Oxidation of 2-Hydroxynevirapine, a Phenolic Metabolite of the Anti-HIV Drug Nevirapine: Evidence for an Unusual Pyridine Ring Contraction
Abstract
:1. Introduction
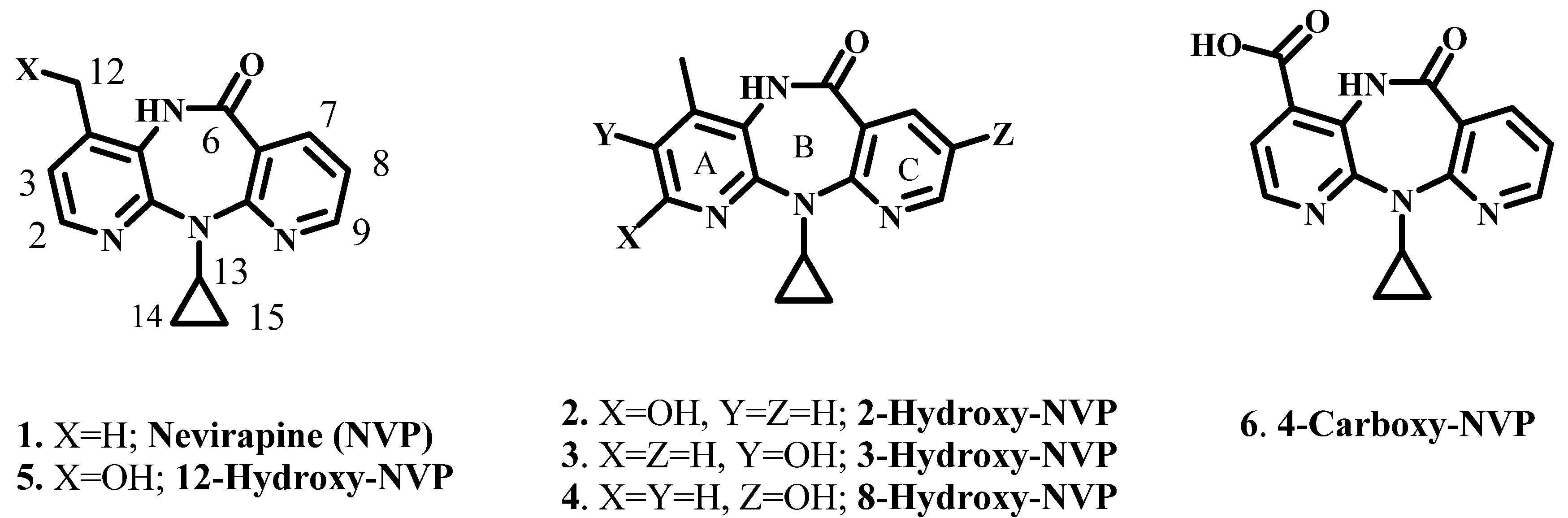
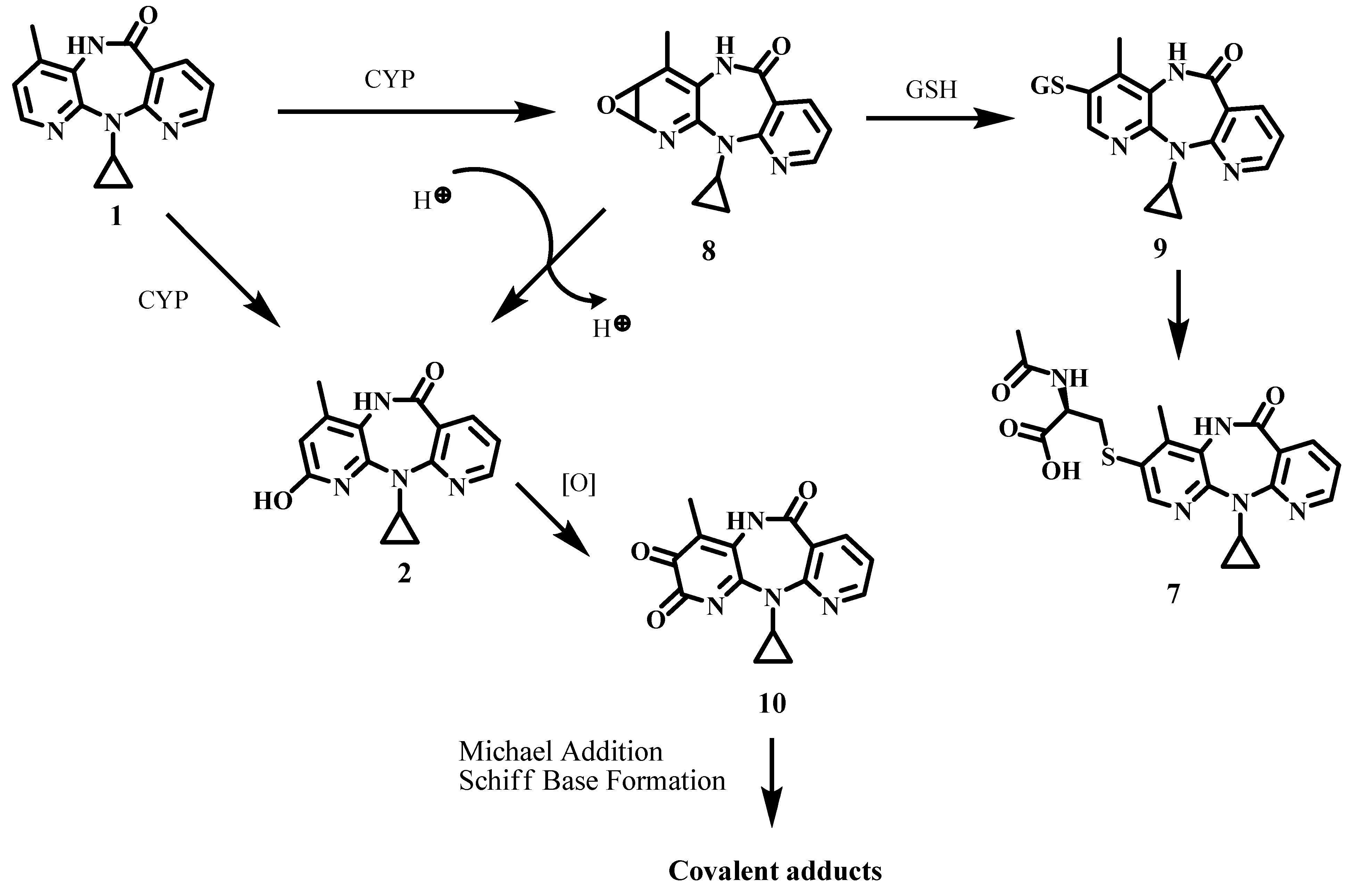
2. Results and Discussion
2.1. Oxidation of the Metabolite 2-Hydroxy-NVP
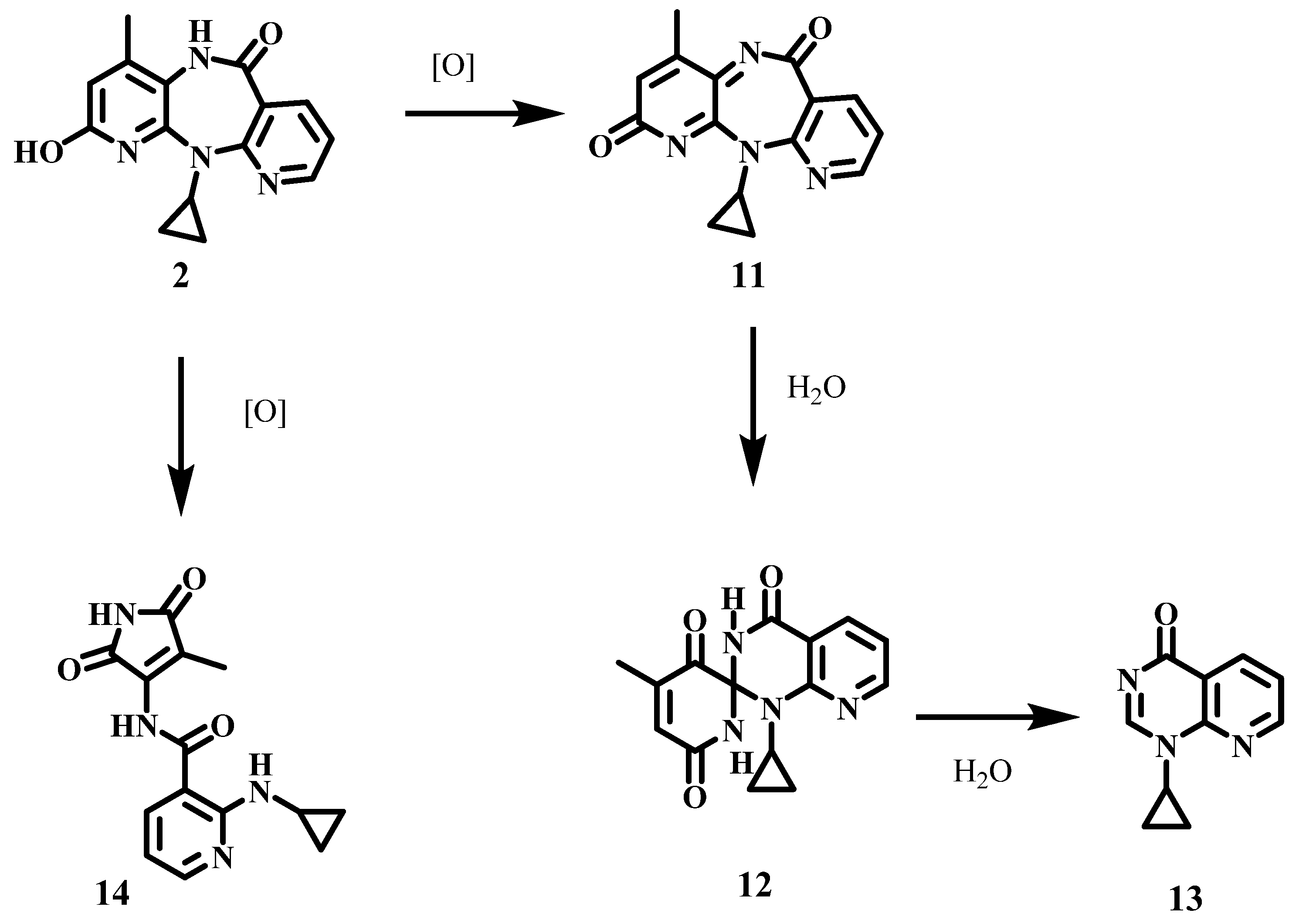
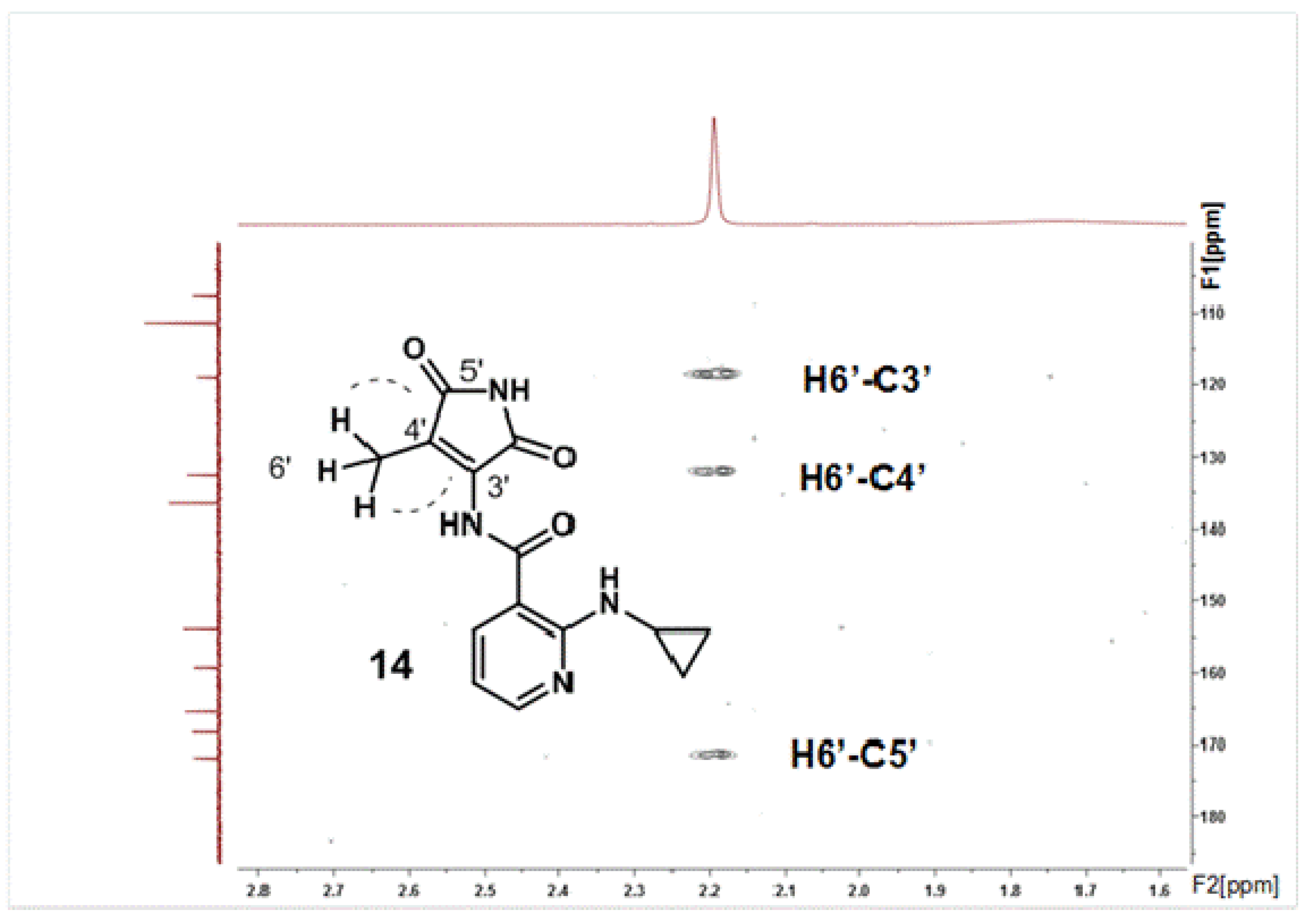
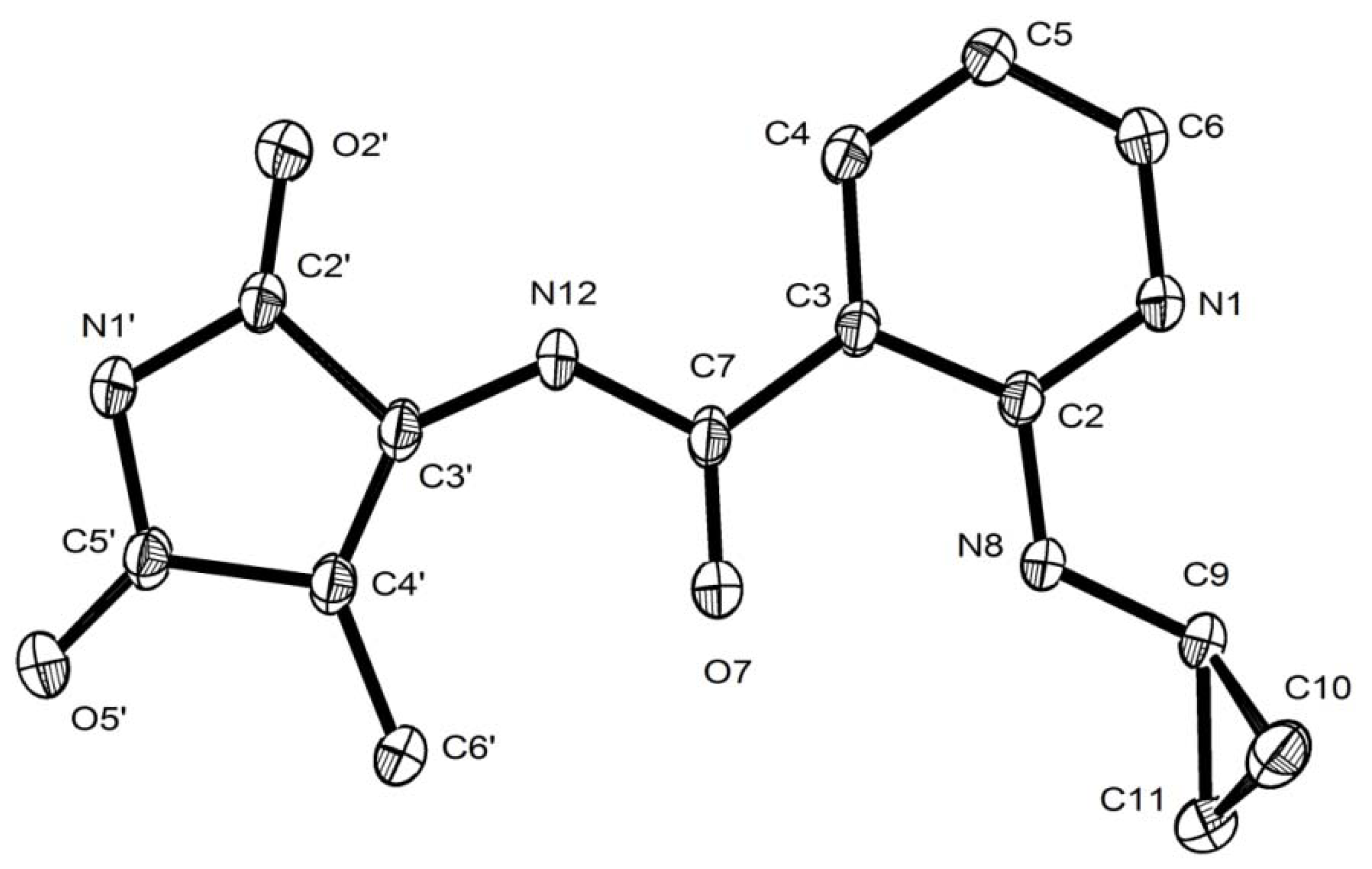
2.2. Structural Characterization of the Oxidation Product 14
3. Experimental
3.1. Chemicals
3.2. General
| Bonds (Å) | Angles (°) | ||||
|---|---|---|---|---|---|
| Molecule 1 | Molecule 2 | Molecule 1 | Molecule 2 | ||
| O5’-C5’ | 1.2130(14) | 1.2138(14) | C2’-N1’-C5’ | 109.18(10) | 109.03(10) |
| O2’-C2’ | 1.2146(14) | 1.2111(14) | N1’-C5’-C4’ | 108.74(10) | 109.01(9) |
| O7-C7 | 1.2234(14) | 1.2219(14) | C3’-C4’-C5’ | 105.96(9) | 105.58(10) |
| N1’-C5’ | 1.3981(15) | 1.3967(15) | C2’-C3’-C4’ | 109.12(10) | 109.50(9) |
| N1’-C2’ | 1.3716(15) | 1.3715(14) | N1’-C2’-C3’ | 106.95(9) | 106.81(9) |
| N12-C3’ | 1.3789(14) | 1.3814(14) | O5’-C5’-C4’ | 126.75(10) | 126.31(11) |
| N12-C7 | 1.3750(14) | 1.3735(15) | O5’-C5’-N1’ | 124.50(10) | 124.67(11) |
| N1-C2 | 1.3553(14) | 1.3497(14) | O2’-C2’-N1’ | 128.46(11) | 128.16(11) |
| N1-C6 | 1.3378(14) | 1.3380(16) | O2’-C2’-C3’ | 124.60(10) | 125.00(10) |
| N8-C2 | 1.3482(14) | 1.3498(14) | C4’-C3’-N12 | 138.20(10) | 137.70(11) |
| N8-C9 | 1.4329(15) | 1.4299(15) | N12-C3’-C2’ | 112.67(10) | 112.79(9) |
| C4’-C5’ | 1.4956(16) | 1.4967(16) | C5’- C4’-C6’ | 119.03(10) | 118.50(10) |
| C3’-C4’ | 1.3517(15) | 1.3480(15) | C3’-C4’-C6’ | 135.01(11) | 135.88(11) |
| C2’-C3’ | 1.5094(15) | 1.5087(16) | C7-N12-C3’ | 128.53(10) | 128.24(10) |
| C3-C7 | 1.4806(16) | 1.4792(15) | O7-C7-N12 | 120.00(11) | 120.10(10) |
| C2-C3 | 1.4408(15) | 1.4363(15) | N12-C7-C3 | 116.55(10) | 116.63(9) |
| C5-C6 | 1.3836(16) | 1.3817(19) | O7- C7-C3 | 123.44(10) | 123.26(10) |
| C4-C5 | 1.3826(17) | 1.3831(18) | C2-C3-C7 | 120.17(9) | 120.31(9) |
| C3-C4 | 1.3925(15) | 1.3855(16) | C4-C3-C7 | 122.84(10) | 122.47(10) |
| C9-C11 | 1.4933(16) | 1.4958(19) | C2-C3-C4 | 116.99(10) | 117.20(10) |
| C9-C10 | 1.4975(18) | 1.4966(18) | N1-C2-C3 | 121.63(10) | 121.96(10) |
| C10-C11 | 1.5029(18) | 1.4973(19) | C2-N1-C6 | 118.19(10) | 117.84(10) |
| Torsion Angles (°) | N1-C6-C5 | 124.34(11) | 124.44(12) | ||
| C4-C5-C6 | 117.81(10) | 117.79(12) | |||
| N8-C9-C11-10 | −110.69(13) 70.12(12) | C3-C4-C5 | 120.92(10) | 120.71(12) | |
| N8-C2-C3 | 121.32(10) | 121.47(10) | |||
| Angles between planes (°) | N1-C2-N8 | 117.04(10) | 116.57(10) | ||
| C2-N8-C9 | 123.66(10) | 123.40(10) | |||
| Cyclopropyl-Main frame | 72.62(7) | 61.55(7) | N8-C9-C11 | 117.54(10) | 116.40(11) |
| N8-C9-C10 | 120.07(11) | 119.08(11) | |||
| C9-C11-C10 | 59.97(8) | 60.00(9) | |||
| C9-C10-C11 | 59.70(8) | 59.95(9) | |||
| C10-C9-C11 | 60.33(8) | 60.05(9) | |||
3.3. General Procedure for 2-Hydroxy-NVP (2) Oxidation
3.3.1. At pH 7.4
3.3.2. At pH 10
4. Conclusions
Acknowledgments
References and Notes
- Marseille, E.; Kahn, J.G.; Mmiro, F.; Guay, L.; Musoke, P.; Fowler, M.G.; Jackson, J.B. Cost effectiveness of single-dose nevirapine regimen for mothers and babies to decrease vertical HIV-1 transmission in sub-Saharan Africa. Lancet 1999, 354, 803–809. [Google Scholar]
- Lallemant, M.; Jourdain, G.; Le Coeur, S.; Mary, J.Y.; Ngo-Giang-Huong, N.; Koetsawang, S.; Kanshana, S.; McInstosh, K.; Thaineua, V. Perinatal HIV Prevention Trial (Thailand) Investigators. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N. Engl. J. Med. 2004, 351, 217–228. [Google Scholar] [CrossRef]
- Taha, T.E.; Kumwenda, N.I.; Hoover, D.R.; Fiscus, S.A.; Kafulafula, G.; Nkhoma, C.; Nour, S.; Chen, S.; Liomba, G.; Miotti, P.G.; Broadhead, R.L. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: A randomized controlled trial. JAMA 2004, 292, 202–209. [Google Scholar]
- Guay, L.A.; Musoke, P.; Fleming, T.; Bagenda, D.; Allen, M.; Nakabiito, C.; Sherman, J.; Bakaki, P.; Ducar, C.; Deseyve, M.; et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999, 354, 795–802. [Google Scholar]
- Jackson, J.B.; Musoke, P.; Fleming, T.; Guay, L.A.; Bagenda, D.; Allen, M.; Nakabiito, C.; Sherman, J.; Bakaki, P.; Owor, M.; et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 2003, 362, 859–868. [Google Scholar]
- Lockman, S.; Shapiro, R.L.; Smeaton, L.M.; Wester, C.; Thior, I.; Stevens, L.; Chand, F.; Makhema, J.; Moffat, C.; Asmelash, A.; et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N. Engl. J. Med. 2007, 356, 135–147. [Google Scholar]
- Ruiz, L.; Negredo, E.; Domingo, P.; Paredes, R.; Francia, E.; Balagué, M.; Gel, S.; Bonjoch, A.; Fumaz, C.R.; Johnston, S.; et al. Spanish Lipodystrophy Group. Antiretroviral treatment simplification with nevirapine in protease inhibitor-experienced patients with HIV-associated lipodystrophy: 1-year prospective follow-up of a multicenter, randomized, controlled study. J. Acquir. Immune. Defic. Syndr. 2001, 27, 229–236. [Google Scholar]
- Perinatal HIV Guidelines Working Group. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2009, pp. 1–90. Available online: http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf (accessed on 11 October 2011).
- Horvath, T.; Madi, B.C.; Iuppa, I.M.; Kennedy, G.E.; Rutherford, G.; Read, J.S. Interventions for preventing late postnatal mother-to-child transmission of HIV. Cochrane Database Syst. Rev. 2009, CD006734. [Google Scholar]
- Pollard, R.B.; Robinson, P.; Dransfield, K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin. Ther. 1998, 20, 1071–1092. [Google Scholar]
- Riska, P.; Lamson, M.; MacGregor, T.; Sabo, J.; Hattox, S.; Pav, J.; Keirns, J. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug. Metab. Dispos. 1999, 27, 895–901. [Google Scholar]
- Riska, P.S.; Joseph, D.P.; Dinallo, R.M.; Davidson, W.C.; Keirns, J.J.; Hattox, S.E. Biotransformation of nevirapine, a nonnucleoside HIV-1 reverse transcriptase inhibitor, in mice, rats, rabbits, dogs, monkeys, and chimpanzees. Drug Metab. Dispos. 1999, 27, 1434–1447. [Google Scholar]
- Ren, C.; Fan-Havard, P.; Schlabritz-Loutsevitch, N.; Ling, Y.; Chan, K.K.; Liu, Z. A sensitive and specific liquid chromatography/tandem mass spectrometry method for quantification of nevirapine and its five metabolites and their pharmacokinetics in baboons. Biomed. Chromatogr. 2010, 24, 717–726. [Google Scholar]
- Chen, J.; Mannargudi, B.M.; Xu, L.; Uetrecht, J. Demonstration of the metabolic pathway responsible for nevirapine induced skin rash. Chem. Res. Toxicol. 2008, 21, 1862–1870. [Google Scholar] [CrossRef]
- Shenton, J.M.; Teranishi, M.; Abu-Asab, M.S.; Yager, J.A.; Uetrecht, J.P. Characterization of a potential animal model of an idiosyncratic drug reaction: nevirapine-induced skin rash in the rat. Chem. Res. Toxicol. 2003, 16, 1078–1089. [Google Scholar] [CrossRef]
- Wen, B.; Chen, Y.; Fitch, W.L. Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab. Dispos. 2009, 37, 1557–1562. [Google Scholar] [CrossRef]
- Srivastava, A.; Lian, L.-Y.; Maggs, J.L.; Chaponda, M.; Pirmohamed, M.; Williams, D.P.; Park, B.K. Quantifying the metabolic activation of nevirapine in patients by integrated applications of NMR and mass spectrometries. Drug Metab. Dispos. 2010, 38, 122–132. [Google Scholar] [CrossRef]
- Antunes, A.M.M.; Novais, D.A; Ferreira da Silva, J.L.; Santos, P.P.; Oliveira, M.C.; Beland, F.A.; Marques, M.M. Synthesis and oxidation of 2-hydroxynevirapine, a metabolite of the HIV reverse transcriptase inhibitor nevirapine. Org. Biomol. Chem 2011, 9, 7822–7835. [Google Scholar]
- Mirochnick, M.; Clarke, D.F.; Dorenbaum, A. Nevirapine: pharmacokinetic considerations in children and pregnant women. Clin. Pharmacokinet. 2000, 39, 281–293. [Google Scholar] [CrossRef]
- Svensson, B.E. Abilities of peroxidases to catalyse peroxidase-oxidase oxidation of thiols. Biochem. J. 1988, 56, 757–762. [Google Scholar]
- Moura, M.D.; Senna, M.I.; Madureira, D.F.; Fonseca, L.M.; Mesquita, R.A. Oral adverse effects due to the use of Nevirapine. J. Contemp. Dent. Pract. 2008, 9, 84–90. [Google Scholar]
- Scully, C.; Diz Dios, P. Orofacial effects of antiretroviral therapies. Oral Diseases 2001, 7, 205–210. [Google Scholar]
- Marrero, J.G.; San Andrés, L.; Luis, J.G. Quinone derivatives by chemical Transformations of 16-hydroxycarnosol from Salvia species. Chem. Pharm. Bull. 2005, 53, 1524–1529. [Google Scholar] [CrossRef]
- Saá, J.M.; Morey, J.; Rubido, C. An Oxidative degradation approach to p-quinones. J. Org. Chem. 1986, 51, 4471–4473. [Google Scholar] [CrossRef]
- Zimmer, H.; Lankin, D.C.; Horgan, S.W. Oxidations with potassium nitrosodisulfonate (Frémy’s radical). The Teuber reaction. Chem. Rev. 1971, 71, 229–246. [Google Scholar]
- Zielonka, J.; Zhao, H.; Xu, Y.; Kalyanaraman, B. Mechanistic similarities between oxidation of hydroethidine by Fremy’s salt and superoxide: Stopped-flow optical and EPR studies. Free Radical Biol. Med. 2005, 39, 853–863. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed; Pergamon Press: Oxford, UK, 1998; pp. 1–391. [Google Scholar]
- SMART and SAINT, Area Detector Control and Integration Software, Bruker AXS, Madison, WI, USA. 2004.
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detectors (Version 2.10). University of Göttingen, Germany, 2004. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, a Computer Program for the Refinement of Crystal Structures, University of Göttingen, GGöttingen, Germany. 1997. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows (v1.076), based on ORTEP-III (v1.03) by Johnson, C.K. and Burnett, M.N. J. Appl. Crystallogr. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Nardelli, M. Parst: A system of Fortran routines for calculating molecular structure parameters from results of crystal structure analyses. Comput. Chem. 1983, 7, 95–98. [Google Scholar] [CrossRef]
- Nardelli, M. PARST95 - an update to PARST: A system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 14 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Antunes, A.M.M.; Sidarus, M.; Novais, D.A.; Harjivan, S.G.; Santos, P.P.; Ferreira da Silva, J.L.; Beland, F.A.; Marques, M.M. Oxidation of 2-Hydroxynevirapine, a Phenolic Metabolite of the Anti-HIV Drug Nevirapine: Evidence for an Unusual Pyridine Ring Contraction. Molecules 2012, 17, 2616-2627. https://doi.org/10.3390/molecules17032616
Antunes AMM, Sidarus M, Novais DA, Harjivan SG, Santos PP, Ferreira da Silva JL, Beland FA, Marques MM. Oxidation of 2-Hydroxynevirapine, a Phenolic Metabolite of the Anti-HIV Drug Nevirapine: Evidence for an Unusual Pyridine Ring Contraction. Molecules. 2012; 17(3):2616-2627. https://doi.org/10.3390/molecules17032616
Chicago/Turabian StyleAntunes, Alexandra M. M., Muna Sidarus, David A. Novais, Shrika G. Harjivan, Pedro P. Santos, João L. Ferreira da Silva, Frederick A. Beland, and M. Matilde Marques. 2012. "Oxidation of 2-Hydroxynevirapine, a Phenolic Metabolite of the Anti-HIV Drug Nevirapine: Evidence for an Unusual Pyridine Ring Contraction" Molecules 17, no. 3: 2616-2627. https://doi.org/10.3390/molecules17032616
APA StyleAntunes, A. M. M., Sidarus, M., Novais, D. A., Harjivan, S. G., Santos, P. P., Ferreira da Silva, J. L., Beland, F. A., & Marques, M. M. (2012). Oxidation of 2-Hydroxynevirapine, a Phenolic Metabolite of the Anti-HIV Drug Nevirapine: Evidence for an Unusual Pyridine Ring Contraction. Molecules, 17(3), 2616-2627. https://doi.org/10.3390/molecules17032616




