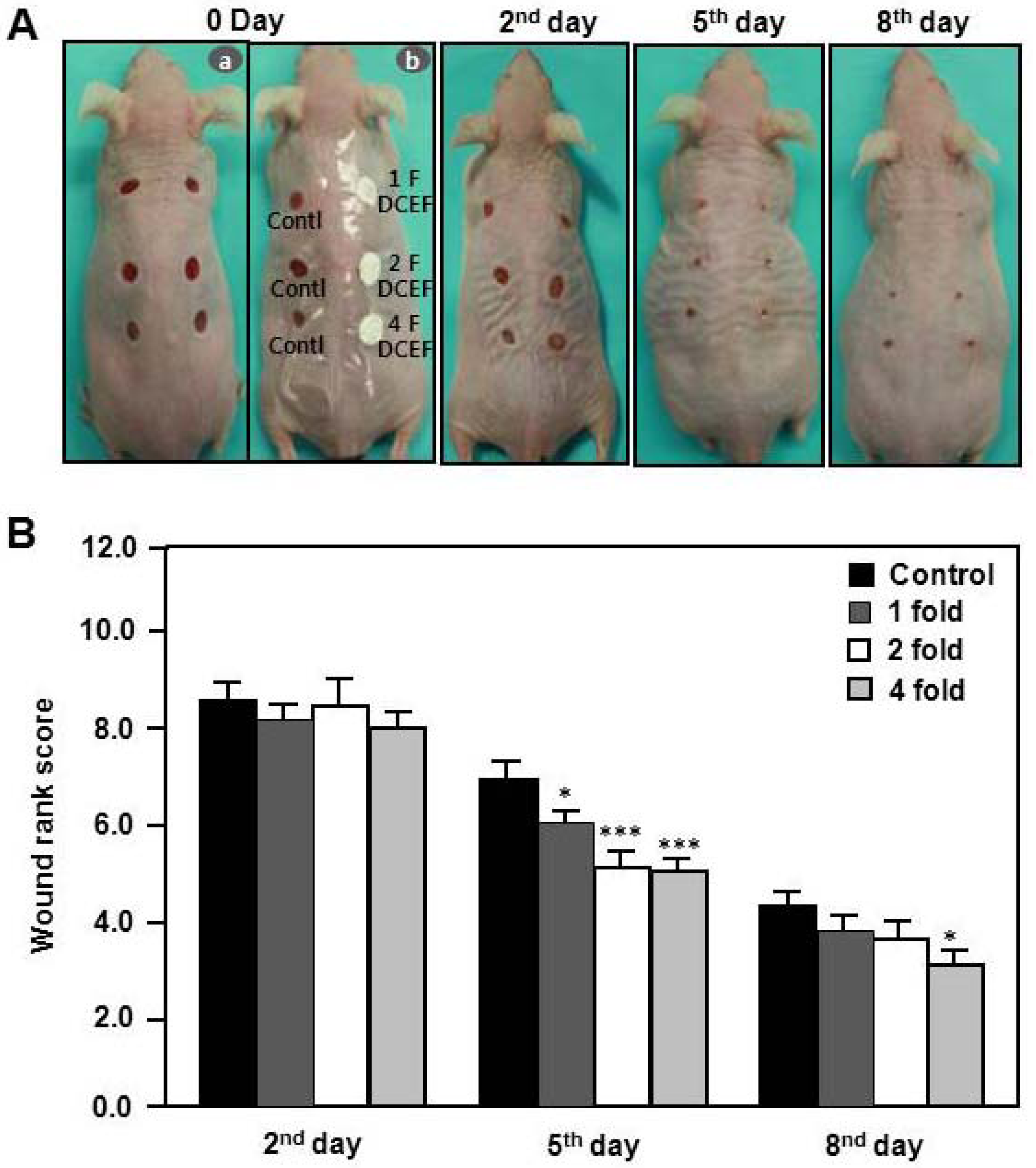
2.4. Effects of DBCNFM on Wound Healing Rank in Hairless Mice
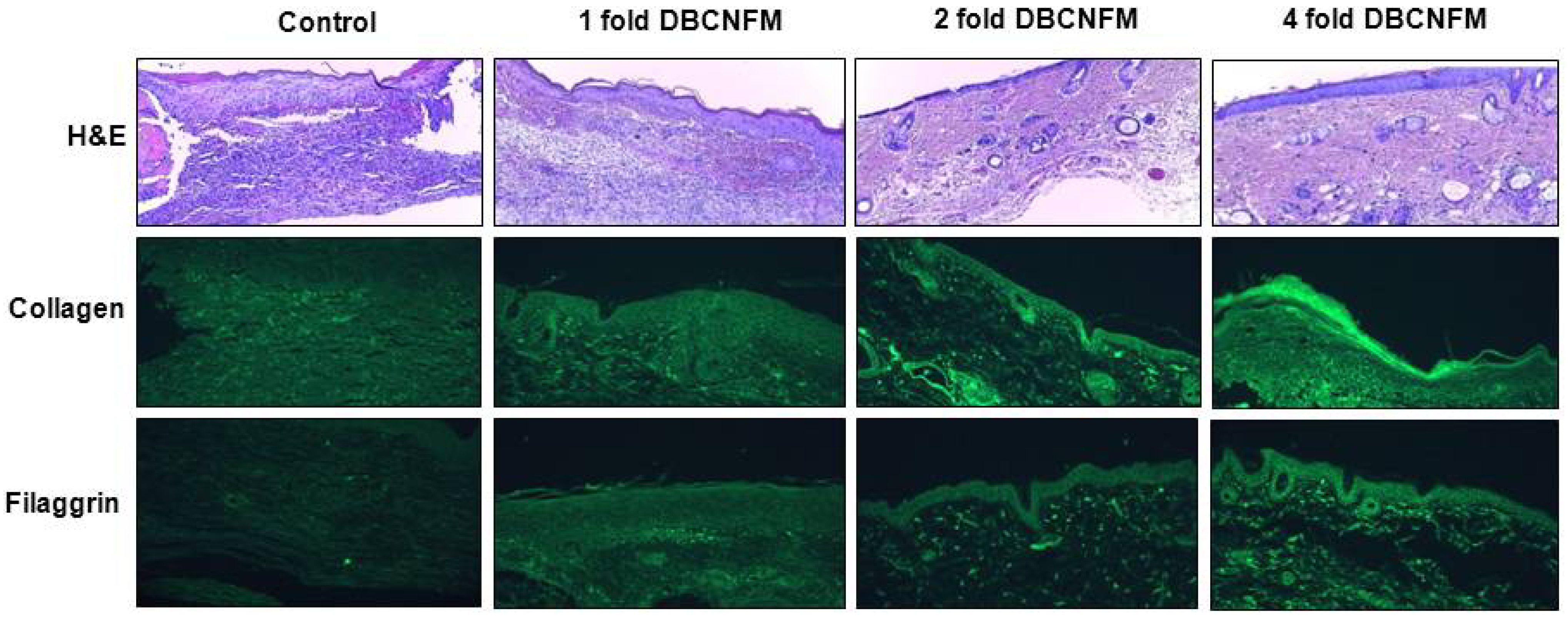
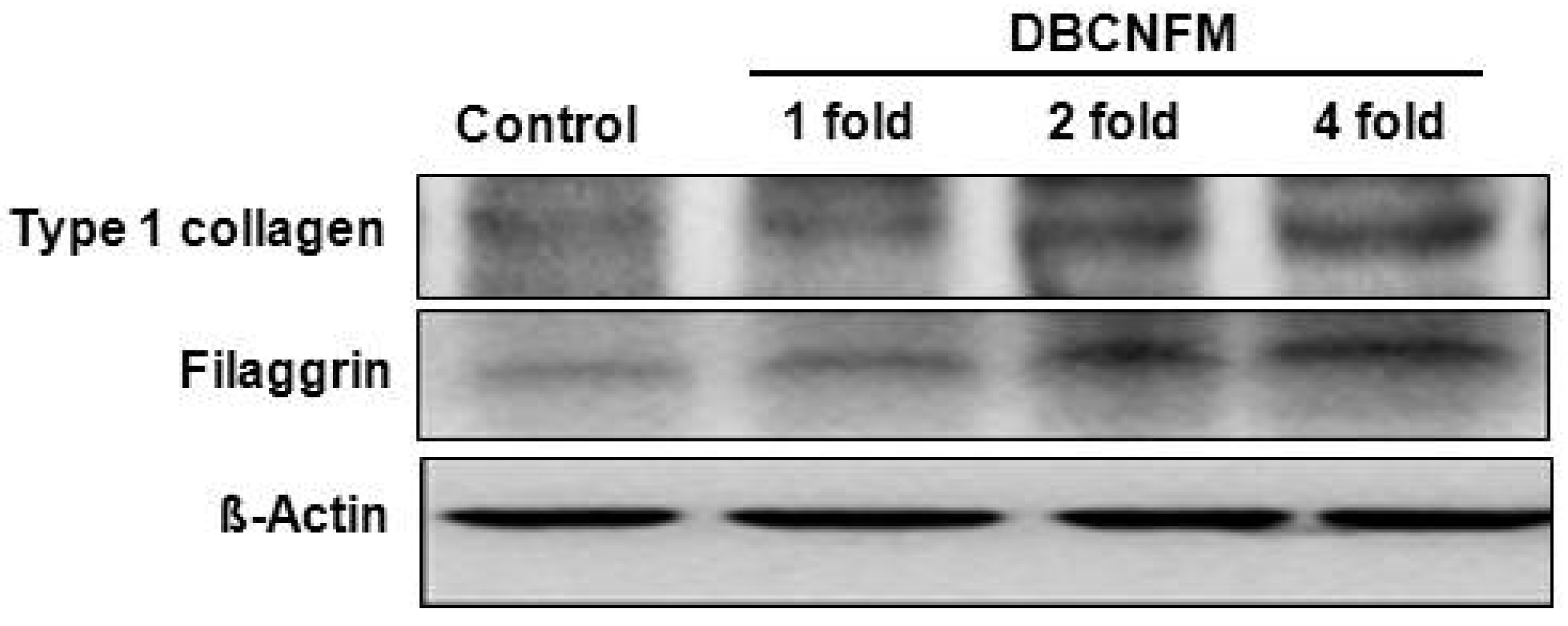
2.5. Effects of DBCNFM on Histological Changes and ECM Expression in the Wounded Skin Tissues
3. Discussion
4. Experimental
4.1. Materials and Methods
4.1.1. Reagents
4.1.2. Identification of DBC
4.1.3. Fabrication of DBCNFM
4.1.4. Characterization of DBCNFM
4.2. Cell Line and Culture
4.3. Scratch and Cell Spreading Assay
4.4. Collagen Type I Assay by ELISA
4.5. Animals
4.6. Induction of Wound
4.7. Wound Rank Scoring System
4.8. Western Blotting Analysis
4.9. Immunohistochemical Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
References and Notes
- Sandjeu, Y.; Haftek, M. Desmosealin and other components of the epidermal extracellular matrix. J. Physiol. Pharmacol. 2009, 4, 23–30. [Google Scholar]
- Rawlings, A.; arding, C. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier function of the skin: “la raison d'etre” of the epidermis. J. Invest. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar]
- Katz, S.I. The epidermal basement membrane: Structure, ontogeny and role in disease. Ciba. Found. Symp. 1984, 108, 243–259. [Google Scholar]
- Uitto, J.; Pulkkinen, L. Molecular complexity of the cutaneous basement membrane zone. Mol. Biol. Rep. 1996, 23, 35–46. [Google Scholar] [CrossRef]
- Senshu, T.; Kan, S.; Ogawa, H.; Manabe, M.; Asaga, H. Preferential deimination of keratin K1 and filaggrin during the terminal differentiation of human epidermis. Biochem. Biophy. Res. Commun. 1996, 225, 712–719. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef]
- Davis, S.C.; Perez, R. Cosmeceuticals and natural products: Wound healing. Clin. Dermatol. 2009, 27, 502–506. [Google Scholar] [CrossRef]
- Fatima, A.D.; Modolo, L.V.; Sanches, A.C.; Porto, R.R. Wound healing agents: The role of natural and non-natural products in drug development. Mini Rev. Med. Chem. 2008, 8, 879–888. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Su, C.H.; Sun, C.S.; Juan, S.W.; Hu, C.H.; Ke, W.T.; Sheu, M.T. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997, 18, 1169–1174. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of chitin and chitosan based nanomaterials—A short review. Carbohyd. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Jayakumar, R.; Chennazhi, K.P.; Sowmya, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef]
- Supaphol, P.; Suwantong, O.; Sansanoh, P.; Sowmya, S.; Jayakumar, R.; Nair, S.V. Electrospinning of biocompatible polymers and their potentials in biomedical applications. Adv. Polym. Sci. 2012, 246, 213–240. [Google Scholar]
- Sudheesh Kumar, P.T.; Abhilash, S.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nano silver composite scaffolds for wound dressing applications. Carbohyd. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Madhumathi, K.; Sudhessh Kumar, P.T.; Abhilash, S.; Tamura, H.; Manzoor, K.; Nair, S.V.; Jayakumar, R. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J. Mater. Sci. Mater. Med. 2010, 21, 807–813. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Bioactivity and cell attachment studies of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 571–576. [Google Scholar] [CrossRef]
- Blasinska, A.; Drobnik, J. Effects of nonwoven mats of di-O-butyrylchitin and related polymers on the process of wound healing. Biomacromolecules 2008, 9, 776–782. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 2010, 8, 292–312. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y.N. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Buckley, A.; Davidson, J.M.; Kamerath, C.D.; Wolt, T.B.; Woodward, S.C. Sustained release of epidermal growth factor accelerates wound repair. Proc. Natl. Acad. Sci. USA 1985, 82, 7340–7344. [Google Scholar]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug. Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Chen, R.N.; Wang, G.M.; Chen, C.H.; Ho, H.O.; Sheu, M.T. Development of N, O-carboxymethyl chitosan/collagen matrixes as a wound dressing. Biomacromolecules 2006, 7, 1058–1064. [Google Scholar] [CrossRef]
- Hay, E.D. Cell Biology of Extracellular Matrix; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Chiu, J.B.; Liu, C.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Functionalization of poly(L-lactide) nanofibrous scaffolds with bioactive collagen molecules. J. Biomed. Mater. Res. Part A 2007, 83, 1117–1127. [Google Scholar]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Leung, D.Y. Our evolving understanding of the functional role of filaggrin in atopic dermatitis. J. Allergy Clin. Immunol. 2009, 124, 494–495. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Noishiki; Wada, M.; Heux, L.; Kuga, S. Esterification of beta-chitin via intercalation by carboxylic anhydrides. Biomacromolecules 2006, 7, 2878–2881. [Google Scholar] [CrossRef]
- Batt, L.R.; Kim, B.M.; Hyun, K.; Kang, K.H.; Lu, C.; Chai, K.Y. Preparation of chitin butyrate by using phosphoryl mixed anhydride system. Carbohydr. Res. 2011, 346, 691–694. [Google Scholar] [CrossRef]
- Qiu, C.; Coutinho, P.; Frank, S.; Franke, S.; Law, L.Y.; Martin, P.; Green, C.R.; Becker, D.L. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 2003, 13, 1697–1703. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jang, S.I.; Mok, J.Y.; Jeon, I.H.; Park, K.-H.; Nguyen, T.T.T.; Park, J.S.; Hwang, H.M.; Song, M.-S.; Lee, D.; Chai, K.Y. Effect of Electrospun Non-Woven Mats of Dibutyryl Chitin/Poly(Lactic Acid) Blends on Wound Healing in Hairless Mice. Molecules 2012, 17, 2992-3007. https://doi.org/10.3390/molecules17032992
Jang SI, Mok JY, Jeon IH, Park K-H, Nguyen TTT, Park JS, Hwang HM, Song M-S, Lee D, Chai KY. Effect of Electrospun Non-Woven Mats of Dibutyryl Chitin/Poly(Lactic Acid) Blends on Wound Healing in Hairless Mice. Molecules. 2012; 17(3):2992-3007. https://doi.org/10.3390/molecules17032992
Chicago/Turabian StyleJang, Seon Il, Ji Ye Mok, In Hwa Jeon, Kwang-Hyun Park, Thuy Thi Thu Nguyen, Jun Seo Park, Hee Min Hwang, Mi-Sun Song, Duckhee Lee, and Kyu Yun Chai. 2012. "Effect of Electrospun Non-Woven Mats of Dibutyryl Chitin/Poly(Lactic Acid) Blends on Wound Healing in Hairless Mice" Molecules 17, no. 3: 2992-3007. https://doi.org/10.3390/molecules17032992
APA StyleJang, S. I., Mok, J. Y., Jeon, I. H., Park, K.-H., Nguyen, T. T. T., Park, J. S., Hwang, H. M., Song, M.-S., Lee, D., & Chai, K. Y. (2012). Effect of Electrospun Non-Woven Mats of Dibutyryl Chitin/Poly(Lactic Acid) Blends on Wound Healing in Hairless Mice. Molecules, 17(3), 2992-3007. https://doi.org/10.3390/molecules17032992




