Azide-Alkyne Huisgen [3+2] Cycloaddition Using CuO Nanoparticles
Abstract
:1. Introduction
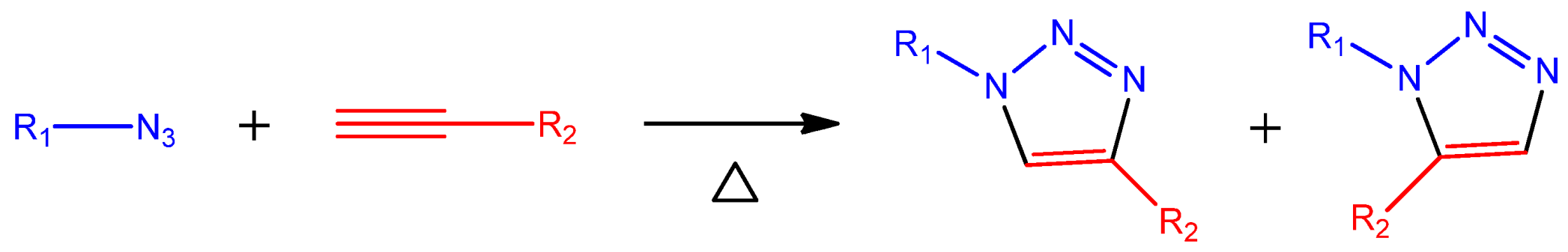
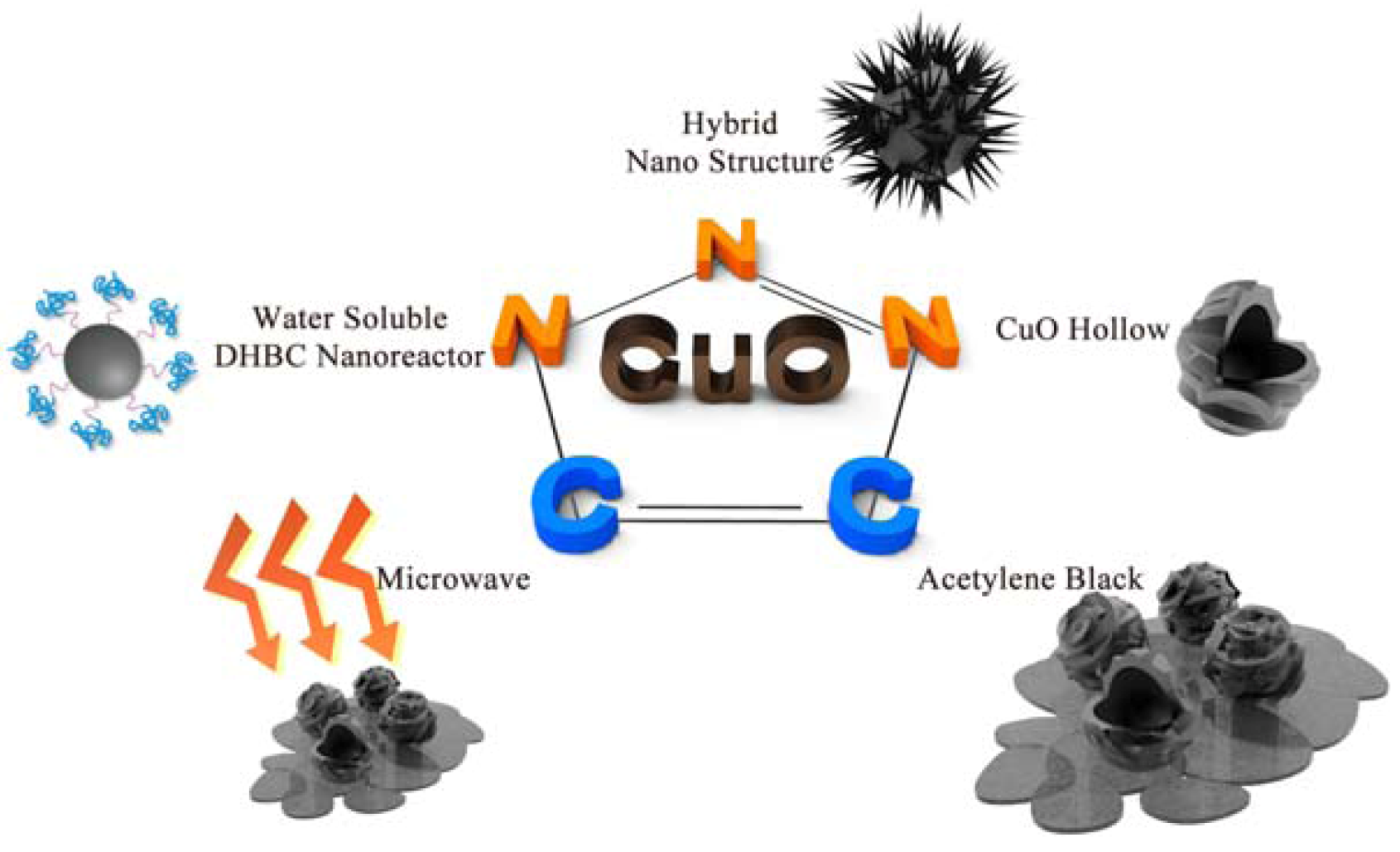
2. Representative Experimental Methods
2.1. Preparation of Cu2O Nanocubes
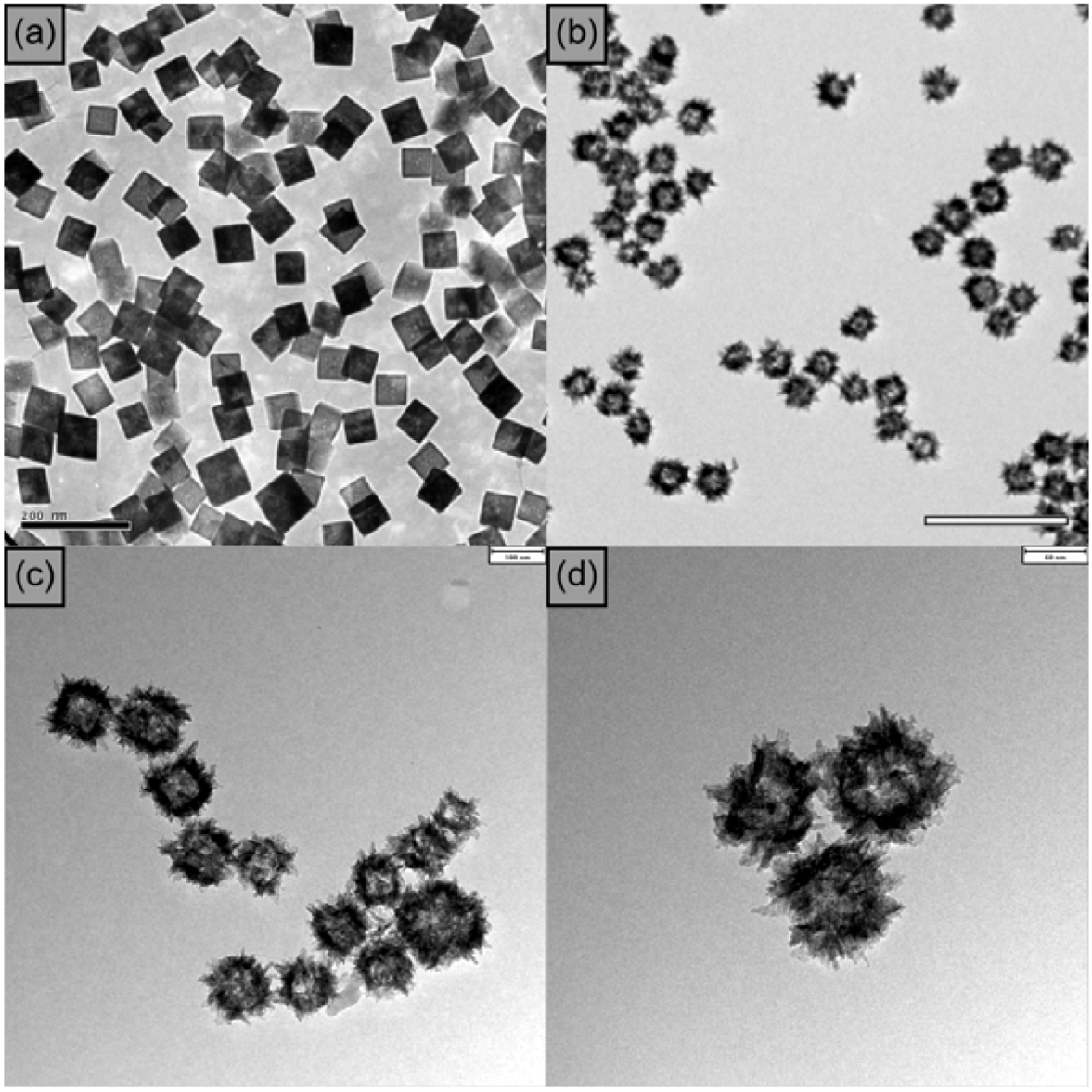
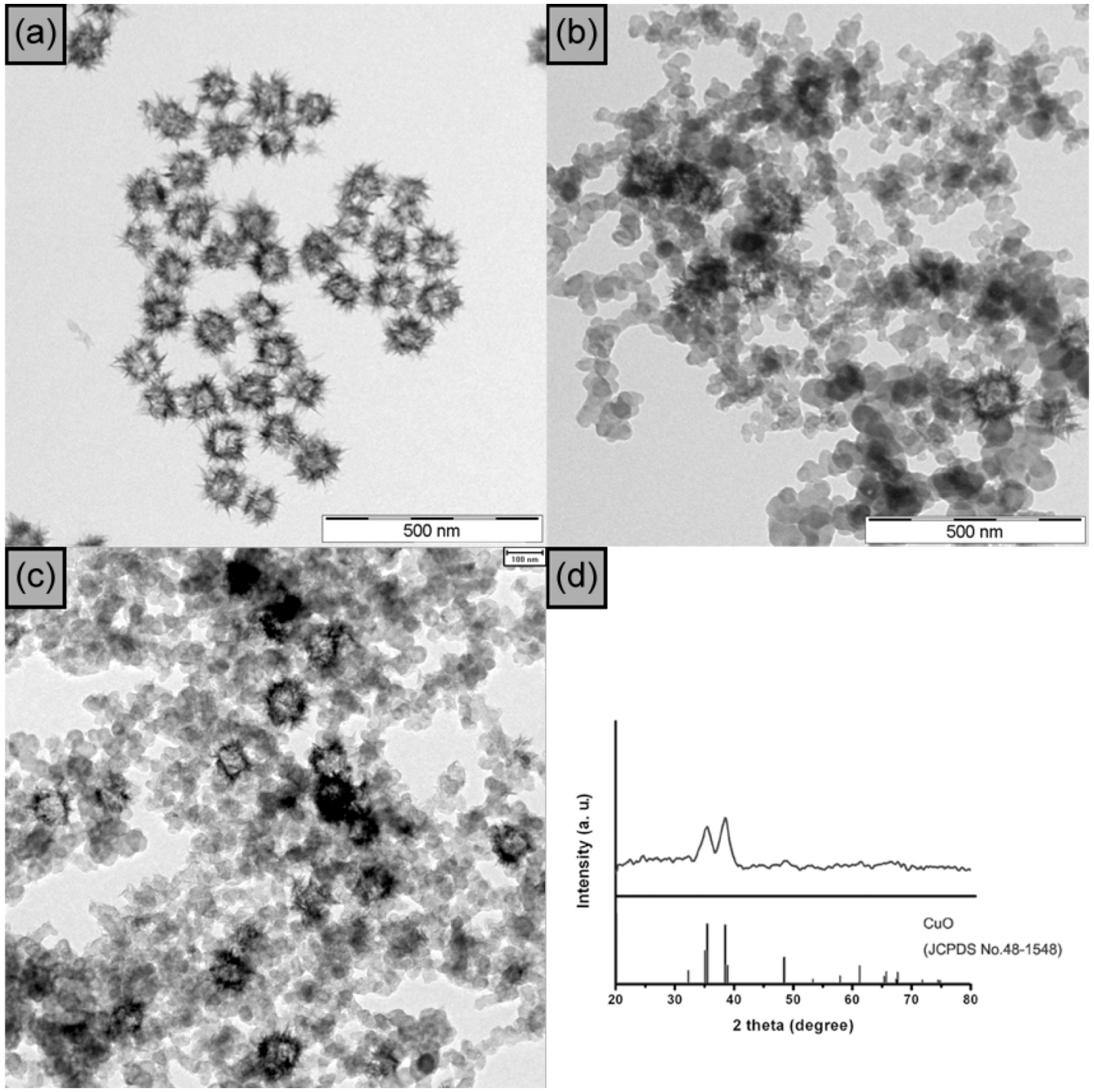
2.2. Synthesis of CuO Hollow and Branched Nanostructures
2.3. Immobilization of CuO Hollow Nanospheres on Acetylene Carbon Black (CuO/AB) and Charcoal (CuO/C)
2.4. Water-Soluble CuO NPs
2.5. Synthesis of Polycrystalline ZnO Nanospheres
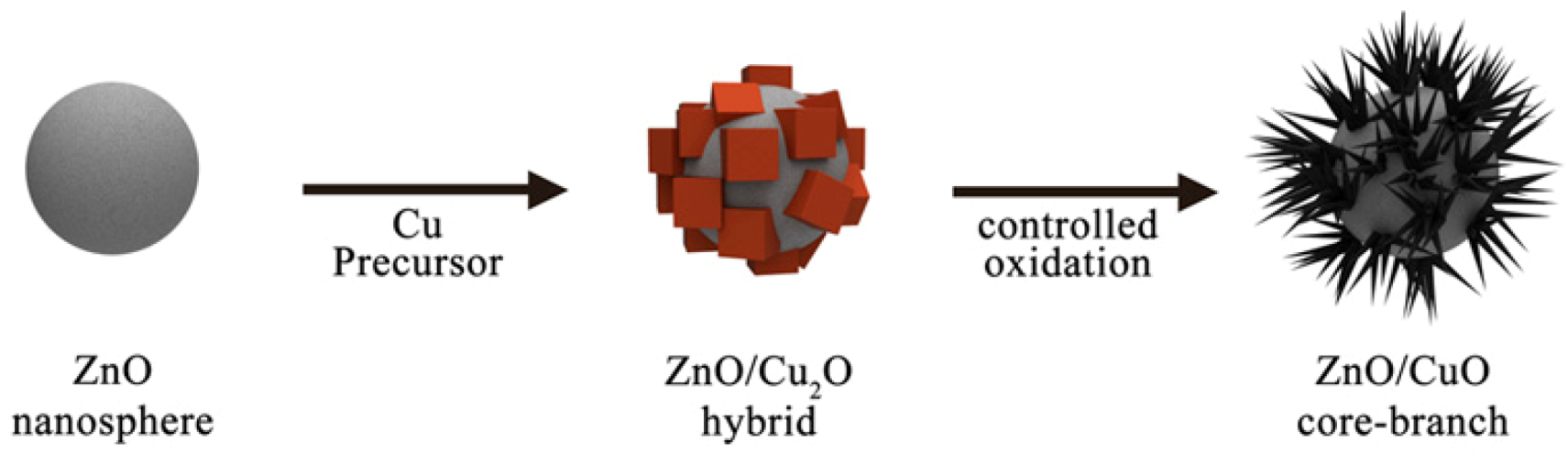
2.6. Synthesis of ZnO/Cu2O Hybrid Nanoparticles
2.7. Synthesis of ZnO/CuO Core-Branch Nanoparticles
3. CuO Hollow Nanoparticles: [3+2] Cycloaddition of Azides with Terminal Alkynes
| Entry | Cat (5 mol%) | Temp (°C) | Time (h) | Solvent | Conv. a (%) |
|---|---|---|---|---|---|
| 1 | CuO urchins | 60 | 12 | THF-H2O (24:1) | 4 |
| 2 | CuO urchins | 100 | 12 | Dioxane/H2O (24:1) | 61 |
| 3 | CuO urchins | 110 | 12 | Toluene/H2O (24:1) | 93 |
| 4 | CuO urchins | 25 | 3 | H2O/t-BuOH (2:1) | 96 |
| 5 | CuO urchins | 25 | 3 | H2O | 90 |
| 6 | CuO urchins | 25 | 3 | t-BuOH | 71 |
| 7 | Commercial CuO b | 25 | 24 | H2O/t-BuOH (2:1) | 35 |
| 8 | Commercial CuO b | 25 | 3 | H2O/t-BuOH (2:1) | <1 |
| 9 | ─ | 25 | 3 | H2O/t-BuOH (2:1) | 0 |
| 10 | CuO urchins | 25 | 3 | H2O/t-BuOH (2:1) | 93 c |
| 11 | CuO hollow spheres | 25 | 3 | H2O/t-BuOH (2:1) | 100 c |
| 12 | CuO hollow cubes | 25 | 3 | H2O/t-BuOH (2:1) | 94 c |
| 13 d | CuO hollow spheres | 25 | 0.5 | H2O/t-BuOH (2:1) | 98 |
4. Immobilized CuO Hollow Nanospheres in Alkyne-Azide Cycloadditions
| Entry | Cat (mol%) | Temp (°C) | Time (h) | Conv (%) a |
|---|---|---|---|---|
| 1 | Blank | 50 | 5 | 7 |
| 2 | CuO (5 mol%) | 25 | 3 | 100 |
| 3 | CuO on AB (1 mol%) | 25 | 3 | >1 |
| 4 | CuO on AB (1 mol%) | 50 | 5 | 22 |
| 5 | CuO on AB (3 mol%) | 25 | 5 | >1 |
| 6 | CuO on AB (3 mol%) | 50 | 5 | 100 |
| 7 | CuO on AB (3 mol%) | 50 | 3 | 60 |
| 8 | CuO on AB (3 mol%) | 40 | 5 | 23 |
| 9 | CuO on AB (3 mol%) | 30 | 5 | 1.1 |
| 10 | CuO on AB (5 mol%) | 50 | 5 | 96 |
| 11 | Recovered from # 6 | 50 | 5 | 100 |
| 12 | Recovered from # 12 | 50 | 5 | 100 |
| 13 | Recovered from # 13 | 50 | 5 | 100 |
| 14 | Recovered from # 14 | 50 | 5 | 100 |
| 15 | Recovered from # 15 | 50 | 5 | 100 |
| 16 | Recovered from # 16 | 50 | 5 | 98 |
| 17 | Recovered from # 17 | 50 | 5 | 100 |
| 18 | Recovered from # 18 | 50 | 5 | 100 |
| 19 | Recovered from # 19 | 50 | 5 | 100 |
5. Solvent-Free Microwave Promoted [3+2] Cycloaddition of Alkyne-Azide
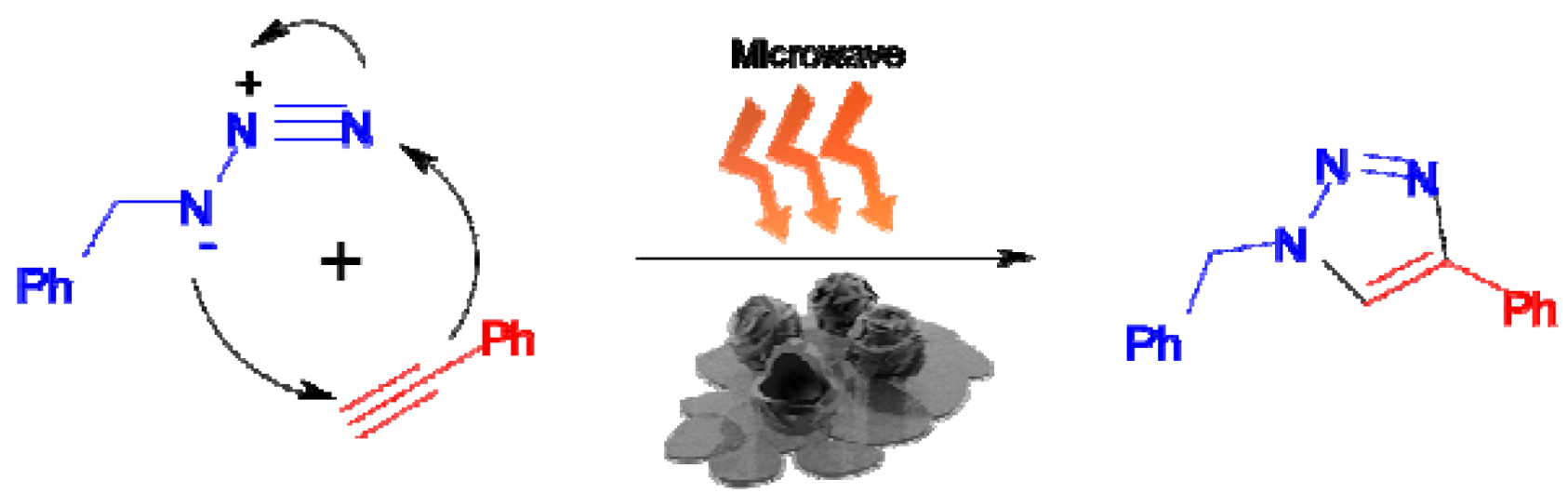
| Entry | Cat (mol%) | Time (min) | Solvent | Conv (%) a |
|---|---|---|---|---|
| 1 | 3 | 300 | H2O/t-BuOH(2:1) | 100 b |
| 2 | 3 | 60 | H2O/t-BuOH(2:1) | 91 c |
| 3 | 3 | 1 | H2O/t-BuOH(2:1) | 100 |
| 4 | 3 | 0.5 | H2O/t-BuOH(2:1) | 10 |
| 5 | 3 | 1 | H2O | 28 |
| 6 | 3 | 1 | t-BuOH | 100 |
| 7 | 3 | 1 | DMSO | 100 |
| 8 | 3 | 1 | DMF | 100 |
| 9 | 3 | 1 | 1-BuOH | 100 |
| 10 | 3 | 1 | 2-BuOH | 11 |
| 11 | 3 | 1 | Toluene | 0 |
| 12 | 3 | 1 | THF | 0 |
| 13 | 1 | 1 | H2O/t-BuOH(2:1) | >99 |
| 14 | 0.5 | 1 | H2O/t-BuOH(2:1) | >99 |
| 15 | 0.3 | 1 | H2O/t-BuOH(2:1) | 37 |
| 16 | 0.3 | 1 | ─ | 100 |
| 17 | 0.3 | 0.5 | ─ | 13 |
| 18 | 0.1 | 1 | ─ | 96 |
| 19 | 0.3 | 1 | DMSO | 100 |
| 20 | 0.3 | 0.5 | DMSO | 100 |
| 21 | 0.1 | 1 | DMSO | 94 |
| 22 | ─ | 1 | ─ | 3 |
6. Water-Soluble Block Copolymer Nanoreactors for the Synthesis of CuO Nanoparticles and Their Application in Click Chemistry
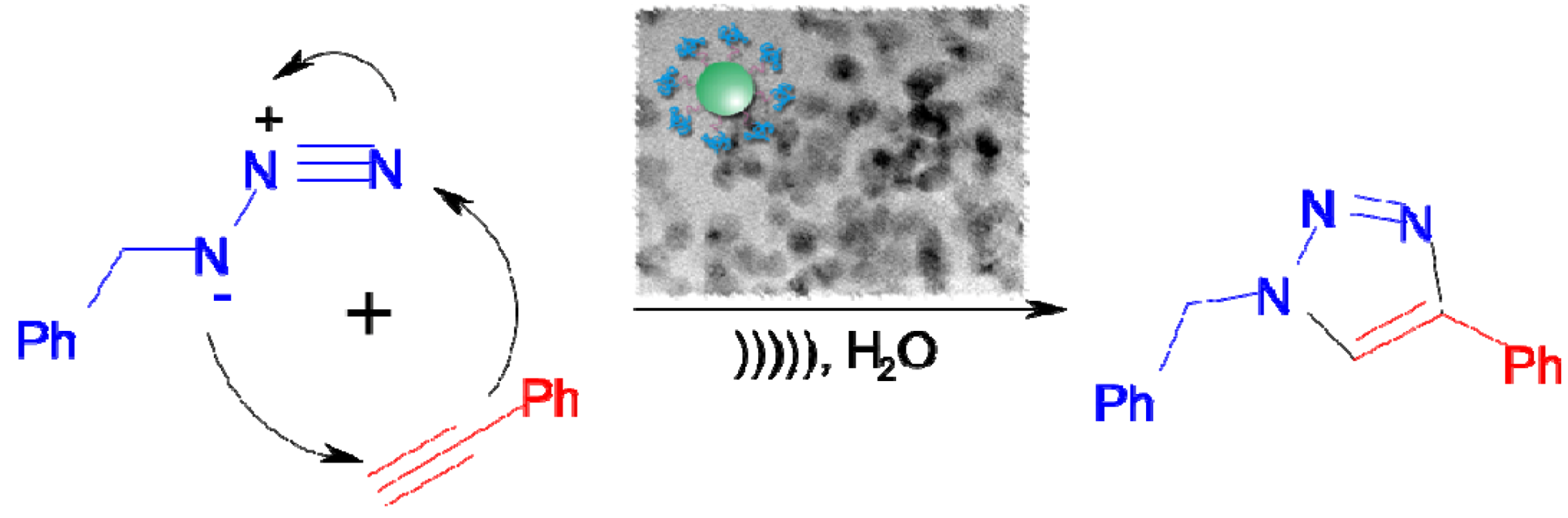
| Entry | Cat (mol %) | Temp (°C) | Time (h) | Conv. (%) a |
|---|---|---|---|---|
| 1 | 1 mol% CuO-poly | 25 | 3 | 3 b |
| 2 | 1 mol% CuO-poly | 50 | 10 min | 17 |
| 3 | 1 mol% CuO-poly | 100 | 5 min | 37 |
| 4 | 1 mol% CuO-poly | 100 | 10 min | >99 |
| 5 | 1 mol% CuO-poly | 100 | 10 min | 27 c |
| 6 | 0.5 mol% CuO-poly | 100 | 10 min | 38 |
| 7 | Recovered # 4 | 100 | 10 min | 99 |
| 8 | Recovered # 7 | 100 | 10 min | 100 |
7. ZnO-CuO Core-Branch Nanocatalysts for Ultrasound-Assisted Click Reaction
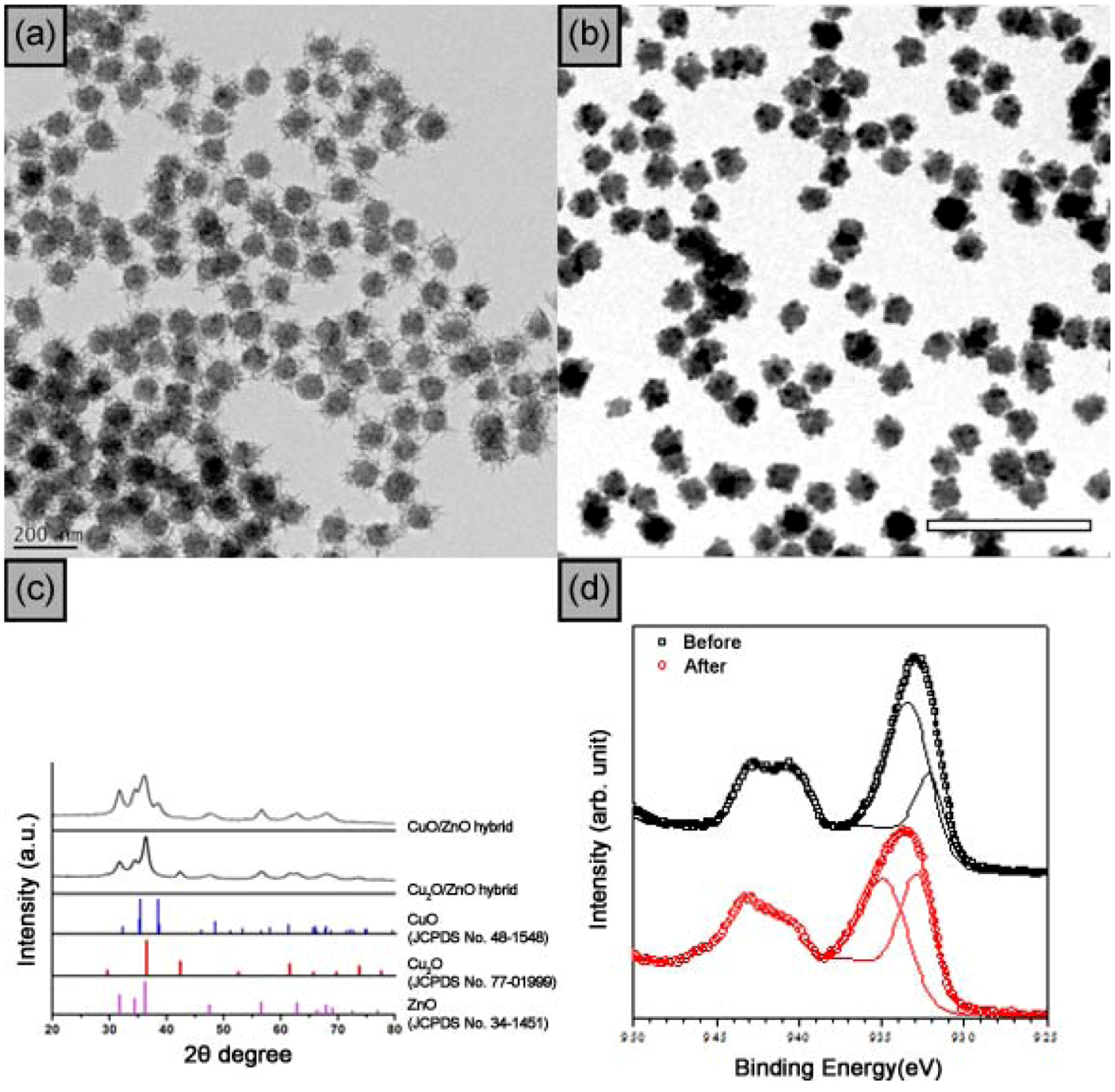
| Entry | Cat (mol%) | Time (min) | Temp. | Conv. b (%) |
|---|---|---|---|---|
| 1 | ZnO-CuO (3 mol%) | 5 | R. T. | 80 |
| 2 | ZnO-CuO (3 mol%) | 10 | R. T. | 100 |
| 3 | ZnO-CuO (1 mol%) | 10 | R. T. | 21 |
| 4 | ZnO nanoparticles (3 mol%) | 10 | R. T. | N.R. |
| 5 | CuO hollows (3 mol%) | 10 | R. T. | 14 |
| 6 | Commercial CuO c (3 mol%) | 10 | R. T. | <1 |
| 7 | ZnO-Cu2O (3 mol%) | 10 | R. T. | 31 |
| 8 | Recovered from #2 | 10 | R. T. | 100 |
| 9 | Recovered from #8 | 10 | R. T. | 100 |
| 10 | Recovered from #9 | 10 | R. T. | 100 |
| 11 | Recovered from #10 | 10 | R. T. | 82 |
| 12 | Recovered from #11 | 10 | R. T. | 76 |
8. Conclusions
Acknowledgements
References
- Burda, C.; Chen, X.B.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Kim, A.Y.; Lee, H.J.; Park, J.C.; Kang, H.; Yang, H.; Song, H.; Park, K.H. Highly Efficient and Reusable Copper-Catalyzed N-Arylation of Nitrogen-Containing Heterocycles with Aryl Halides. Molecules 2009, 14, 5169–5178. [Google Scholar] [CrossRef]
- Lin, K.-S.; Pan, C.-Y.; Chowdhury, S.; Tu, M.-T.; Hong, W.-T.; Yeh, C.-T. Hydrogen Generation Using a CuO/ZnO-ZrO2 Nanocatalyst for Autothermal Reforming of Methanol in a Microchannel Reactor. Molecules 2011, 16, 348–366. [Google Scholar] [CrossRef]
- Monopoli, A.; Nacci, A.; Calò, V.; Ciminale, F.; Cotugno, P.; Mangone, A.; Giannossa, L.C.; Azzone, P.; Cioffi, N. Palladium/Zirconium Oxide Nanocomposite as a Highly Recyclable Catalyst for C-C Coupling Reactions in Water. Molecules 2010, 15, 4511–4525. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–498. [Google Scholar] [CrossRef]
- Izaki, M.; Shinagawa, T.; Mizuno, K.; Ida, Y.; Inaba, M.; Tasaka, A. Electrochemically constructed p-Cu2O/n-ZnO heterojunction diode for photovoltaic device. J. Phys. D Appl. Phys. 2007, 40, 3326–3329. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly Monodisperse Cu2O and CuO Nanospheres: Preparation and Applications for Sensitive Gas Sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Elayadi, H.; Ali, M.A.; Mehdi, A.; Lazrek, H.B. Nanoscrystalline CuO: Synthesis and application as an efficient catalyst for the preparation of 1,2,3-triazole acyclic nucleosides via 1,3-dipolar cycloaddition. Catal. Commun. 2012, 26, 155–158. [Google Scholar] [CrossRef]
- Molteni, G.; Bianchi, C.L.; Marinoni, G.; Santo, N.; Ponti, A. Cu/Cu-oxide nanoparticles as catalyst in the “click” azide–alkyne cycloaddition. New J. Chem. 2006, 30, 1137–1139. [Google Scholar] [CrossRef]
- Botteselle, G.V.; Godoi, M.; Galetto, F.Z.; Bettanin, L.; Singh, D.; Rodrigues, O.E.D.; Braga, A.L. Microwave-assisted one-pot synthesis of symmetrical diselenides, Ditellurides and disulfides from organoyl iodides and elemental chalcogen catalyzed by CuO nanoparticles. J. Mol. Catal. A 2012, 365, 186–193. [Google Scholar] [CrossRef]
- Rout, S.K.; Guin, S.; Nath, J.; Patel, B.K. An “on-water” exploration of CuO nanoparticle catalysed synthesis of 2-aminobenzothiazoles. Green Chem. 2012, 14, 2491–2498. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.C.; Kang, H.; Song, H.; Park, K.H. CuO hollow nanostructures catalyze [3 + 2] cycloaddition of azides with terminal alkynes. Chem. Commun. 2010, 46, 439–441. [Google Scholar] [CrossRef]
- Kang, H.; Jung, H.S.; Kim, J.Y.; Park, J.C.; Kim, M.; Song, H.; Park, K.H. Immobilized CuO Hollow Nanospheres Catalyzed Alkyne-Azide Cycloadditions. J. Nanosci. Nanotechnol. 2010, 10, 6504–6509. [Google Scholar] [CrossRef]
- Kang, H.; Lee, H.J.; Park, J.C.; Song, H.; Park, K.H. Solvent-Free Microwave Promoted [3+2] Cycloaddition of Alkyne-Azide in Uniform CuO Hollow Nanospheres. Top Catal. 2010, 53, 523–528. [Google Scholar] [CrossRef]
- Kantam, M.L.; Jaya, V.S.; Sreedhar, B.; Rao, M.M.; Choudary, B.M. Preparation of alumina supported copper nanoparticles and their application in the synthesis of 1,2,3-triazoles. J. Mol. Catal. A Chem. 2006, 256, 273–277. [Google Scholar] [CrossRef]
- Sharghi, H.; Khalifeh, R.; Doroodmand, M.M. Copper Nanoparticles on Charcoal for Multicomponent Catalytic Synthesis of 1,2,3-Triazole Derivatives from Benzyl Halides or Alkyl Halides, Terminal Alkynes and Sodium Azide in Water as a “Green” Solvent. Adv. Synth. Catal. 2009, 351, 207–218. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, C.; Yang, C.; Hu, D.; Long, J.; Wang, L.; Li, H.; Chen, Y.; Kong, D. Stabilized Copper(I) Oxide Nanoparticles Catalyze Azide-Alkyne Click Reactions in Water. Adv. Synth. Catal. 2010, 352, 1600–1604. [Google Scholar]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Multicomponent Synthesis of 1,2,3-Triazoles in Water Catalyzed by Copper Nanoparticles on Activated Carbon. Adv. Synth. Catal. 2010, 352, 3208–3214. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Click chemistry from organic halides, Diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org. Biomol. Chem. 2011, 9, 6385–6395. [Google Scholar]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Multicomponent Click Synthesis of 1,2,3-Triazoles from Epoxides in Water Catalyzed by Copper Nanoparticles on Activated Carbon. J. Org. Chem. 2011, 76, 8394–8405. [Google Scholar]
- Kim, A.; Sharma, B.; Kim, B.-S.; Park, K.H. Double-Hydrophilic Block Copolymer Nanoreactor for the Synthesis of Copper Nanoparticles and for Application in Click Chemistry. J. Nanosci. Nanotechnol. 2011, 11, 6162–6166. [Google Scholar]
- Park, J.C.; Kim, A.Y.; Kim, J.Y.; Park, S.; Park, K.H.; Song, H. ZnO–CuO core–branch nanocatalysts for ultrasound-assisted azide–alkyne cycloaddition reactions. Chem. Commun. 2012, 48, 8484–8486. [Google Scholar]
- Lee, L.V.; Michaell, L.; Huang, S.J.; Fokin, V.V.; Sharpless, K.B.; Wong, C.H. A Potent and Highly Selective Inhibitor of Human α-1,3-Fucosyltransferase via Click Chemistry. J. Am. Chem. Soc. 2003, 125, 9588–9589. [Google Scholar]
- Appukkuttan, P.; Dehaen, W.; Fokin, V.V.; Eycken, V. A Microwave-Assisted Click Chemistry Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via a Copper(I)-Catalyzed Three-Component Reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef]
- Feldman, A.K.; Colasson, B.; Fokin, V.V. One-Pot Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from In Situ Generated Azides. Org. Lett. 2004, 6, 3897–3899. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-Scale Synthesis of Cu2O Nanocubes and Subsequent Oxidation to CuO Hollow Nanostructures for Lithium-Ion Battery Anode Materials. Adv. Mater. 2009, 21, 803–807. [Google Scholar] [CrossRef]
- Orgueira, H.A.; Fokas, D.; Isome, Y.; Chan, P.C.; Baldino, C.M. Regioselective synthesis of [1,2,3]-triazoles catalyzed by Cu(I) generated in situ from Cu(0) nanosize activated powder and amine hydrochloride salts. Tetrahedron Lett. 2005, 46, 2911–2914. [Google Scholar]
- Hamilton, D.J.C. Homogeneous Catalysis--New Approaches to Catalyst Separation, Recovery, and Recycling. Science 2003, 299, 1702–1706. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, C.S.; Zhou, C.Y.; Ma, H.; Chen, J. Metallic Magnesium Nano/Mesoscale Structures: Their Shape-Controlled Preparation and Mg/Air Battery Applications. Angew. Chem. Int. Ed. 2006, 45, 6009–6012. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar] [CrossRef]
- Gupta, G.K.; Rani, N.; Kumar, V. Microwave assisted synthesis of imidazoles—A review. Mini-Rev. Org. Chem. 2012, 9, 270–284. [Google Scholar]
- Ullah, F.; Zang, Q.; Javed, S.; Porubsky, P.; Neuenswander, B.; Lushington, G.H.; Hanson, P.R.; Organ, M.G. Synthesis of an isoindoline-annulated, Tricyclic sultam library via microwave-assisted, continuous-flow organic synthesis (MACOS). Synthesis 2012, 44, 2547–2554. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanotechnology with Soft Materials. Angew. Chem. Int. Ed. 2003, 42, 1692–1712. [Google Scholar] [CrossRef]
- Basina, G.; Mountrichas, G.; Devlin, E.; Boukos, N.; Niarchos, D.; Petridis, D.; Pispas, S.; Tzitzios, V. Synthesis and Magnetic Properties of Fe3O4 Nanoparticles Coated with Biocompatible Double Hydrophilic Block Copolymer. J. Nanosci. Nanotechnol. 2009, 9, 4753–4759. [Google Scholar] [CrossRef]
- Zhang, Z.; Zha, Z.; Gan, C.; Pan, C.; Zhou, Y.; Wang, Z.; Zhou, M. Catalysis and Regioselectivity of the Aqueous Heck Reaction by Pd(0) Nanoparticles under Ultrasonic Irradiation. J. Org. Chem. 2006, 71, 4339–4342. [Google Scholar] [CrossRef]
- Cintas, P.; Barge, A.; Tagliapietra, S.; Boffa, L.; Cravotto, G. Alkyne–azide click reaction catalyzed by metallic copper under ultrasound. Nat. Protoc. 2010, 5, 607–616. [Google Scholar]
- Wang, Z.X.; Qin, H.L. Regioselective synthesis of 1,2,3-triazole derivatives via 1,3-dipolar cycloaddition reactions in water. Chem. Commun. 2003, 39, 2450–2451. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Lee, K.; Ouyang, M. Nonepitaxial Growth of Hybrid Core-Shell Nanostructures with Large Lattice Mismatches. Science 2010, 327, 1634–1638. [Google Scholar] [CrossRef]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal Strategies for Preparing Oxide-Based Hybrid Nanocrystals. Eur. J. Inorg. Chem. 2008, 6, 837–854. [Google Scholar]
- Somorjai, G.A.; Park, J.Y. Colloid Science of Metal Nanoparticle Catalysts in 2D and 3D Structures, Challenges of Nucleation, Growth, Composition, Particle Shape, Size Control and Their Influence on Activity and Selectivity. Top. Catal. 2008, 49, 126–135. [Google Scholar]
- Rolison, D.R. Catalytic Nanoarchitectures--the Importance of Nothing and the Unimportance of Periodicity. Science 2003, 299, 1698–1701. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Woo, H.; Kang, H.; Kim, A.; Jang, S.; Park, J.C.; Park, S.; Kim, B.-S.; Song, H.; Park, K.H. Azide-Alkyne Huisgen [3+2] Cycloaddition Using CuO Nanoparticles. Molecules 2012, 17, 13235-13252. https://doi.org/10.3390/molecules171113235
Woo H, Kang H, Kim A, Jang S, Park JC, Park S, Kim B-S, Song H, Park KH. Azide-Alkyne Huisgen [3+2] Cycloaddition Using CuO Nanoparticles. Molecules. 2012; 17(11):13235-13252. https://doi.org/10.3390/molecules171113235
Chicago/Turabian StyleWoo, Hyunje, Hyuntae Kang, Aram Kim, Seongwan Jang, Ji Chan Park, Sungkyun Park, Byeong-Su Kim, Hyunjoon Song, and Kang Hyun Park. 2012. "Azide-Alkyne Huisgen [3+2] Cycloaddition Using CuO Nanoparticles" Molecules 17, no. 11: 13235-13252. https://doi.org/10.3390/molecules171113235
APA StyleWoo, H., Kang, H., Kim, A., Jang, S., Park, J. C., Park, S., Kim, B.-S., Song, H., & Park, K. H. (2012). Azide-Alkyne Huisgen [3+2] Cycloaddition Using CuO Nanoparticles. Molecules, 17(11), 13235-13252. https://doi.org/10.3390/molecules171113235






