New Triterpenes from Maytenus robusta: Structural Elucidation Based on NMR Experimental Data and Theoretical Calculations
Abstract
:1. Introduction
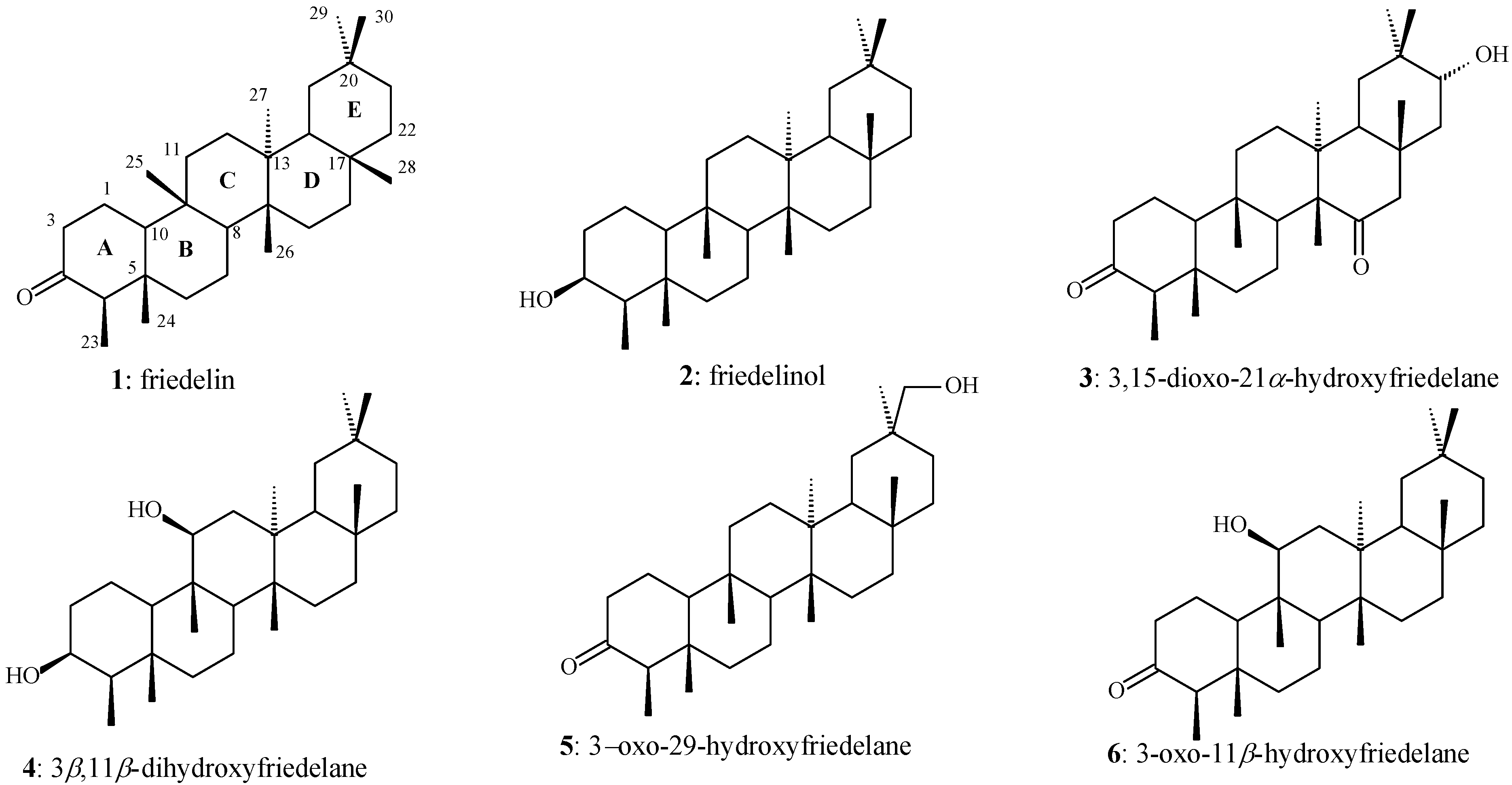

2. Results and Discussion
2.1. Structural Analysis
2.1.1. Compounds 1 and 7
| Carbon | Compound/ δC | |||||
|---|---|---|---|---|---|---|
| 7 | 12 | 13 | 10 | 14 | 15 | |
| 1 | 39.6 | 33.2 | 39.6 | 33.8 | 33.8 | 40.4 |
| 2 | 34.2 | 25.4 | 34.2 | 28.4 | 28.3 | 18.7 |
| 3 | 218.2 | 76.3 | 217.9 | 180.1 | 180.6 | 42.1 |
| 4 | 47.4 | 37.2 | 47.4 | 25.4 | 25.9 | 33.3 |
| 5 | 54.9 | 50.1 | 54.9 | 40.7 | 40.7 | 56.1 |
| 6 | 19.7 | 18.3 | 19.8 | 18.3 | 25.0 | 18.7 |
| 7 | 33.7 | 33.2 | 32.7 | 32.0 | 29.6 | 33.3 |
| 8 | 41.6 | 41.9 | 41.6 | 40.0 | 40.5 | 42.1 |
| 9 | 49.6 | 49.5 | 49.7 | 47.2 | 47.1 | 50.4 |
| 10 | 36.8 | 37.5 | 36.8 | 41.5 | 39.1 | 37.5 |
| 11 | 21.6 | 20.9 | 21.6 | 21.7 | 21.5 | 20.9 |
| 12 | 23.9 | 23.9 | 23.9 | 24.0 | 24.9 | 24.0 |
| 13 | 49.6 | 48.9 | 48.8 | 49.6 | 38.0 | 49.5 |
| 14 | 42.1 | 42.1 | 42.3 | 42.5 | 42.9 | 41.9 |
| 15 | 32.6 | 33.6 | 32.7 | 32.7 | 27.3 | 33.6 |
| 16 | 21.6 | 21.6 | 20.8 | 21.8 | 35.4 | 21.6 |
| 17 | 54.9 | 54.9 | 53.9 | 54.9 | 43.1 | 54.9 |
| 18 | 44.7 | 44.7 | 44.2 | 44.8 | 48.1 | 44.8 |
| 19 | 41.9 | 41.9 | 40.2 | 42.0 | 47.8 | 41.9 |
| 20 | 27.3 | 27.4 | 27.8 | 27.4 | 150.6 | 27.4 |
| 21 | 46.4 | 46.5 | 47.9 | 46.5 | 29.7 | 46.5 |
| 22 | 148.6 | 148.7 | 148.0 | 148.7 | 39.8 | 148.7 |
| 23 | 26.6 | 28.6 | 26.6 | 19.4 | 19.5 | 33.4 |
| 24 | 21.1 | 22.5 | 21.1 | 18.8 | 18.7 | 21.6 |
| 25 | 15.7 | 15.7 | 15.7 | 16.5 | 15.8 | 15.9 |
| 26 | 16.4 | 16.6 | 16.5 | 24.8 | 20.0 | 16.7 |
| 27 | 16.6 | 16.8 | 16.5 | 16.5 | 14.3 | 16.7 |
| 28 | 16.1 | 16.1 | 15.2 | 16.2 | 17.9 | 16.1 |
| 29 | 110.1 | 110.1 | 109.5 | 110.1 | 109.3 | 110.1 |
| 30 | 25.0 | 25.3 | 19.7 | 25.0 | 19.2 | 25.0 |
2.1.2. Compound 2
2.1.3. Compound 8
| Triterpene 8 | Compound 11 | |||||
|---|---|---|---|---|---|---|
| Atom | Type | δC | δH | HMBC | COSY | δC |
| 1 | CH2 | 19.1 | 1.73 (H α); 1.58 (Hβ) | H-2 α; H-2β | 33.2 | |
| 2 | CH2 | 34.5 | 2.52 (H α); J = 13.2; t 2.62 (H β); J = 13.8 and 6.6 Hz; dd | H-1 α; H-1β | H-1 α | 25.4 |
| 3 | C | 175.6 | H-1α; H-1 β; H-2β | 76.3 | ||
| 4 | CH2 | 36.1 | 1.18 (Ha); 1.32 (Hb) | H-23; H-24 | 37.2 | |
| 5 | C | 36.8 | H-23; H-24 | 50.1 | ||
| 6 | CH2 | 38.8 | 1.38 (H α ); 1.59 (H β) | H-24 | 18.3 | |
| 7 | CH2 | 18.0 | 1.51 (H α and Hβ) | 33.2 | ||
| 8 | CH | 52.6 | 1.34 (H α) | H-25; H-26 | 41.9 | |
| 9 | C | 42.9 | H-25 | 49.5 | ||
| 10 | CH | 58.2 | 1.25 (H α) | H-1 α; H-1β; H-2α; H-2β; H-11; H-24; H-25 | 37.5 | |
| 11 | CH | 84.1 | 4.25 (H α); J = 5.2 and 11.2 Hz; dd | H-12 α; H-12β; H-25 | H-12 | 20.9 |
| 12 | CH2 | 37.6 | 1.61 (H α ); 1.67 (H β) | H-11; H-27 | 23.9 | |
| 13 | C | 40.7 | H-26; H-27 | 48.9 | ||
| 14 | C | 37.9 | H-26 | 42.1 | ||
| 15 | CH2 | 32.1 | 1.54 (H α and Hβ) | H-26 | 33.6 | |
| 16 | CH2 | 35.9 | 1.39 (H β ); 1.56 (H α) | H-28 | 21.6 | |
| 17 | C | 30.0 | 54.9 | |||
| 18 | CH | 42.6 | 1.61 (H β) | H-27; H-28 | 44.7 | |
| 19 | CH2 | 35.3 | 1.39 (H α ); 1.24 (H β) | H-29; H-30 | 41.9 | |
| 20 | C | 28.1 | H-29; H-30 | 27.4 | ||
| 21 | CH2 | 32.7 | 1.48 (H α); | H-29; H-30 | H-22 β | 46.5 |
| 22 | CH2 | 39.2 | 1.49 (H α ); 0.97 (H β) | H-28 | 148.7 | |
| 23 | CH3 | 7.7 | 0.78; J = 7.4 Hz; t | H-4 | 28.6 | |
| 24 | CH3 | 22.1 | 0.79; s | 22.5 | ||
| 25 | CH3 | 13.6 | 0.97; s | H-11 | 15.7 | |
| 26 | CH3 | 19.9 | 1.04; s | 16.6 | ||
| 27 | CH3 | 19.3 | 1.09; s | 16.8 | ||
| 28 | CH3 | 32.0 | 1.18; s | 16.1 | ||
| 29 | CH3 | 34.9 | 0.95; s | H-30 | 110.1 | |
| 30 | CH3 | 31.7 | 0.99; s | H-29 | 25.3 | |
2.1.4. Compound 9
| Triterpene 9 | Compound 12 | |||||
|---|---|---|---|---|---|---|
| Atom | Type | δC | δH | HMBC | COSY | δC |
| 1 | CH2 | 38.9 | 0.94 (H α);1.70 (Hβ) | H-25 | 33.2 | |
| 2 | CH2 | 27.5 | 1.63 (H α and Hβ) | 25.4 | ||
| 3 | CH | 78.4 | 3.23 (H α); m | H-23; H-24 | H-2 | 76.3 |
| 4 | C | 39.0 | H-3; H-23; H-24 | 37.2 | ||
| 5 | CH | 55.3 | 0.69 (H α) | H-23; H-24; H-25 | 50.1 | |
| 6 | CH2 | 18.5 | 1.40 (H α); 1.53 (Hβ) | H-5 | 18.3 | |
| 7 | CH2 | 33.4 | 1.47 (H α); 1.62 (Hβ) | H-26 | 33.2 | |
| 8 | C | 41.7 | H-26; H-27 | 41.9 | ||
| 9 | CH | 50.4 | 1.24 (H α) | H-25; H-26 | 49.5 | |
| 10 | C | 37.2 | H-5; H-25 | 37.5 | ||
| 11 | CH2 | 21.1 | 1.51 (H α); 1.32 (Hβ) | 20.9 | ||
| 12 | CH2 | 24.0 | 1.43 (H α); 1.49 (Hβ) | 23.9 | ||
| 13 | CH | 49.5 | 1.37 (H β) | H-27; H-28 | 48.9 | |
| 14 | C | 42.1 | H-26; H-27 | 42.1 | ||
| 15 | CH2 | 33.7 | 1.42 (H α); 1.24 (Hβ) | H-27 | 33.6 | |
| 16 | CH2 | 21.7 | 1.74 (H α); 1.65 (Hβ) | 21.6 | ||
| 17 | CH | 54.9 | 1.39 (H β) | H-21; H-28 | 54.9 | |
| 18 | C | 44.8 | H-21; H-28 | 44.7 | ||
| 19 | CH2 | 41.9 | 1.60 (H α); 1.04 (Hβ) | H-28 | 41.9 | |
| 20 | CH2 | 27.4 | 1.84 (H α); 1.97 (Hβ) | H-21 | H-19 | 27.4 |
| 21 | CH | 46.5 | 2.67 (H β); J = 16.6 and 9.0 Hz; dd | H-29; H-30 | H-17; H-20 | 46.5 |
| 22 | C | 148.6 | H-21; H-29; H-30 | 148.7 | ||
| 23 | CH3 | 28.2 | 1.02; s | H-3; H-24 | 28.6 | |
| 24 | CH3 | 15.7 | 0.81; s | H-3; H-23 | 22.5 | |
| 25 | CH3 | 16.7 | 0.83; s | 15.7 | ||
| 26 | CH3 | 15.9 | 0.94; s | 16.6 | ||
| 27 | CH3 | 16.7 | 0.97; s | 16.8 | ||
| 28 | CH3 | 16.1 | 0.73; s | 16.1 | ||
| 29 | CH2 | 110.2 | 4.79; s | H-21; H-30 | H-30; H-21 | 110.1 |
| 30 | CH3 | 25.0 | 1.75; s | H-21; H-29 | 25.3 | |
2.1.5. Compounds 10 and 11
2.2. In Vitro AChE Inhibitory Activity
3. Experimental
3.1. General Procedures
3.2. Phytochemical Methodology
3.2.1. Plant Material
3.2.2. Extraction and Isolation of Constituents
3.3. Theoretical Methodology
3.4. In Vitro AChE Inhibitory Activity
4. Conclusions
Supplementary Material
Acknowledgements
- Sample Availability: Samples of the compounds 1, 2 and 9 are available from the authors.
References
- Chin, Y.H.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease revalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Yang, Z.-D.; Duan, D.-Z.; Xue, W.-W.; Yao, X.-J.; Li, S. Steroidal alkaloids from Holarrhena antidysenterica as acetylcholinesterase inhibitors and the investigation for structure–activity relationships. Life Sci. 2012, 90, 929–933. [Google Scholar] [CrossRef]
- Dumont, M.; Wille, E.; Calingasan, N.Y.; Tampellini, D.; Williams, C.; Gouras, G.K.; Liby, K.; Sporn, M.; Nathan, C.; Flint-Beal, M.; et al. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2009, 109, 502–512. [Google Scholar]
- Patil, S.P.; Maki, S.; Khedkar, S.A.; Rigby, A.C.; Chan, C. Withanolide A and Asiatic acidmodulate multiple targets associated with amyloid-beta precursor protein processing and amyloid-beta clearance. J. Nat. Prod. 2010, 73, 1196–1202. [Google Scholar] [CrossRef]
- Wilkinson, K.; Boyd, J.D.; Glicksman, M.; Moore, K.J.; El Khoury, J. A high content drug screen identifies ursolic acid as an inhibitor of amyloid beta protein interactions with its receptor CD36. J. Biol. Chem. 2011, 286, 34914–34922. [Google Scholar]
- Zhang, X.; Wu, J.; Xia, B.; Rong, W.; Rimbach, G.; Lou, Y. Asiatic acid protects primary neurons against C2-ceramide-induced apoptosis. Eur. J. Pharmacol. 2012, 679, 51–59. [Google Scholar]
- Corsino, J.; Silva, D.H.S.; Zanoni, M.V.B.; Bolzani, V.S.; França, S.C.; Pereira, A.M.S.; Furlan, M. Antioxidant flavan-3-ols and flavonol glycosides from Maytenus Aquifolium. Phytother. Res. 2003, 17, 913–916. [Google Scholar] [CrossRef]
- Yariwake, J.H.; Lanças, F.M.; Cappelaro, E.A.; Vasconcelos, E.C.; Tiberti, L.A.; Pereira, A.M.S.; França, S.C. Seasonal variability of chemical compounds (triterpenes, flavonoids, and polyphenols) from Maytenus aquifolium Mart. (Celastraceae) leaves. Braz. J. Pharmacogn. 2005, 15, 162–168. [Google Scholar]
- Ravelo, A.G.; Delgado-Mendes, P.; Herrera, N.; Chaves, H.; Estévez-Braun, A.; Cortes, F.; Castanys, S.; Gamarro, R. New terpenoids from Maytenus aurimacensis as MDR reversal agents in the parasite Leishmania. Bioorg. Med. Chem. 2008, 16, 1425–1430. [Google Scholar]
- De Sousa, D.P.; Silva, M.S.; Medeiros, V.M.; Folly, M.A.B.; Tavares, J.F.; Barbosa-Filho, J.M. Alkaloid, flavonoids, and pentacyclic triterpenoids of Maytenus obtusifolia Mart. Biochem. Syst. Ecol. 2008, 36, 500–503. [Google Scholar] [CrossRef]
- Chabariberi, R.A.O.; Pozzi, A.C.S.; Zeraik, M.L.; Yariwake, J.H. Spectrometric determination of flavonoids from Maytenus (Celastraceae) and Passiflora (Passifloraceae) leaves and comparison with a HPLC-UV method. Braz. J. Pharmacogn. 2009, 19, 860–864. [Google Scholar]
- Leite, J.P.V.; Braga, F.C.; Romussi, G.; Persoli, R.M.; Tabach, R.; Carlini, E.A.; Oliveira, A.B. Constituents from Maytenus ilicifolia and bioguided fractionation for gastroprotective activity. J. Braz. Chem. Soc. 2010, 21, 248–357. [Google Scholar] [CrossRef]
- Niero, R.; Andrade, S.F.; Cechinel Filho, V. A Review of the ethnopharmacology, phytochemistry, and pharmacology of plants of the Maytenus Genus. Curr. Pharm. Des. 2011, 17, 1851–1871. [Google Scholar] [CrossRef]
- Niero, R.; Moser, R.; Busato, A.C.B.; Yunes, R.A.; Reis, A.; Cechinel-Filho, V.A. Comparative chemical study of Maytenus ilicifolia Rart. Reiss and Maytenus robusta Reiss (Celastraceae). J. Biosci. 2001, 56, 158–161. [Google Scholar]
- Niero, R.; Mafra, A.P.; Lenzi, A.C.; Cechinel-Filho, V.; Tisher, C.A.; Malheiros, A.; De Sousa, M.M.; Yunes, R.A.; Delle Monache, F. A new triterpene with antinociceptive activity from Maytenus robusta. Nat. Prod. Res. Part B 2006, 20, 1315–1320. [Google Scholar] [CrossRef]
- De Andrade, S.F.; Lemos, M.; Comunello, E.; Noldin, V.; Delle Monache, F.; Chechinel-Filho, V.; Niero, R. Antiulcerogenic activity of fractions and 3,15-dioxo-21α-hydroxyfriedelane isolated from Maytenus robusta (Celastraceae). Arch. Pharm. Res. 2008, 31, 41–46. [Google Scholar]
- Sousa, G.F.; Ferreira, F.L.; Duarte, L.P.; Silva, G.D.F.; Messias, M.C.T.B.; Vieira Filho, S.A. Structural determination of 3β,11β-dihydroxyfriedelane from Maytenus robusta (Celastraceae) by 1D and 2D NMR. J. Chem. Res. 2012, 36, 203–205. [Google Scholar] [CrossRef]
- De Andrade, S.F.; Lemos, M.; Comunello, E.; Noldin, V.F.; Chechinel-Filho, V.; Niero, R. Evaluation of the antiulcerogenic activity of Maytenus robusta (Celastraceae) in different experimental ulcer models. J. Ethnopharmacol. 2007, 113, 252–257. [Google Scholar] [CrossRef]
- Santos, V.L.; Costa, V.B.M.; Agra, M.F.; Silva, B.A.; Batista, L.M. Pharmacological studies of ethanolic extract of Maytenus rigida Mar. (Celastraceae) in animal models. Braz. J. Pharmacogn. 2007, 17, 336–342. [Google Scholar]
- González, A.G.; Alvarenga, N.L.; Estévez-Braun, A.; Ravelo, A.G.; Bazzocchi, I.L. Structure and absolute configuration of triterpene dimers from Maytenus scutioides. Tetrahedron 1996, 52, 9597–9608. [Google Scholar]
- Avilla, J.; Teixidó, A.; Velásquez, N.A.; Ferro, E.; Canela, R. Insectidal activity of Maytenus species (Celastraceae) nortriterpene quinone methides against codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae). J. Agric. Food Chem. 2001, 48, 88–92. [Google Scholar]
- Shirota, O.; Morita, H.; Takeya, K.; Itokawa, H. Sesquiterpene pyridine alkaloids from Maytenus ilicifolia. Heterocycles 1994, 38, 383–389. [Google Scholar] [CrossRef]
- González, A.G.; Tincusi, B.M.; Bazzocchi, S.L.; Tokuda, J.; Nishino, H.; Konoshima, T.; Jiménez, J.A.; Ravelo, A.G. Anti-tumor promoting effects of sesquiterpenes from Maytenus cuzcoina (Celastraceae). Bioorg. Med. Chem. 2000, 8, 1773–1778. [Google Scholar]
- Madrigal, R.V.; Zilkowski, B.W.; Smith, C.R. Structure-activity-relationship among maytansinoides in their effect on the european corn-borer, Ostrinia-Bubilalis (Hubner) (Lipidoptera, Pyralidae). J. Chem. Ecol. 1985, 11, 501–506. [Google Scholar] [CrossRef]
- Kiem, P.V.; Minh, C.V.; Huong, H.T.; Nam, N.H.; Lee, J.J.; Kim, Y.H. Pentacyclic Triterpenoids from Mallotus apelta. Arch. Pharm. Res. 2004, 27, 1109–1113. [Google Scholar]
- David, J.P.; Meira, M.; David, J.M.; Guedes, M.L.S. Triterpenos e Ferrulatos de Alquila de Maprounea guianensis. Quim. Nova 2004, 27, 62–65. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, A.; Mitra, P.; Banerji, A.; Banerji, J.; Mandal, S.; Das, M. Crystal structure and DFT calculations of 3,4-seco-lup-20(29)-en-3-oic acid isolated from Wrightia tinctoria: Stacking of supramolecular dimmers in the crystal lattice. J. Mol. Struct. 2010, 980, 7–12. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Rhee, I.K.; Van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Queiroga, C.L.; Silva, G.F.; Dias, P.C.; Possenti, A.; Carvalho, J.E. Evaluation of the antiulcerogenic activity of friedelan-3β-ol and friedelin isolated from Maytenus ilicifolia (Celastraceae). J. Ethnopharmacol. 2000, 72, 465–468. [Google Scholar] [CrossRef]
- Salazar, G.C.M.; Silva, G.D.F.; Duarte, L.P.; Vieira-Filho, S.A.; Lula, I.S. Two epimeric friedelane triterpenes isolated from Maytenus truncata Reiss: 1H and 13C chemical shift assignments. Magn. Reson. Chem. 2000, 38, 977–980. [Google Scholar] [CrossRef]
- David, J.M.; Santos, F.A.; Guedes, M.L.S.; David, J.P. Flavonóides e triterpenos de Stigmaphyllom paralias. Quim. Nova 2003, 26, 484–487. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003; Volume Revision B.04. [Google Scholar]
- Santos, F.J.L.; Alcântara, A.F.C.; Ferreira-Alves, D.L.; Piló-Veloso, D. Theoretical and experimental NMR studies of the Swern oxidation of methyl 6α,7β-dihydroxyvouacapan-17β-oate. Struct. Chem. 2008, 19, 625–631. [Google Scholar] [CrossRef]
- De Abreu, H.A.; Lagos, I.A.S.; Souza, G.P.; Piló-Veloso, D.; Duarte, H.A.; Alcântara, A.F.C. Antioxidant activity of (+)-bergenin—A phytoconstituent isolated from the bark of Sacoglottis uchi Huber (Humireaceae). Org. Biomol. Chem. 2008, 6, 2713–2718. [Google Scholar] [CrossRef]
- Euzébio, F.P.G.; Santos, F.J.L.; Piló-Veloso, D.; Alcântara, A.F.C.; Ruiz, A.L.G.; Carvalho, J.E.; Foglio, M.A.; Ferreira-Alves, D.L.; Fátima, A. Synthesis, Antiproliferative activity in cancer cells and theoretical studies of novel 6α,7β-dihydroxyvouacapan-17β-oic acid Mannich base derivatives. Bioorg. Med. Chem. 2010, 18, 8172–8177. [Google Scholar]
- Souza, G.P.; Konzen, C.; Simões, T.R.G.; Rodrigues, B.L.; Alcântara, A.F.C.; Stumpf, H.O. Structural characterization of a new dioxamic acid derivative by experimental (FT-IR, NMR, and X-ray) analyses and theoretical (HF and DFT) investigations. J. Mol. Struct. 2012, 1016, 13–21. [Google Scholar] [CrossRef]
- Alcântara, A.F.C.; Piló-Veloso, D.; Almeida, W.B.; Maltha, C.R.A.; Barbosa, L.C.A. Conformational analysis of 8-oxabicyclo[3.2.1]oct-6-en-3-one derivatives by NMR and theoretical calculations. J. Mol. Struct. 2006, 791, 180–185. [Google Scholar] [CrossRef]
- Pérez-Rebolledo, A.; Mendes, I.C.; Speziali, N.L.; Bertani, P.; Resende, J.M.; Alcântara, A.F.C.; Beraldo, H. N(4)-Methyl-4-nitroacetophenone thiosemicarbazone and its nickel(II) complex: Experimental and theoretical structural studies. Polyhedron 2007, 26, 1449–1458. [Google Scholar] [CrossRef]
- Pacheco, A.G.; Abreu, V.C.; De Abreu, H.A.; Piló-Veloso, D.; Alcântara, A.F.C. Structural analysis of aristolochic acids and aristolactams by correlations between calculated and experimental 13C-NMR chemical shifts. Struct. Chem. 2012, 23, 703–710. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sousa, G.F.; Duarte, L.P.; Alcântara, A.F.C.; Silva, G.D.F.; Vieira-Filho, S.A.; Silva, R.R.; Oliveira, D.M.; Takahashi, J.A. New Triterpenes from Maytenus robusta: Structural Elucidation Based on NMR Experimental Data and Theoretical Calculations. Molecules 2012, 17, 13439-13456. https://doi.org/10.3390/molecules171113439
Sousa GF, Duarte LP, Alcântara AFC, Silva GDF, Vieira-Filho SA, Silva RR, Oliveira DM, Takahashi JA. New Triterpenes from Maytenus robusta: Structural Elucidation Based on NMR Experimental Data and Theoretical Calculations. Molecules. 2012; 17(11):13439-13456. https://doi.org/10.3390/molecules171113439
Chicago/Turabian StyleSousa, Grasiely F., Lucienir P. Duarte, Antônio F. C. Alcântara, Grácia D. F. Silva, Sidney A. Vieira-Filho, Roqueline R. Silva, Djalma M. Oliveira, and Jacqueline A. Takahashi. 2012. "New Triterpenes from Maytenus robusta: Structural Elucidation Based on NMR Experimental Data and Theoretical Calculations" Molecules 17, no. 11: 13439-13456. https://doi.org/10.3390/molecules171113439
APA StyleSousa, G. F., Duarte, L. P., Alcântara, A. F. C., Silva, G. D. F., Vieira-Filho, S. A., Silva, R. R., Oliveira, D. M., & Takahashi, J. A. (2012). New Triterpenes from Maytenus robusta: Structural Elucidation Based on NMR Experimental Data and Theoretical Calculations. Molecules, 17(11), 13439-13456. https://doi.org/10.3390/molecules171113439




