Abstract
A new iridoid glycoside, named loganic acid ethyl ester (1), together with five known compounds: chlorogenic acid (2), caffeic acid (3), loganin (4), cantleyoside (5) and syringaresinol-4′,4′′-O-bis-β-D-glucoside (6) were isolated from the roots of Dipsacus asper. The structure of compound 1 was elucidated on the basis of detailed spectroscopic analyses. Lignan is isolated from Dipsacaceae species for the first time. Compounds 1, 4 and 5 had moderate neuroprotective effects against the Aβ25–35 induced cell death in PC12 cells.
1. Introduction
Dipsacus asper Wall., (Dipsacaceae), is a perennial herb widely distributed in the southwest of the People's Republic of China. The roots of D. asper have been used in Traditional Chinese Medicine for hundreds of years as an antiosteoporosis, tonic and antiaging agent for the therapy of low back pain, traumatic hematoma, threatened abortion and bone fractures [1]. In previous studies, dozens of chemical constituents, including triterpene saponins, iridoids, phenolics and alkaloids have been identified from the roots of D. asper [2]. Pharmacological studies so far have demonstrated that, saponins isolated from the roots of this plant possess anticomplementary, antinociceptive, cytotoxic, osteoprotective, cardioprotective and inhibition of Alzheimer's disease activities, while phenolics possess neuroprotective and antioxidant effects [2]. Herein we report on the isolation from the roots of D. asper and structural elucidation of a new iridoid glycoside, together with five known compounds: chlorogenic acid (2) [3], caffeic acid (3) [4], loganin (4) [5], cantleyoside (5) [6] and syringaresinol-4′,4′′-O-bis-β-D-glucoside (6) [7] (Figure 1). Lignan is isolated from Dipsacaceae species for the first time. Compounds 1, 4–6 were evaluated for their neuroprotective effects in PC12 cells against the Aβ25–35 induced cell death.
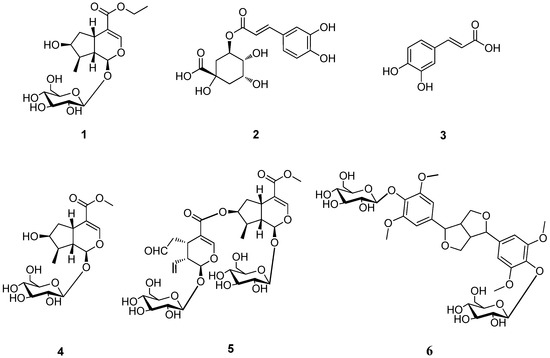
Figure 1.
The chemical structures of compounds 1–6.
2. Results and Discussion
The air-dried roots of D. asper were pulverized and refluxed with 70% MeOH. The 70% MeOH extract was evaporated in vacuo to remove the MeOH and then diluted with H2O. The soluble fraction was subjected to column chromatography over macroporous resin HPD-722 and ODS to afford compounds 1–6.
Loganic acid ethyl ester (1) was obtained as a white, amorphous powder. The TOF-MS gave a molecular ion at m/z 449.1818 ([M+HCOO]−), which corresponds to a molecular formula of C18H28O10. The IR spectrum (KBr) showed absorptions for hydroxyl (3420 cm−1), carboxyl (1688 cm−1), double bond (1634cm−1) and C–O–C (1076 cm−1) groups. The 1H-NMR spectrum of 1 showed one anomeric proton at δH 4.65 (d, J = 8.0 Hz), and together with the corresponding carbon resonances at δC 100.1, it was easily deduced that compound 1 contained a β-glucopyranose moiety. Meanwhile, the 1H-NMR spectrum of 1 showed the presence of an iridoid structure with one acetal proton at δH 5.27 (1H, d, J = 4.5 Hz), one characteristic H-3 proton at δH 7.38 (1H, s), one oxygenated methylene proton at δH 4.15 (2H, m), one oxygenated methine proton at δH 4.04 (1H, m), one methylene proton at δH 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz) and 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz,), three methine protons at δH 3.11 (1H, q, J = 8.0 Hz), 1.88 (1H, m) and 2.03 (1H, dt, J = 4.5, 9.0 Hz), and two methyl protons at δH 1.09 (3H, d, J = 7.0 Hz) and 1.27 (3H, t, 7.1). The 13C-NMR spectrum of 1 showed 18 resonances, including six for a sugar unit and twelve for an iridoid aglycone moiety.
It is found that the NMR chemical shifts of 1 were very similar to those of the known compound loganic acid [8], which has 28 mass units less than 1. Compound 1 might be an ethyl ester of loganic acid, as indicated by additional signals of δC 61.0 (C-12) and δC 14.6 (C-13), with the corresponding δH 4.15 (2H, m, H-12) and δH 1.27 (3H, t, 7.1Hz, H-13). HMBC correlations of H-13 (δH 1.27) with C-12 (δC 61.0) and H-12 (δH 4.15) with C-11 (δC 169.1) were also observed (Figure 2), which confirmed the above structure. The proton and carbon signals were assigned unambiguously using 1H, 13C, HSQC, HMBC NMR experiments.
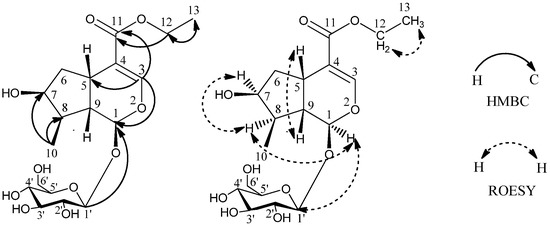
Figure 2.
Key HMBC and ROESY correlations of 1.
The relative configuration of 1 was further determined by the NOESY experiment. The NOE correlations of δH 5.27 (H-1) with δH 1.88 (H-8), δH 3.11 (H-5) with δH 2.03 (H-9), δH 4.04 (H-7) with δH 1.88 (H-8) and no correlation between δH 1.88 (H-8) and δH 2.03 (H-9), revealed the relative configuration of 1 (Figure 2). Thus, the structure of 1 was elucidated as shown in Figure 1 determined as loganic acid ethyl ester.
Compounds 2-6 were identified as chlorogenic acid (2) [3], caffeic acid (3) [4], loganin (4) [5], cantleyoside (5) [6] and syringaresinol-4′,4′′-O-bis-β-D-glucoside (6) [7], respectively, by comparison of the spectroscopic data with those reported in the literature.
Zhang et al. found that Dipsacus asper extract significantly ameliorated animal’s performance impairment in the passive avoidance task and suppressed the overexpression of hippocampal Aβ immunoreactivity [9]. It has been demonstrated that the phenolics and saponins isolated from the roots of D. asper all possess neuroprotective effects [10,11], so the iridoids and lignan of compounds 1, 4–6 were examined for their neuroprotective effects against the Aβ25-35 induced cytotoxicity in PC12 cells by the MTT method using salvianolic acid B as positive control [12,13]. The result showed that compounds 1, 4 and 5 had moderate protective effects against the Aβ25-35 induced cell death (Table 1).

Table 1.
Neuroprotective effects of the compounds 1, 4–6 against the Aβ25-35 induced PC12 cell death.
| Compound | Inhibition ratio (%) a | ||
|---|---|---|---|
| 10 μM | 30 μM | 100 μM | |
| 1 | 27.66 ± 1.72 b | 18.59 ± 1.10 b | 14.17 ± 0.38 b |
| 4 | 26.40 ± 2.05 b | 19.17 ± 1.34 b | 15.98 ± 2.11 b |
| 5 | 23.17 ± 1.87 b | 17.24 ± 0.87 b | 12.02 ± 1.46 b |
| 6 | 38.73 ± 2.01 | 35.24 ± 2.33 | 36.59 ± 2.33 |
| salvianolic acid B c | 18.28 ± 1.02 b | 7.28 ± 0.87 b | 4.28 ± 0.58 b |
Results are presented as mean ± SEM (n = 3). a Inhibition ratio (%): 35.06 ± 0.86 (treated only with Aβ25-35, p < 0.01 compared with the control group); b p < 0.01 compared with group treated only with Aβ25-35; c positive control.
3. Experimental
3.1. General
Optical rotations were measured with a JASCO P-1020 polarimeter. UV spectra were obtained on a Shimadzu UV-2450 UV-visible spectrophotometer. IR spectra were measured on a Bruker Tensor-27 spectrophotometer. NMR spectra were recorded at 303k on a Bruker AV-500 NMR (1H-NMR, 500 MHz; 13C-NMR, 125 MHz) instrument with TMS as internal standard, and chemical shifts were recorded as δ values. The 2D-NMR experiments, HSQC, HMBC and ROESY were performed using standard Bruker pulse sequences. The ESIMS experiment was performed on an Agilent SL G1946D single quadrupole mass spectrometer with an ESI source in negative-ion mode. the TOF-MS experiment was performed on Agilent orthogonal TOF/MS equipped with ESI source [drying gas, N2; flow rate, 9.0 L/min; temperature, 325 °C; nebulizer, 35 psig; capillary, 3,000 V; skimmer, 60 V; OCT RFV, 250 V; the sample was dissolved in MeOH; analyzed in negative-ion mode; fragment voltage, 120 V]. Column chromatography (CC) were performed on macroporous resin HPD722 (Cangzhou Bon Adsorber Technology Co., Ltd., China) and ODS (40–63 μm, YMC, Japan).
3.2. Plant Material
The air-dried roots of D. asper were collected from Lianshan County, Sichuan Province of China in July 2009, and authenticated by Professor Ping Li, Department of Traditional Chinese Medicine, China Pharmaceutical University. Voucher specimens (NO.20090701) are deposited in the State Key laboratory of Natural Products and Functions, China Pharmaceutical University.
3.3. Extraction and Isolation
The air-dried roots of D. asper (3 kg) were powdered and successively extracted two times with 70% MeOH (2 × 10 L) under reflux. After removal of the solvents in vacuo, the residue (456 g) was suspended in H2O (30 L) to afford H2O-soluble and H2O-insoluble fractions. The soluble fraction was chromatographied on a macroporous resin HPD722 column eluted with H2O, followed by step gradients with EtOH to obtain four fractions (Fractions 1–4). Fraction 1 (water eluent) was concentrated to subject to ODS column chromatography and eluted with MeOH–H2O (0:1–2:8) to afford compounds 2 (25 mg) and 3 (16 mg). Fraction 2 (20% EtOH eluent) was concentrated to subject to ODS column chromatography and eluted with MeOH–H2O (0:1–3:7) to afford compounds 5 (10 mg), 4 (24 mg), 6 (5 mg), and 1 (12 mg).
Loganic acid ethyl ester (1). White, amorphous powder; 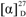 −86.3(c 0.082, MeOH); UV (MeOH) λmax (log ε) 198 (4.07), 235 (4.36) nm; IR (KBr) γmax 3420, 2933, 1688, 1634, 1399, 1288, 1076 cm−1; 1H-NMR (CD3OD) δ 5.27 (1H, d, J = 4.5 Hz, H-1), 7.38 (1H, s, H-3), 3.11 (1H, q, J = 8.0 Hz, H-5), 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz, H-6a), 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz, H-6b), 4.04 (1H, m, H-7), 1.88 (1H, m, H-8), 2.03 (1H, dt, J = 4.5, 9.0 Hz, H-9), 1.09 (3H, d, J = 7.0 Hz, H-10), 4.15 (2H, m, H-12), 1.27 (3H, t, J = 7.1 Hz, H-13), 4.65 (1H, d, J = 8.0 Hz, H-1′), 3.20 (m, H-2′), 3.30 (m, H-3′), 3.28 (m, H-4′), 3.37 (1H, m, H-5′), 3.66 (1H, m, H-6′a), 3.89 (1H, dd, J = 1.7, 12.0 Hz, H-6′b); 13C-NMR (CD3OD) δ 97.7 (C-1), 151.9 (C-3), 114.2 (C-4), 32.1 (C-5), 42.8 (C-6), 75.0 (C-7), 42.1 (C-8), 46.5 (C-9), 13.4 (C-10), 169.1 (C-11), 61.0 (C-12), 14.6 (C-13), 100.1 (C-1′), 74.7 (C-2′), 78.3 (C-3′), 71.6 (C-4′), 78.0 (1H, m, C-5′), 62.8 (C-6′); negative ESI-MS m/z 449 [M+HCOO]−; negative TOF-MS m/z 449.1818 [M+HCOO]− (calcd for C19H29O12, 449.1817).
−86.3(c 0.082, MeOH); UV (MeOH) λmax (log ε) 198 (4.07), 235 (4.36) nm; IR (KBr) γmax 3420, 2933, 1688, 1634, 1399, 1288, 1076 cm−1; 1H-NMR (CD3OD) δ 5.27 (1H, d, J = 4.5 Hz, H-1), 7.38 (1H, s, H-3), 3.11 (1H, q, J = 8.0 Hz, H-5), 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz, H-6a), 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz, H-6b), 4.04 (1H, m, H-7), 1.88 (1H, m, H-8), 2.03 (1H, dt, J = 4.5, 9.0 Hz, H-9), 1.09 (3H, d, J = 7.0 Hz, H-10), 4.15 (2H, m, H-12), 1.27 (3H, t, J = 7.1 Hz, H-13), 4.65 (1H, d, J = 8.0 Hz, H-1′), 3.20 (m, H-2′), 3.30 (m, H-3′), 3.28 (m, H-4′), 3.37 (1H, m, H-5′), 3.66 (1H, m, H-6′a), 3.89 (1H, dd, J = 1.7, 12.0 Hz, H-6′b); 13C-NMR (CD3OD) δ 97.7 (C-1), 151.9 (C-3), 114.2 (C-4), 32.1 (C-5), 42.8 (C-6), 75.0 (C-7), 42.1 (C-8), 46.5 (C-9), 13.4 (C-10), 169.1 (C-11), 61.0 (C-12), 14.6 (C-13), 100.1 (C-1′), 74.7 (C-2′), 78.3 (C-3′), 71.6 (C-4′), 78.0 (1H, m, C-5′), 62.8 (C-6′); negative ESI-MS m/z 449 [M+HCOO]−; negative TOF-MS m/z 449.1818 [M+HCOO]− (calcd for C19H29O12, 449.1817).
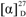 −86.3(c 0.082, MeOH); UV (MeOH) λmax (log ε) 198 (4.07), 235 (4.36) nm; IR (KBr) γmax 3420, 2933, 1688, 1634, 1399, 1288, 1076 cm−1; 1H-NMR (CD3OD) δ 5.27 (1H, d, J = 4.5 Hz, H-1), 7.38 (1H, s, H-3), 3.11 (1H, q, J = 8.0 Hz, H-5), 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz, H-6a), 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz, H-6b), 4.04 (1H, m, H-7), 1.88 (1H, m, H-8), 2.03 (1H, dt, J = 4.5, 9.0 Hz, H-9), 1.09 (3H, d, J = 7.0 Hz, H-10), 4.15 (2H, m, H-12), 1.27 (3H, t, J = 7.1 Hz, H-13), 4.65 (1H, d, J = 8.0 Hz, H-1′), 3.20 (m, H-2′), 3.30 (m, H-3′), 3.28 (m, H-4′), 3.37 (1H, m, H-5′), 3.66 (1H, m, H-6′a), 3.89 (1H, dd, J = 1.7, 12.0 Hz, H-6′b); 13C-NMR (CD3OD) δ 97.7 (C-1), 151.9 (C-3), 114.2 (C-4), 32.1 (C-5), 42.8 (C-6), 75.0 (C-7), 42.1 (C-8), 46.5 (C-9), 13.4 (C-10), 169.1 (C-11), 61.0 (C-12), 14.6 (C-13), 100.1 (C-1′), 74.7 (C-2′), 78.3 (C-3′), 71.6 (C-4′), 78.0 (1H, m, C-5′), 62.8 (C-6′); negative ESI-MS m/z 449 [M+HCOO]−; negative TOF-MS m/z 449.1818 [M+HCOO]− (calcd for C19H29O12, 449.1817).
−86.3(c 0.082, MeOH); UV (MeOH) λmax (log ε) 198 (4.07), 235 (4.36) nm; IR (KBr) γmax 3420, 2933, 1688, 1634, 1399, 1288, 1076 cm−1; 1H-NMR (CD3OD) δ 5.27 (1H, d, J = 4.5 Hz, H-1), 7.38 (1H, s, H-3), 3.11 (1H, q, J = 8.0 Hz, H-5), 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz, H-6a), 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz, H-6b), 4.04 (1H, m, H-7), 1.88 (1H, m, H-8), 2.03 (1H, dt, J = 4.5, 9.0 Hz, H-9), 1.09 (3H, d, J = 7.0 Hz, H-10), 4.15 (2H, m, H-12), 1.27 (3H, t, J = 7.1 Hz, H-13), 4.65 (1H, d, J = 8.0 Hz, H-1′), 3.20 (m, H-2′), 3.30 (m, H-3′), 3.28 (m, H-4′), 3.37 (1H, m, H-5′), 3.66 (1H, m, H-6′a), 3.89 (1H, dd, J = 1.7, 12.0 Hz, H-6′b); 13C-NMR (CD3OD) δ 97.7 (C-1), 151.9 (C-3), 114.2 (C-4), 32.1 (C-5), 42.8 (C-6), 75.0 (C-7), 42.1 (C-8), 46.5 (C-9), 13.4 (C-10), 169.1 (C-11), 61.0 (C-12), 14.6 (C-13), 100.1 (C-1′), 74.7 (C-2′), 78.3 (C-3′), 71.6 (C-4′), 78.0 (1H, m, C-5′), 62.8 (C-6′); negative ESI-MS m/z 449 [M+HCOO]−; negative TOF-MS m/z 449.1818 [M+HCOO]− (calcd for C19H29O12, 449.1817).3.4. Neuroprotective Effects
Rat pheochromocytoma PC12 cell line, TCR 3, was obtained from the Committee on Type Culture collection of Chinese Academy of Sciences (CTCCAS, Shanghai, China) and grown in RPMI1640-based medium (Gibco, Gaithersburg, MD, USA) supplemented with 10% NBCS (Gibco). Cells were maintained at 37 °C in an atmosphere of 95% air/5% CO2 saturated with H2O. At 24 h after seeding in 96-well microplates, cells were incubated for 48 h with 15 μM Aβ25-35 (Sigma, St. Louis, MO, USA) in the absence or presence of the purified compounds. Cellular viability was evaluated by the MTT reduction assay. Briefly, 4 h after incubation with 0.5% MTT (20 μL) at 37 °C, the formazan crystals were lysed in 150 μL dimethyl sulfoxide (DMSO), and the microplates were shaken vigorously to ensure complete solubilization. The optical density at 570 nm (OD570 nm) was determined in a Microplate Reader (Dynatec Laboratories, Alexandria, VA, USA). All values were obtained from 6 wells and from 3 separate experiments. The inhibition rates (%) were calculated by comparing the treatment group with the tested compounds and the solvent control group. The percent of inhibition rate (%) = [1 − OD value of sample well/ OD value of control well] × 100.
4. Conclusions
In summary, three iridoid glycosides 1, 4–5, two phenolic acids 2, 3 and a lignan glycoside 6 were isolated from the roots of D. asper. Lignan is isolated for the first time from Dipsacaceae species. Compound 1 was found to be a new iridoid glycoside by detailed spectroscopic analyses. Iridoid glycosides isolated from the roots of D. asper exhibited moderate neuroprotective effects against the Aβ25-35 induced cell death in PC12 cells. As the fraction containing compound 1 was obtained by using EtOH-H2O mixture, its presence could an artifact of the isolation process.
Acknowledgments
This work was supported by the Key Project of the National Natural Science Foundation of China (Grant no.30730113). The assistance of the staff is gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seng, Y.H.; Bae, K.H. Antioxidant activity of caffeoylquinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y. Phytochemicals and biological activities of Dipsacus species. Chem. Biodiv. 2011, 8, 414–430. [Google Scholar] [CrossRef]
- Sun, Y.R.; Dong, J.X.; Wu, S.G. Studies on chemical constituents from Eucommia ulmoides Oliv. J. Chin. Med. Mater. 2004, 27, 341–343. [Google Scholar]
- Li, Y.M.; Wang, T.Z.; Wang, Z.X. Studies on chemical constituents in dried buds of Lonicera similis Hemsl. China J. Chin. Mat. Med. 2001, 26, 45–47. [Google Scholar]
- Qin, S.J.; Li, H.J.; Li, P.; Tang, D. Studies on chemical constituents of aerial parts of Lonicera dasystyla Rehd. Chin. Pharm. J. 2008, 43, 662–664. [Google Scholar]
- Wei, F.; Lou, Z.C. Structure determination of sylvestroside III from Dipsacus asper Wall. China Tradit. Herbal Drugs 1996, 27, 265–266. [Google Scholar]
- Ovodov, Y.S.; Frolova, G.M.; Nefedova, M.Y.; Elyakov, G.B. Glycosides of Eleutherococcussenticosus II, the structure of eleutherosides A, B1, C and D. Khim. Prir. Soedin. 1967, 3, 63–64. [Google Scholar]
- Tomita, H.; Mouri, Y. An iridoidglucoside from dipsacus asperoides. Phytochemistry 1996, 42, 239–240. [Google Scholar]
- Zhang, Z.J.; Qian, Y.H.; Hu, H.T.; Yang, J.; Yang, G.D. The herbal medicine Dipsacus asper wall. extract reduces the cognitive deficits and overexpression of beta-amyloid protein induced by aluminum exposure. Life Sci. 2003, 73, 2443–2454. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Yang, Z.L.; Xu, L.; Li, P.; Hu, Y.Z. Akebia saponin D, a saponin component from Dipsacus asper Wall., protects PC 12 cells against amyloid-beta induced cytotoxicity. Cell Biol. Int. 2009, 33, 1102–1110. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liu, A.H.; Wu, H.L.; Westenbroek, C.; Song, Q.L.; Yu, H.M. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Aβ25-35-induced reduction in BPRP in PC12 cells. Biochem. Bioph. Res. Commun. 2006, 348, 593–599. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, N.H.; Zhang, J.T. Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by salvianolic acid B, a new compound isolated from Radix Salviaemiltiorrhizae. Phytomedicine 2007, 14, 492–497. [Google Scholar]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).