Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil
Abstract
:1. Introduction
2. Results and Discussion
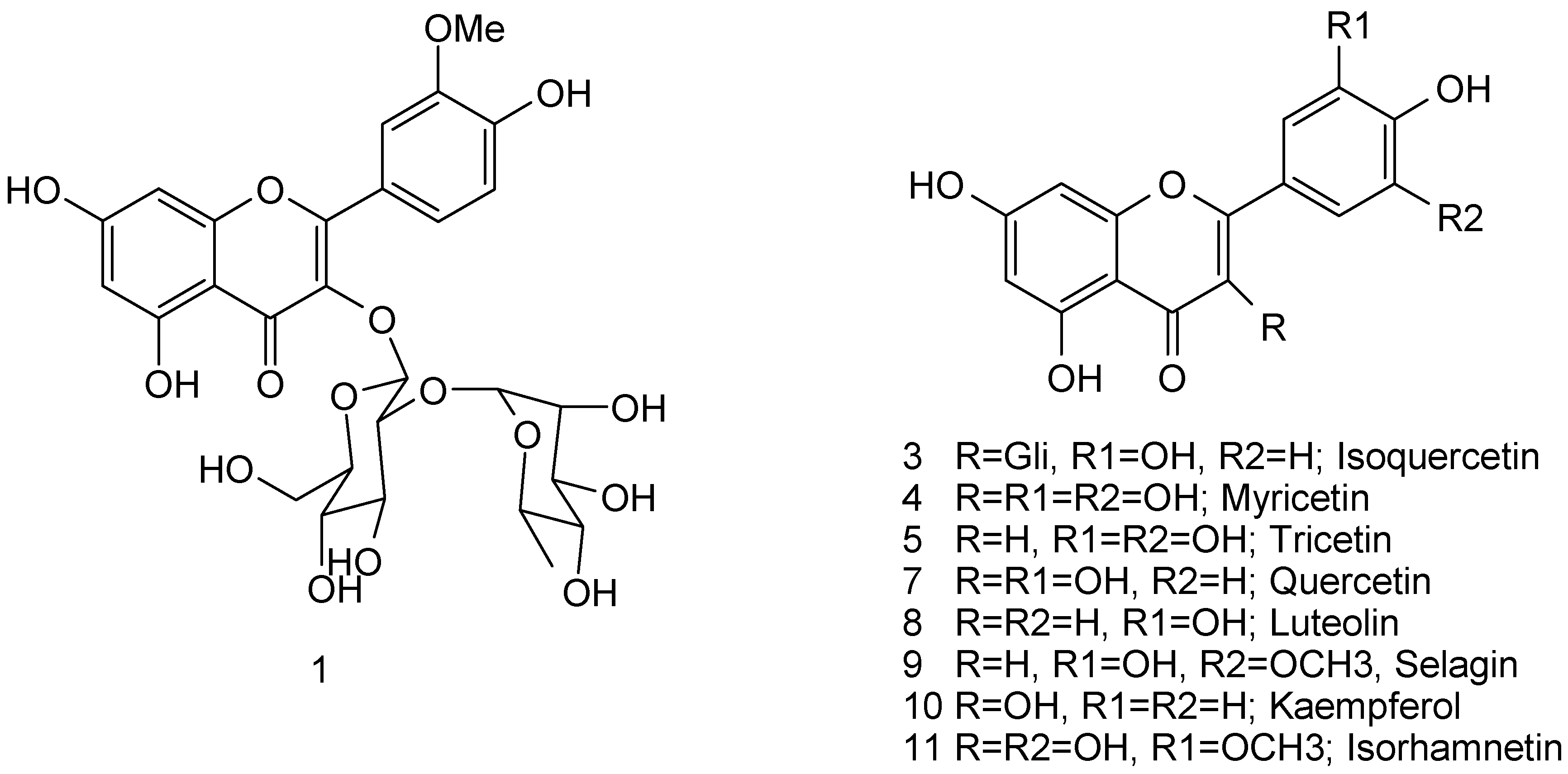
2.1. Palynological and Flavonoid Analysis
| Palynoteca registry (PUEFS) | Pollen types | Flavonoids identified * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Isoq | Myri | Tri | Quer | Lut | Sel | Kae | Isor | |||
| 1-Feb | 528 | Scrophulariaceae 2 | 35.85 | X | X | X | X | ||||
| Eupatorium | 16.82 | ||||||||||
| 2-Feb | 529 | Ricinus | 43.70 | X | X | ||||||
| M. arenosa | 26.73 | ||||||||||
| 3-Feb | 530 | Cecropia | 64.23 | X | X | X | X | ||||
| Eupatorium | 12.19 | ||||||||||
| 1-Mar | 531 | Eucalyptus | 71.73 | X | X | X | X | X | X | X | X |
| Cecropia | 21.80 | ||||||||||
| 2-Mar | 532 | Eucalyptus | 66.80 | X | X | X | X | X | X | X | X |
| Cecropia | 24.60 | ||||||||||
| 3-Mar | 533 | Eucalyptus | 50.47 | X | X | X | X | X | X | X | X |
| Cecropia | 32.33 | ||||||||||
| 4-Mar | 534 | Elaeis | 84.00 | X | X | X | X | ||||
| Melast./Crombretaceae | 10.80 | ||||||||||
| 1-Apr | 535 | M. pudica | 74.60 | X | X | X | X | ||||
| Elaeis | 16.16 | ||||||||||
| 2-Apr | 536 | Elaeis | 75.53 | X | X | X | |||||
| Eupatorium | 15.33 | ||||||||||
| May | 537 | M. pudica | 60.20 | X | X | X | |||||
| Elaeis | 30.60 | ||||||||||
| 1-Jun | 540 | Eupatorium | 77.73 | X | X | X | |||||
| Elaeis | 13.00 | ||||||||||
| 2-Jun | 912 | M. pudica | 77.07 | X | |||||||
| Non-identified | 8.52 | ||||||||||
| 3-Jun | 913 | Elaeis | 73.46 | X | |||||||
| Elephantopus | 12.89 | ||||||||||
| 1-Jul | 542 | M. pudica | 97.13 | X | X | X | X | ||||
| Elaeis | 1.40 | ||||||||||
| 2-Jul | 545 | Cecropia | 65.40 | X | X | X | X | ||||
| Elaeis | 25.87 | ||||||||||
| 3-Jul | 546 | M. pudica | 93.87 | X | X | ||||||
| Eupatorium | 3.00 | ||||||||||
| 1-Aug | 547 | M. pudica | 77.53 | X | X | X | X | X | |||
| Elaeis | 12.27 | ||||||||||
| 2-Aug | 549 | Elaeis | 60.73 | X | X | X | X | X | X | ||
| Cecropia | 22.93 | ||||||||||
| 3-Aug | 550 | M. pudica | 76.07 | X | X | X | |||||
| Cecropia | 14.60 | ||||||||||
| Sep | 551 | Eucalyptus | 36.07 | X | X | X | X | ||||
| Elaeis | 32.87 | ||||||||||
| 1-Oct | 552 | Elaeis | 48.63 | X | X | X | |||||
| Cecropia | 32.82 | ||||||||||
| 2-Oct | 553 | M. pudica | 74.80 | X | X | X | X | X | |||
| Cecropia | 15.87 | ||||||||||
| 3-Oct | 554 | Elaeis | 99.33 | X | |||||||
| Elephantopus | 0.53 | ||||||||||
| 1-Nov | 555 | Scoparia | 98.53 | X | |||||||
| Eucalyptus | 0.67 | ||||||||||
| 2-Nov | 556 | Eucalyptus | 57.80 | X | X | X | X | X | |||
| Cecropia | 34.53 | ||||||||||
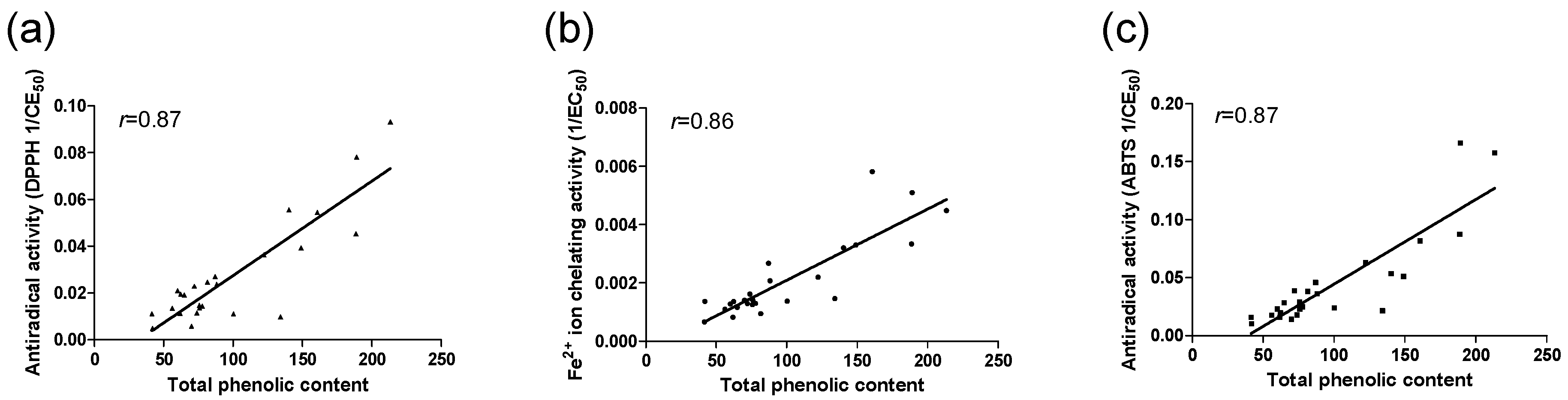
2.2. Total Phenolic Content, Antioxidant Activity, and Correlations between Total Phenolic and Metal Chelating Activity and Antiradical Activity
| Sample | Total phenolic content (mg GAE/g) a | DPPH (EC50) a,b | ABTS (EC50) a,b | Fe2+ ion chelating activity a,b |
|---|---|---|---|---|
| Feb 01 | 88.1 ± 0.5 | 42.3 ± 1,1 | 27.7 ± 0.4 | 483.6 ± 7.6 |
| Feb 02 | 188.6 ± 1.7 | 22.0 ± 0.0 | 11.4 ± 0.1 | 299.7 ± 6.3 |
| Feb 03 | 122.1 ± 1.3 | 27.5 ± 0.0 | 15.8 ± 0.5 | 455.5 ± 21.4 |
| Mar 01 | 188.9 ± 2.0 | 12.8 ± 0.1 | 6.0 ± 0.1 | 196.2 ± 4.3 |
| Mar 02 | 213.2 ± 1.1 | 10.7 ± 0.0 | 6.4 ± 0.1 | 223.0 ± 4.8 |
| Mar 03 | 160.6 ± 1.1 | 18.3 ± 0.5 | 12.2 ± 0.2 | 171.9 ± 5.9 |
| Mar 04 | 75.7 ± 1.6 | 73.2 ± 0.7 | 43.5 ± 0.8 | 706.2 ± 13.3 |
| Apr 01 | 77.6 ± 1.3 | 69.7 ± 0.8 | 40.4 ± 1.1 | 772.5 ± 3.5 |
| Apr 02 | 69.9 ± 0.3 | 17.9 ± 0.2 | 71.2 ± 1.7 | 712.1 ± 14.4 |
| May | 61.8 ± 2.0 | 88.7 ± 1.6 | 63.4 ± 0.9 | 1214.1 ± 40.3 |
| Jun 01 | 140.1 ± 0.6 | 18.0 ± 0.0 | 18.8 ± 0.7 | 312.4 ± 9.3 |
| Jun 02 | 75.6 ± 1.3 | 68.0 ± 0.4 | 34.4 ± 1.8 | 795.9 ± 21.6 |
| Jun 03 | 149.0 ± 0.8 | 25.5 ± 10.8 | 19.6 ± 0.3 | 303.3 ± 6.1 |
| Jul 01 | 59.9 ± 0.8 | 47.8 ± 0.3 | 43.7 ± 0.4 | 781.8 ± 3.9 |
| Jul 02 | 62.2 ± 0.5 | 51.1 ± 0.5 | 51.1 ± 3.2 | 737.8 ± 11.4 |
| Jul 03 | 81.4 ± 2.8 | 40.6 ± 0.3 | 26.2 ± 2.0 | 1063.6 ± 7.9 |
| Aug 01 | 41.5 ± 0.2 | 90.1 ± 0.4 | 64.1 ± 3.5 | 1507.0 ± 40.8 |
| Aug 02 | 55.9 ± 0.4 | 74.6 ± 0.1 | 57.6 ± 1.2 | 909.9± 9.6 |
| Aug 03 | 73.8 ± 0.3 | 87.1 ± 0.5 | 56.3 ± 2.5 | 6196 ± 12.9 |
| Sep | 64.8 ± 1.7 | 52.2 ± 0.0 | 35.5 ± 1.8 | 861.6 ± 7.8 |
| Oct 01 | 100.2 ± 1.1 | 90.4 ± 0.1 | 41.9 ± 1.8 | 727.3 ± 7.5 |
| Oct 02 | 71.9 ± 1.3 | 43.8 ± 0.0 | 25.8 ± 1.2 | 777.1 ± 19.4 |
| Oct 03 | 41.9 ± 1.1 | 209.1 ± 0.5 | 97.2 ± 3.8 | 734.4 ± 8.8 |
| Nov 01 | 134.1 ± 1.2 | 102.1 ± 0.5 | 46.7 ± 0.2 | 684.24 ± 5.3 |
| Nov 02 | 86.9 ± 1.1 | 37.1 ± 0.1 | 21.8 ± 0.3 | 373.4 ± 11.7 |
| Ascorbic acid | 2.5 ± 0.0 | |||
| Trolox | 2.7 ± 0.0 | |||
| EDTA | 5.1 ± 0.1 |
3. Experimental
3.1. Materials and Methods
3.1.1. Reagents and Standards
3.1.2. General Procedures
3.2. Pollen Samples and Their Classification
3.3. High Performance Liquid Chromatography-diode-array Detection (HPLC-DAD)
3.4. Extraction and Separation
3.5. Determination of Total Phenolic Content
3.6. DPPH• Radical Scavenging Assay
3.7. ABTS+• Radical Cation Decolorization Assay
3.8. Metal Chelating Activity on Ferrous Ions (Fe2+)
3.9. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 1–11 are available from the authors.
References and Notes
- Borges, R.L.B.; Lima, L.C.L.; Oliveira, P.P.; Silva, F.H.M.; Novais, J.S.; Dórea, M.C.; Santos, F.A.R. O pólen no mel do Semi-Árido brasileiro. In Apium Plantae; Santos, F.A.R., Ed.; IMSEAR: Recife, Brasil, 2006; pp. 103–118. [Google Scholar]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese natural parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Estevinho, L.M.; Rodriguesa, S.; Dias, T.; Da Silva, J.P.; Feás, X. Botanical, nutritional and microbiological characterisation of honeybee-collected pollen from Portugal. Food Chem. Toxicol. 2012, in press. [Google Scholar]
- Dórea, M.C.; Novais, J.S.; Santos, F.A.R. Botanical profile of bee pollen from the southern coastal region of Bahia, Brazil. Acta Bot. Bras. 2010, 24, 862–867. [Google Scholar] [CrossRef]
- Kroyer, G.; Hegedus, N. Evaluation of bioactive properties of pollen extracts as functional dietary food supplement. Innov. Food Sci. Emerg. Technol. 2001, 2, 171–174. [Google Scholar] [CrossRef]
- Lins, A.C.S.; Silva, T.M.S.; Câmara, C.A.; Silva, E.M.S.; Freitas, B.M. Flavonóides isolados do pólen coletado pela abelha Scaptotrigona bipunctata (canudo). Rev. Bras. Farmacogn. 2003, 13, 40–41. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; Lins, A.C.S.; Barbosa, J.M.; Silva, E.M.S.; Freitas, B.M.; Santos, F.A.R. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Comp. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; Lins, A.C.S.; Agra, M.F.; Silva, E.M.S.; Reis, I.T.; Freitas, B.M. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela). Ann. Acad. Bras. Cienc. 2009, 81, 173–178. [Google Scholar] [CrossRef]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; Cunha, A.P. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Conde, E.; Cadahia, E.; Garciavallejo, M.C. Low molecular weight polyphenols in leaves of Eucalyptus camaldulensis, E. globulus and E. rudis. Phytochem. Anal. 1997, 8, 186–193. [Google Scholar]
- Zhaobang, S.; Jianping, X. Chemical constituents of Eucalyptus citriodora leaves. Part II. Isolation and identification of flavonoid compounds. Linchan Huaxue Yu Gongye 1987, 7, 28–34. [Google Scholar]
- Yao, L.H.; Jiang, Y.M.; D’arcy, B.; Singanusong, R.T.; Datta, N.; Caffin, N.; Raymont, K. Quantitative high-performance liquid chromatography analyses of flavonoids in Australian Eucalyptus honeys. J. Agric. Food Chem. 52, 210–214.
- Lobstein, A.; Weniger, B.; Um, B.H.; Steinmetz, M.; Declercq, L.; Anton, R. 4 ''-Hydroxymaysin and cassiaoccidentalin B, two unusual C-glycosylflavones from Mimosa pudica (Mimosaceae). Biochem. Sys. Ecol. 2002, 30, 375–377. [Google Scholar] [CrossRef]
- Kawasaki, M.; Hayashi, T.; Arisawa, M.; Morita, N.; Berganza, L.H. 8-Hydroxytricetin 7-glucuronide, a β-glucuronidase inhibitor from Scoparia dulcis. Phytochemistry 1988, 27, 3709–3711. [Google Scholar]
- Albuquerque, M.; Pires, A.M.L.; Pessoa, O.D.L.; Silveira, E.R. Terpenoids, flavonoids and other constituents of Eupatorium betonicaeforme (Asteraceae). J. Braz. Chem. Soc. 2006, 17, 68–72. [Google Scholar]
- Suksamrarn, A.; Chotipong, A.; Suavansri, T.; Boongird, S.; Timsulksai, P.; Vimuttilpong, S.; Chuaynugul, A. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharmacal. Res. 2004, 27, 507–511. [Google Scholar] [CrossRef]
- Militao, G.C.G.; Albuquerque, M.; Pessoa, O.D.L.; Pessoa, C.; Moraes, M.E.A.; de Moraes, M.O.; Costa-Lotufo, L.V. Cytotoxic activity of nepetin, a flavonoid from Eupatorium ballotaefolium HBK. Pharmazie 2004, 59, 965–966. [Google Scholar]
- Aqil, M.; Khan, I.Z.; Otukoyoung, E.E. Three flavonol glycosides from Ricinus communis. Bull. Chem. Soc. Ethiopia 1997, 11, 51–53. [Google Scholar]
- Upasani, S.M.; Kotkar, H.M.; Mendki, P.S.; Maheshwari, V.L. Partial characterization and insecticidal properties of Ricinus communis L. foliage flavonoids. Pest. Manag. Sci. 2003, 59, 1349–1354. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon 13 NMR of Flavonoids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Norbñk, R.; Kondo, T. Flavonol glycosides from flowers of Crocus speciosus and C-antalyensis. Phytochemistry 1999, 51, 1113–1119. [Google Scholar]
- Tao, W.; Yang, N.; Duan, J.; Wu, D.; Guo, J.M.; Tang, Y.; Qian, D.; Zhu, Z. Simultaneous determination of eleven major flavonoids in the pollen of Typha angustifolia by HPLC-PDA-MS. Phytochem. Anal. 2011, 22, 455–461. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar]
- Duh, P.D.; Tu, Y.Y.; Yen, G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). Food Sci. Technol. 1999, 32, 269–277. [Google Scholar]
- Erdtman, G. The acetolysis method. A revised description. Svensk Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Santos, F.A.R. Identificação botânica do pólen apícola. Magistra 2011, 23, 5–9. [Google Scholar]
- Medeiros, K.C.P.; Figueiredo, C.A.V.; Figueredo, T.B.; Freire, K.R.L.; Santos, F.A.R.; Alcantara-Neves, N.M.; Silva, T.M.S.; Piuvezam, M.R. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid-Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Freire, K.R.L.; Lins, A.C.S.; Dórea, M.C.; Santos, F.A.R.; Camara, C.A.; Silva, T.M.S. Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil. Molecules 2012, 17, 1652-1664. https://doi.org/10.3390/molecules17021652
Freire KRL, Lins ACS, Dórea MC, Santos FAR, Camara CA, Silva TMS. Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil. Molecules. 2012; 17(2):1652-1664. https://doi.org/10.3390/molecules17021652
Chicago/Turabian StyleFreire, Kristerson R. L., Antonio C. S. Lins, Marcos C. Dórea, Francisco A. R. Santos, Celso A. Camara, and Tania M. S. Silva. 2012. "Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil" Molecules 17, no. 2: 1652-1664. https://doi.org/10.3390/molecules17021652
APA StyleFreire, K. R. L., Lins, A. C. S., Dórea, M. C., Santos, F. A. R., Camara, C. A., & Silva, T. M. S. (2012). Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil. Molecules, 17(2), 1652-1664. https://doi.org/10.3390/molecules17021652




