Abstract
Solvent-free Claisen-Schmidt reactions of cycloalkanones with various substituted benzaldehydes (aryl aldehydes) using solid NaOH (20 mol%) and applying a grinding technique were studied. Quantitative yields (96–98%) of α,α'-bis-(substituted-benzylidene)cycloalkanones were obtained. Aliphatic aldehydes also provided α,α'-bis-(substituted-alkylidene)cycloalkanones in very good yields with minor amounts of α-(substituted-alkylidene)cycloalkanones. The catalytic performance of solid NaOH was examined. The molar ratio of NaOH was optimized. The catalytic effect of solid NaOH was also evaluated by comparing it with KOH, NaOAc, and NH4OAc and it turns out that 20 mol% of solid NaOH was good enough to catalyze the Claisen-Schmidt reactions of cycloalkanones with various substituted benzaldehydes. Additionally, the regioselectivity of the Claisen-Schmidt reaction of acetone with benzaldehyde was examined. Using the same method, we could synthesize the corresponding bis-benzylidene- and mono-benzylideneacetone separately in 98% and 96% yields, respectively.
1. Introduction
The Claisen-Schmidt reaction (crossed-aldol reaction) is a condensation reaction of aldehydes and carbonyl compounds leading to β-hydroxycarbonyl compounds and it has played an important role in synthetic organic chemistry [1,2,3,4,5,6]. Subsequent dehydration of the β-hydroxycarbonyl compounds afford α-alkylidene or α-arylidene compounds. Although studies on the Claisen-Schmidt reaction have been focused on α-alkylidene- and α-arylidene-carbonyl compounds, interest in α,α'-bisalkylidene- and α,α'-bisarylidene-carbonyl compounds is increasing. Particularly, α,α'-bis-(substituted-benzylidene)-cycloalkanones have been attracting much more attention, not only due to their intriguing biological activities such as antiangiogenic [7,8], quinine reductase inducer [9], arginine methyltransferase inhibitor [10], cytotoxicity [11,12], cholesterol-lowering activity [13], uses in agrochemicals, pharmaceuticals and perfumes [14], in bis-spiropyrrolidines [14,15,16], and as liquid crystalline polymer units [17], but also as important precursors for the synthesis of pyrimidine derivatives [18], 2,7-disubstituted tropones [19], and they are the synthetic intermediates of choice to functionalize the α, β-position during the total synthesis of natural products such as the cystodytins [20]. They have also been reported to possess drug resistance reversal properties [21,22].
The Claisen-Schmidt reactions of cycloalkanones leading to α,α'-bis-(benzylidene)cycloalkanones are classically catalyzed by strong acids [23,24] and more likely by base with or without solvent [25,26,27,28,29,30,31,32,33,34,35]. Various reagents have been introduced as the methodology was developed during last few decades, such as Cp2ZrH2 [36], Cp2TiPh2 [37], bis(p-methoxyphenyl)telluroxide (BMPTO) [38], RuCl3 [39], SmI3 [40,41], TiCl3(CF3SO3) [42], La3+-immobilized organic solid [43], KF-Al2O3 [44], Mg(HSO4)2 [45], FeCl3 [46], BF3·OEt2 [47], InCl3 [48], TMSCl/NaI [49], TMSCl/Pd-C [50], SOCl2 [51], Yb(OTf)3 [52], K2CO3/PEG-400 [53], molecular I2 [54], Cu(OTf)2 [55], silica chloride [56], silica-supported phosphorus pentoxide (P2O5/SiO2) or silicaphosphinoxide (silphox, [POCl3-n(SiO2)n]) as heterogeneous reagents [57], 1-methyl-3(2-(sulfooxy)ethyl)-1H-imidazol-3-ium chloride [58] and Et3N in the presence of LiClO4 [59]. The crossed-aldol condensation for the preparation of α,α'-bis(benzylidene) cycloalkanones is also catalyzed by animal bone meal (ABM) or Na/ABM [60], ionic liquid [61,62], sodium-modified-hydroxyapatite (Na-HAP) [63], micellar media [64], ethanolic KOH [65], 2,4,6-trichloro [1,3,5]triazine [66], polymer-supported sulphonic acid [67], lithium hydroxide monohydrate (LiOH•H2O) [68], and rare earth(III) perfluorooctane sulfonates [RE(OPf)3] [69]. However, most of the reactions suffer from reverse and/or side reactions [70,71,72] resulting in low yields of the desired products. Later, different complexes of metal (II) ions were used as catalysts to replace acids or bases but satisfactory yields were not obtained [73].
In our recent studies, we have synthesized α,α'-bis-(substituted-benzylidene)-cycloalkanones and substituted-benzylidene heteroaromatics using NaOAc [74] and NH4OAc [75] as catalysts. Due to the importance of the Claisen-Schmidt reaction in synthetic organic chemistry and of α,α'-bis-(substituted-benzylidene)-cycloalkanones as precursor for various natural products, we wish to report herein a facile solvent-free Claisen-Schmidt reaction using a grinding technique for the synthesis of α,α'-bis-(substituted-benzylidene)cycloalkanones, di- and/or mono- benzylidene acetone and benzylidene camphor using solid NaOH as catalyst.
2. Results and Discussion
2.1. α,α'-bis-(Substitutedbenzylidene)cycloalkanones
The Claisen-Schmidt reaction of cyclopentanone (1a, 10 mmol) or cyclohexanone (1b, 10 mmol) with benzaldehyde (2a, 20 mmol) in the presence of an equimolar amount of solid NaOH without any solvent after grinding with a mortar and pestle for 5 min. afforded the corresponding α,α'-bis-benzylidenecyclopentanone (3a) or α,α'-bis-benzylidenecyclohexanone (3e), both in 99% yield (Scheme 1).
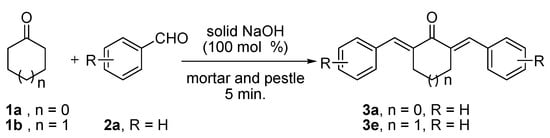
Scheme 1.
Solvent free Claisen-Schmidt reactions of 1a/1b with 2a in presence of NaOH (100 mol%) by grinding with a mortar and pestle for 5 min.
We next examined the catalytic ability of NaOH by grinding cyclohexanone (1b, 10 mmol) with benzaldehyde (2a, 20 mmol) in presence of various molar ratios of NaOH (1–100) using the same procedure to afford the corresponding α,α'-bis-benzylidenecyclohexanone (3e). The results indicate that 20 mol% of NaOH gave satisfactory yield (98%) compared with a stoichiometric amount of NaOH. The results are summarized in Table 1. It should be noted that 10 mol% of NaOH gave 95% of the corresponding target compound 3e, while 80 mol% and an equimolar amount of NaOH gave 99% yields.

Table 1.
Claisen-Schmidt reactions of 1b with 2a using various mol% of NaOH by grinding in a mortar and pestle for 5 min.
| Entry | NaOH (mol%) [a] | Time (min.) | Yield [b] |
|---|---|---|---|
| 1 | 100 | 5 | 99 |
| 2 | 80 | 5 | 99 |
| 3 | 40 | 5 | 98 |
| 4 | 20 | 5 | 98 |
| 5 | 10 | 5 | 95 |
| 6 | 1 | 5 | 70 |
[a] Relative to benzaldehyde; [b] Isolated yields, and were confirmed by proton NMR spectroscopy, which are not optimized.
Furthermore, we evaluated the effect of NaOH on the Claisen-Schmidt reaction of cyclohexanone (1b) with benzaldehyde (2a) over KOH and our previously reported catalysts NaOAc [74], and NH4OAc [75], (Table 2). The highest yield (98%) was achieved using 20 mol% of solid NaOH after grinding with a mortar and pestle for 5 minutes (entry 1, Table 2), while a slightly lower yield (85%) was obtained with 20 mol% of solid KOH (entry 2, Table 2). In addition, different experimental conditions were also applied to optimize the catalytic performance of solid NaOH by introducing solvent (EtOH) at room temperature as well as under refluxing conditions. When we stirred the reaction mixture with 20 mol% of NaOH in ethanol at room temperature for 24 hours (entry 3, Table 2), the product was obtained, but with low yield (40%), and much longer time (5 days) was required to obtain a 66% yield (entry 5, Table 2). After having no promising results with stirring at room temperature for 5 days, we then heated the reaction mixture of cyclohexanone (1b) and benzaldehyde (2a) to reflux for 8 hours with 20 mol% of NaOH in ethanol and this afforded the corresponding α,α'-bis-benzylidenecyclohexanone (3e) in 93% yield (entry 3, Table 2). Comparing all the results (entries 1 to 6, Table 2) with our previously reported methods (entries 7 and 8, Table 2) [74,75], we found that 20 mol% of solid NaOH and grinding with a mortar and pestle for 5 minutes is better than any other catalyst (such as KOH, NaOAc and NH4OAc tested) for the Claisen-Schmidt reaction of cyclohexanone (1b) with benzaldehyde (2a).

Table 2.
Effect of the catalysts, solvents, temperature on the Claisen-Schmidt reactions of 1b with 2a.
| Entry | Catalysts | Time | Yield [a] (%) |
|---|---|---|---|
| [a] Isolated yields which are not optimized. | |||
| 1 | NaOH (20 mmol), mortar and pestle | 5 min | 98 |
| 2 | KOH (20 mmol), mortar and pestle | 5 min | 85 |
| 3 | NaOH (20 mmol), r.t, EtOH | 24 h | 40 |
| 4 | NaOH (20 mmol), r.t, EtOH | 96 h | 60 |
| 5 | NaOH (20 mmol), r.t, EtOH | 5 d | 66 |
| 6 | NaOH (20 mmol), reflux , EtOH | 8 h | 93 |
| 7 | NaOAc (20 mmol), AcOH, 120 °C | 8 h | 81–93[74] |
| 8 | NH4OAc (4 mmol), AcOH, 120 °C | 8 h | 83–95[75] |
Subsequently, we examined the scope and limitation of NaOH (20 mol%) as catalyst for the Claisen-Schmidt reaction of selected cycloalkanones (1a and 1b) and a number of electronically modified aryl aldehydes 2a–h employing grinding with a mortar and pestle for 5 minutes without any solvent to afford the corresponding α,α'-bis(substituted-benzylidene)cycloalkanones 3a–h; the results are summarized in Table 3.

Table 3.
The Claisen-Schmidt reaction of 1a–b with 2a–h in presence of solid NaOH (20 mol%) by grinding in a mortar and pestle for 5 min.
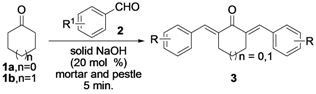 | ||||
|---|---|---|---|---|
| Entry | R | n | Yield (%) | mp (°C) (lit.value) [references] |
| 3a | H | 0 | 98 | 188, (188–190) [ 74], (188–189) [38] |
| 3b | 2-Br | 0 | 96 | 165, (163–165) [ 74], (162–163) [40] |
| 3c | 4-Me | 0 | 98 | 184, (183–184) [ 40] |
| 3d | 4-OMe | 0 | 98 | 211, (210–211) [ 74], (210–211) [38] |
| 3e | H | 1 | 98 | 119, (119–120) [ 74], (117–118) [38] |
| 3f | 2-NO2 | 1 | 98 | 159, (158–159) [ 74], (158–159) [74] |
| 3g | 3-Cl | 1 | 97 | 104, (103–105) [ 74] |
| 3h | 4-Me | 1 | 98 | 168, (165–167) [ 74], (170.1) [76] |
The electronic nature of the substituent on the benzene ring of compounds 2a–h and the ring size of cycloalkanones (compounds 1a and 1b) did not affect the reaction and high yields (96–98%) were obtained for all the entries. We then attempted to prepare substituted-alkylidenecycloalkanones 3i and 3j from the reactions of cyclohexanone (1b) with acetaldehyde (2g) and iso-propanal (2h) using the optimized molar ratio of solid NaOH (20 mol%) to obtain the corresponding 2,6-bis-ethylidene-cyclohexanone (3i) and 2,6-bis-isobutylidenecyclohexanone (3j). It should be noted that we obtained a small amount of mono-substituted alkylidenecycloalkanone (4a and 4b) along with the desired major products 3i and 3j (Scheme 2) and the conversion of 1b was 100%.
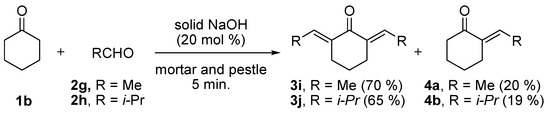
Scheme 2.
Solvent free Claisen-Schmidt reactions of 1b with 2g/2h in the presence of NaOH (20 mol%) by grinding with a mortar and pestle for 5 min.
2.2. Reaction of Acetone (5) with Benzaldehyde (2a)
The Claisen-Schmidt reaction for acetone (5, 10 mmol) and benzaldehyde (2a, 20 mmol) was conducted for the preparation of bis-benzylidene acetone (6) using similar reaction conditions as shown in scheme 2, but the reaction time was only for 2 min. instead of 5 (Scheme 3). The reaction of 5 with 2a gave a mixture of 6 and 7 in 53% and 42% yields, respectively.
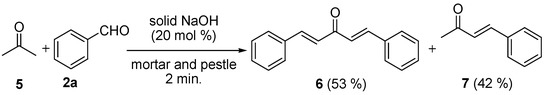
Scheme 3.
Claisen-Schmidt reactions of 5 with 2a in the presence of NaOH (20 mol%) by grinding with a mortar and pestle for 5 min.
Then we examined the regioselectivity of the reaction and the results are summarized in Table 4. Use of excess amount of 5 (>5 equiv.) resulted in mainly 7 (96%) with a trace amount of 6, while on the other hand, when we used an excess of 2a (>3 equiv.) bis-benzylideneacetone was obtained in 98% yield as a single product.

Table 4.
The Claisen-Schmidt reaction of acetone (5) with benzaldehyde (2a) in presence of 20 mol% of solid NaOH by grinding in a mortar and pestle of 5 min.
| Molar ratio | Conversion(%) | Yield [a] (%) | ||
|---|---|---|---|---|
| 5 | 2a | 6 | 7 | |
| 10 mmol | 20 mmol | 100 b | 53 | 42 |
| Excess (>5 equiv.) | 10 mmol | 100 b | trace | 96 |
| 10 mmol | Excess (>3 equiv.) | 100 c | 98 | 0 |
[a] Isolated yields which are not optimized; [b] conversion of benzaldehyde; [c] conversion of acetone.
2.3. Reaction of 1,7,7-Trimethyl[2,2,1]hexan-2-one (Camphor, 8) with Substituted-benzaldehyde (2a and 2c)
We further examined the scope and limitations of this grinding technique for Claisen-Schmidt reactions by using substituted-benzaldehydes (compounds 2a and 2c) with 1,7,7-trimethyl[2,2,1]hexan-2-one (camphor, 8) in the presence of 20 mol% NaOH. The reactions afforded the desired products benzylidene-1,7,7-trimethylbiyclo[2,2,1]hexan-2-one (9a) and 3-chloro-benzylidene-1,7,7-trimethylbicyclo[2,2,1]hexan-2-one (9b) in 86% and 82% yields, respectively (Scheme 4).
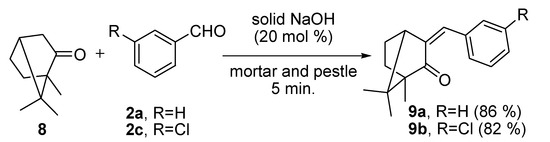
Scheme 4.
Claisen-Schmidt reactions of 8 with 2a/2c in the presence of solid NaOH (20 mol%) by grinding with a mortar and pestle for 5 min.
3. Experimental
3.1. General
Melting points were recorded on a Fisher–Jones melting point apparatus and are uncorrected. Nuclear magnetic resonance (NMR) spectra were recorded using a Bruker 250 spectrometer (250 MHz for 1H-NMR and 62.5 MHz for 13C-NMR) and are reported in parts per million (ppm) from the internal standard tetramethylsilane. Electrospray ionization (ESI) mass spectrometry (MS) experiments were performed on LCQ advantage-trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA).
3.2. Chemistry
3.2.1. General Procedure for the Preparation of α,α'-bis-(Substituted-benzylidene)cycloalkanones 3a–h
A mixture of cyclopentanone/cyclohexanone (1a/1b, 5.0 mmol), substituted benzaldehyde (2a–h, 10.0 mmol) and solid NaOH (20 mol%) was ground with a mortar and pestle at room temperature, under the hood for 5 minutes. The reaction mixture was poured into 2 N HCl, and the solid materials were collected and purified by flash chromatography on silica gel eluting with CH2Cl2-hexane (1:1) to give analytically pure α,α'-bis-(substituted-benzylidene)cycloalkanones 3a–h.
2,5-bis-(Benzylidene)cyclopentanone (3a). Yellow solid (98%), mp. 188 °C, (188–190 °C) [24],(188–189 °C) [38].
2,5-bis-(2-Bromobenzylidene)cyclopentanone (3b). Yellow solid (96%). mp. 165 °C, (163–165 °C) [74], (162–163 °C) [40].
2,5-bis-(4-Methylbenzylidene)cyclopentanone (3c). Yellow solid (98%). mp. 184 °C, (183–184 °C) [40].
2,5-bis-(4-Methoxylbenzylidene)cyclopentanone (3d). Yellow solid (98%), mp. 211 °C, (210–211 °C) [74], (210–211 °C) [38].
2,6-bis-(Benzylidene)cyclohexanone (3e). Yellow solid (98%), mp. 119 °C, 119–120 °C [74],(117–118 °C) [38].
2,6-bis(2-Nitrobenzylidene)cyclohexanone (3f). Yellow needles (98%), mp. 159 °C, (158–159 °C) [74].
2,6-bis(3-Chlorobenzylidene)cyclohexanone (3g). Yellow needles (97%). mp. 104 °C, (103–105 °C) [74].
2,6-bis(3-Methylbenzylidene)cyclohexanone (3h). Yellow solid (98%), mp. 168 °C, (165–167 °C) [74] (170.1 °C) [76].
3.2.2. General Procedure for the Preparation of 3i, 3j and 4
2,6-bis-Alkylcycloalkanones 3i and 3j and α-(mono)alkylcycloalkanones 4a and 4b were obtained as solid/oily materials following the procedure adopted for 3a–h from a mixture of cyclohexanone (1b, 5.0 mmol) and acetaldehyde (2g) or iso-propanal (2h) (10.0 mmol) and were purified by flash chromatography on silica gel eluting with CH2Cl2-hexane (1:1).
2,6-bis-Ethylidene-cyclohexanone (3i). Colorless oil (70%): bp. 130 °C (0.5 mm Hg) [75].
2,6-bis-Isobutylidene-cyclohexanone (3j). Colorless oil (65%) [68,75].
(E)-2-Ethylidenecyclohexanone (4a). Colorless oil (20%), bp. 76–80 °C (14 mm Hg) [77], bp. 87–89 °C (18 mm Hg) [75].
2-Isobutylidenecyclohexanone (4b). Colorless oil (19%) [78].
3.2.3. Procedure for the Preparation of (1E,4E)-1,5-Diphenylpenta-1,4-dien-3-one (6) and (E)-4-Phenylbut-3-en-2-one (7)
(1E,4E)-1,5-diphenylpenta-1,4-dien-3-one (6) and (E)-4-phenylbut-3-en-2-one (7) were obtained following the procedure adopted for 3a–h from mixture of acetone (5, 5.0 mmol) and benzaldehyde (2a, 10.0 mmol). The oily material was collected and purified by flash chromatography on silica gel.
(1E,4E)-1,5-Diphenylpenta-1,4-dien-3-one (6). Yellow solid: mp 109–111 °C, (107 °C) [79], (112 °C). [80] 1H NMR (CDCl3, 250 MHz): δ 7.73 (d, J = 15.9 Hz, 2H), 7.61–7.59 (m, 4H), 7.41–7.38 (m, 6H), 7.07 (d, J = 15.9 Hz, 2H).
(E)-4-Phenylbut-3-en-2-one (7). Low melting solid mp 39–42 °C) 1H-NMR (CDCl3, 250 MHz): 7.55–7.51 (m, 2H), δ 7.50 (d, J = 16.4 Hz, 1H), 7.39–7.36 (m, 3H), 6.70 (d, J = 16.2 Hz, 1H), 2.37 (s, 3H). 13C-NMR (CDCl3, 62.5 MHz): δ 198.5, 143.47, 134.31, 130.5, 128.93, 128.22, 127.07, 27.49.
3.2.4. Preparation of (E)-3-Substitutedbenzylidene-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one (9)
(E)-3-Benzylidene-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one (9a) and (E)-3-(3-chloro-benzylidene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one (9b) were obtained following the procedure adopted for 3a–h from a mixture of 1,7,7-trimethyl[2,2,1]hexan-2-one (camphor, 8, 5.0 mmol) and substituted benzaldehyde (2a/2c, 5.0 mmol). The oily material was collected and purified by flash chromatography on silica gel eluting with EtOAc-hexane (1:19).
(E)-3-Benzylidene-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one (9a). White solid (86%). mp. 74 °C, (71–73 °C) [81], (74–75 °C) [82]; 1H-NMR (CDCl3, 250 MHz): δ 7.48–7.28 (m, 5H), 7.22 (s, 1H), 3.09 (d, J = 4.1 Hz, 1H), 2.21–2.12 (m, 1H), 1.96–1.71 (m, 1H), 1.63–1.46 (m, 2H), 1.01 (s, 3H), 0.98 (s, 3H), 0.78 (s, 3H). 13C-NMR (CDCl3, 62.5 MHz): δ 208.33, 142.09, 135.67, 129.76, 128.68, 128.63, 127.52, 57.11, 49.17, 46.70, 30.67, 25.94, 20.57, 18.32, 9.30.
(E)-3-(3-chlorobenzylidene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one (9b). White solid (82%). 1H-NMR (CDCl3, 250 MHz): δ 8.07 (s, 1H), 7.97 (d, J = 7.8 Hz, 1H), 7.55 (d, J = 7.8 Hz, 1H), 7.40 (t, J = 7.8 Hz, 1H), 7.35 (s, 1H), 2.40–2.23 (m, 1H), 2.07–1.95 (m, 1H), 1.85–1.78 (m, 1H), 1.68–1.62 (m, 1H), 1.40–1.30 (m, 1H), 0.93 (s, 3H), 0.98 (s, 3H), 0.81(s, 3H).
4. Conclusions
A facile solvent-free Claisen-Schmidt reaction between cyclopentanone (1a)/cyclohexanonoe (1b) and different substituted benzaldehydes 2a–h catalyzed by solid NaOH (20 mol%) by applying a grinding technique using a mortar and pestle for 5 minutes was performed, resulting in excellent yields (96–98%) of the corresponding α,α'-bis(substituted-benzylidene)cyclo-alkanones 3a–h. The Claisen-Schmidt reaction using NaOH was optimized and it turned out that 20 mol% of NaOH is sufficient to perform the reactions. The catalytic effect was also examined and we found 20 mol% of solid NaOH is better than any other catalyst tested such as KOH, NaOAc and NH4OAc, for the Claisen-Schmidt reaction under solvent free condition. Beside aryl aldehydes, alkyl aldehydes were also converted to their corresponding bis-alkylidenecycloalkanones along with a little mono alkylidene cycloalkanone by the method in question. Additionally, we examined the regioselectivity of the Claisen-Schmidt reaction by reacting acetone (5) with benzaldehyde (2a) leading to give the corresponding bis-benzylideneacetone in 98% yield using the same method.
References and Notes
- Nielsen, A.T.; Houlihan, W.J. Organic Reactions. In The Aldol Condensation; Adams, R., Blatt, A.H., Boekelheide, V., Cairns, T.L., Cram, D.J., House, H.O., Eds.; J. Wiley & Sons: New York, NY, USA, 1968; Volume 16, pp. 1–438. [Google Scholar]
- Mukaiyama, T. Organic Reactions; Dauben, W.G., Ed.; J. Wiley & Sons: New York, NY, USA, 1982; Volume 28, pp. 203–331. [Google Scholar]
- Heathcock, C.H. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 2, pp. 133–179. [Google Scholar]
- Gennari, C. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 2, pp. 629–660. [Google Scholar]
- Mahrwald, R., Ed. ; Modern Aldol Reactions; Wiley-VCH: Weinheim, Germany, 2004; Volume 1 and 2. [Google Scholar]
- Reeves, R.L. Chemistry of Carbonyl Group; Patai, S., Ed.; Wiley-Intersciences: New York, NY, USA, 1966; pp. 580–600. [Google Scholar]
- Robinson, T.P.; Ehlers, T.; Hubbard, R.B.; Bai, X.; Arbiser, J.L.; Goldsmith, D.J.; Bowena, J.P. Design, synthesis, and biological evaluation of angiogenesis inhibitors: Aromatic enone and dienone analogues of curcumin. Bioorg. Med. Chem. Lett. 2003, 13, 115–117. [Google Scholar] [CrossRef]
- Robinson, T.P.; Hubbard, R.B.; Ehlers, T.J.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg. Med. Chem. 2005, 13, 4007–4013. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abeygunawardana, C.; Talalay, P. Chemoprotective Properties of Phenylpropenoids, Bis(benzylidene)cycloalkanones, and Related Michael Reaction Acceptors: Correlation of Potencies as Phase 2 Enzyme Inducers and Radical Scavengers. J. Med. Chem. 1998, 41, 5287–5296. [Google Scholar] [CrossRef]
- Cheng, D.; Valente, S.; Castellano, S.; Sbardella, G.; Di Santo, R.; Costi, R.; Bedford, M.T.; Mai, A. Novel 3,5-Bis(bromohydroxybenzylidene)piperidin-4-ones as Coactivator-Associated Arginine Methyltransferase 1 Inhibitors: Enzyme Selectivity and Cellular Activity. J. Med. Chem. 2011, 54, 4928–4932. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Padmanilayam, M.P.; Zello, G.A.; Nienaber, K.H.; Allen, T.M.; Santos, C.L.; De Clercq, E.; Balzarini, J.; Manavathu, E.K.; Stables, J.P. Cytotoxic analogues of 2,6-bis(arylidene)cyclohexanones. Eur. J. Med. Chem. 2003, 38, 169–177. [Google Scholar] [CrossRef]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef]
- Piantadosi, C.; Hall, I.H.; Irvine, J.L.; Carlson, G.L. Cycloalkanones. 2. Synthesis and biological activity of alpha, alpha'-dibenzylcycloalkanones. J. Med. Chem. 1973, 16, 770–795. [Google Scholar] [CrossRef]
- Ogawa, M.; Ishii, Y.; Nakano, T.; Irifune, S. Production of 2-alkylidenecycloalkanone derivative. Jpn. Kohai Tokkyo JP 1988, 63238034–A2. [Google Scholar]
- Raj, A.A.; Raghunathan, R. double 1,3-dipolar cycloaddition of 2,6-bis(arylmethylidene)-cyclohexanones: A novel entry into the synthesis of 2′,6′- bis-spiro(1-n-methyl-4-aryl-pyrrolidine)cyclohexanones. Synth. Commun. 2001, 32, 3295–3300. [Google Scholar]
- Raj, A.A.; Raghunathan, R.; Sridevi Kumari, M.R.; Raman, N. Synthesis, Antimicrobial and Antifungal Activity of a New Class of Spiro pyrrolidines. Bioorg. Med. Chem. 2003, 11, 407–419. [Google Scholar] [CrossRef]
- Gangadhara, K.K. Synthesis and characterization of photo-crosslinkable main-chain liquid-crystalline polymers containing bis(benzylidene)cycloalkanone units. Polymer 1995, 36, 1903–1910. [Google Scholar] [CrossRef]
- Deli, J.; Lorand, T.; Szabo, D.; Foldesi, A. Potential bioactive pyrimidine derivatives, part 1: 2-Amino-4-aryl-8-arylidene-3,4,5,6,7,8 hexahydroquinazolines. Pharmazie 1984, 39, 539–540. [Google Scholar]
- Leonard, N.J.; Miller, L.A.; Berry, J.W. The Synthesis of 2,7-Disubstituted Tropones via Aromatization. J. Am. Chem. Soc. 1957, 79, 1482–1485. [Google Scholar] [CrossRef]
- Ciufolini, M.A.; Byrne, N.E. The total synthesis of cystodytins. J. Am. Chem. Soc. 1991, 113, 8016–8024. [Google Scholar] [CrossRef]
- Das, U.; Kawase, M.; Sakagami, H.; Ideo, A.; Shimada, J.; Molnar, J.; Barath, Z.; Bata, Z.; Dimmock, J.R. 3-(3,4,5-Trimethoxyphenyl)-1-oxo-2-propene: A novel pharmacophore displaying potent multidrug resistance reversal and selective cytotoxicity. Bioorg. Med. Chem. 2007, 15, 3373–3380. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Hamon, N.W.; Hindmarsh, K.W.; Sellar, A.P.; Turner, W.A.; Rank, G.H.; Robertson, A.J. Evaluation of 2-benzylidenecyclohexanones and 2,6-bis(benzylidene)cyclohexanones for antitumor and cytotoxic activity and as inhibitors of mitochondrial function in yeast: Metabolism studies of (E)-2-benzylidenecyclohexanone. J. Pharm. Sci. 1976, 65, 538–543. [Google Scholar] [CrossRef]
- Dhar, D.N.; Barton, D. The Chemistry of Chalcones and Related Compounds; J. Wiley & Sons: New York, NY, USA, 1981; p. 8. [Google Scholar]
- Gall, E.L.; Texier-Boullet, F.; Hamelin, J. Simple Access to α, β Unsaturated Ketones by Acid-Catalyzed Solvent-Free Reactions. Synth. Commun. 1999, 29, 3651–3657. [Google Scholar] [CrossRef]
- Geissman, T.A.; Clinton, R.O. Flavanones and Related Compounds. I. The Preparation of Polyhydroxychalcones and Flavanones. J. Am. Chem. Soc. 1946, 68, 697–700. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, A.K.; Paul, S.; Kachroo, P.L. Improved Microwave-Induced Synthesis of Chalcones and Related Enones. Indian J. Chem. Sect. B 1995, 34, 61–62. [Google Scholar]
- Lin, T.; Cromwell, N.H.; Kingsbury, C.A. The synthesis and chemistry of a bispyrazolinyl ketone and the structure determination. J. Heterocycl. Chem. 1985, 22, 21–24. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pani, G.; Piermatti, O.; Pizz, F. Condensation reactions in water of active methylene compounds with arylaldehydes. One-pot synthesis of flavonols. Tetrahedron 1994, 50, 11499–11508. [Google Scholar] [CrossRef]
- Li, J.-T.; Chen, G.-F.; Wang, X.-J.; Li, T.-S. Ultrasound Promoted Synthesis of α,α'-bis(Substituted Furfurylidene) Cycloalkanones and Chalcones. Synth. Commun. 1999, 29, 965–971. [Google Scholar] [CrossRef]
- Sinistierra, J.V.; Garcia-Raso, A.; Cabello, J.A.; Marinas, J.M. An Improved Procedure for the Claisen-Schmidt Reaction. Synthesis 1984, 6, 502–504. [Google Scholar]
- Sun, Y.-F.; Wang, Z.-Y.; Zhao, X.; Zheng, Z.-B.; Li, J.-K.; Wu, R.-T.; Cui, Y.-P. The synthesis, spectroscopic characterization and structure of three bis(arylmethylidene)cyclopentanones. Dyes Pigments 2010, 86, 97–105. [Google Scholar] [CrossRef]
- Raston, C.L.G.; Cave, W.V. Green Chemistry Laboratory: Benign Synthesis of 4,6-Diphenyl[2,2']bipyridine via Sequential Solventless Aldol and Michael Addition Reactions. J. Chem. Educ. 2005, 82, 468. [Google Scholar] [CrossRef]
- Raston, C.L.; Scott, J.L. Chemoselective, solvent-free aldol condensation reaction. Green Chem. 2000, 2, 49–52. [Google Scholar] [CrossRef]
- Mogilailah, K.; Swamy, T.K.; Chandra, A.V.; Srivani, N.; Vidya, K. Claisen—Schmidt Condensation under Solvent-Free Conditions. Indian J. Chem. Sec. B 2010, 49, 382–385. [Google Scholar]
- Shan, Z.-X.; Luo, X.-X.; Hu, L.; Hu, X.-Y. New observation on a class of old reactions: Chemoselectivity for the solvent-free reaction of aromatic aldehydes with alkylketones catalyzed by a double-component inorganic base system. Sci. China. Chem. 2010, 53, 1095–1101. [Google Scholar] [CrossRef]
- Nakano, T.; Irifune, S.J.; Umano, S.; Inada, A.; Ishii, Y.; Ogawa, M. Cross-condensation reactions of cycloalkanones with aldehydes and primary alcohols under the influence of zirconocene complexes. J. Org. Chem. 1987, 52, 2239–2244. [Google Scholar] [CrossRef]
- Nakano, T.; Migita, T. A Convenient Synthesis of α,α′-Bis(substitutedbenzylidene)cycloalkanones. Chem. Lett. 1993, 12, 2157–2158. [Google Scholar]
- Zheng, M.; Wang, L.; Shao, J.; Zhong, Q. A Facile Synthesis of α, α'-bis(Substituted Benzylidene)cycloalkanones Catalyzed by bis(p-methoxyphenyl)telluroxide(bmpto) Under Microwave Irradiation. Synth. Commun. 1997, 27, 351–354. [Google Scholar] [CrossRef]
- Iranpoor, N.; Kazemi, F. RuCI3 Catalyses Aldol Condensations of Aldehydes and Ketones. Tetrahedron 1998, 54, 9475–9480. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Y.; Ying, T. A Facile Route to Synthesize α,α′-bis(Substituted-benzylidene) Cycloalkanones Promoted by SmI3. Synth. Commun. 1996, 26, 503–507. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y. SmI3 Catalyzed Condensation of Aliphatic Cycloketones and Aldehydes in Ionic Liquid. Synth. Commun. 2003, 33, 161–165. [Google Scholar] [CrossRef]
- Iranpoor, N.; Zeynizadeh, B.; Aghapour, A. Aldol Condensation of Cycloalkanones with Aromatic Aldehydes Catalysed with TiCl3(SO3CF3). J. Chem. Res, Synop 1999, 9, 554–555. [Google Scholar]
- Dewa, T.; Saiki, T.; Aoyama, Y. Enolization and Aldol Reactions of Ketone with a La3+-Immobilized Organic Solid in Water. A Microporous Enolase Mimic. J. Am. Chem. Soc. 2001, 123, 502–503. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Nagaraju, A.; Sarma, J.A.R.P. Microwave assisted synthesis of α,α′-bis(benzylidene)ketones in dry media. Synth. Commun. 2002, 32, 893–896. [Google Scholar] [CrossRef]
- Salehi, P.; Khodaei, M.M.; Zolfigol, M.A.; Keyvan, A. Solvent-Free Crossed Aldol Condensation of Ketones with Aromatic Aldehydes Mediated by Magnesium Hydrogensulfate. Monatsh. Chem. 2002, 133, 1291–1295. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Niu, H.; Wang, J. An ionic liquid as a recyclable medium for the green preparation of α,α′-bis (substituted benzylidene)cycloalkanones catalyzed by FeCl3·6H2O. Green Chem. 2003, 5, 267–269. [Google Scholar] [CrossRef]
- Huang, D.F.; Wang, J.X.; Hu, Y.L. A New Solvent-free Synthesis of α,α′-Dibenzylidene-cycloalkanones from Acetals with Cycloalkanones under Microwave Irradiation. Chin. Chem. Lett. 2003, 14, 333–334. [Google Scholar]
- Deng, G.; Ren, T. Indium Trichloride Catalyzes Aldol-Condensations of Aldehydes and Ketones. Synth. Commun. 2003, 33, 2995–3001. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.K.; Reddy, K.B.; Yadav, J.S. Iodotrimethylsilane-Mediated Cross-Aldol Condensation: A Facile Synthesis of α,α′-Bis(substituted benzylidene)cycloalkanones. Synthesis 2004, 263–266. [Google Scholar]
- Zhu, Y.; Pan, Y. A New Lewis Acid System Palladium/TMSCl for Catalytic Aldol Condensation of Aldehydes with Ketones. Chem. Lett. 2004, 33, 668–669. [Google Scholar] [CrossRef]
- Hu, Z.G.; Liu, J.; Zeng, P.L.; Dong, Z.B. Synthesis of α,α′-Bis(substituted benzylidene)ketones Catalyzed by a SOCl2/EtOH Reagent. J. Chem. Res. Synop. 2004, 1, 55–56. [Google Scholar]
- Wang, L.; Sheng, J.; Tian, H.; Han, J.; Fan, Z.; Qian, C. A Convenient Synthesis of α,α′-Bis(substituted benzylidene)cycloalkanones Catalyzed by Yb(OTf)3 Under Solvent-Free Conditions. Synthesis 2004, 18, 3060–3064. [Google Scholar]
- Cao, Y.-Q.; Zhi, D.; Zhang, R.; Chen, B.-H. Aldol Condensations Catalyzed by PEG400 and Anhydrous K2CO3 without Solvent. Synth. Commun. 2005, 35, 1045–1049. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P.; Mahender, I.; Reddy, K.R. Convenient and facile cross-Aldol condensation catalyzed by molecular iodine: An efficient synthesis of α,α′-bis(substituted-benzylidene) cycloalkanones. J. Mol. Catal. A Chem. 2006, 247, 182–185. [Google Scholar] [CrossRef]
- Li, J.; Su, W.; Li, N. Copper Triflate–Catalyzed Cross‐Aldol Condensation: A Facile Synthesis of α,α′‐Bis(Substituted Benzylidene) Cycloalkanones. Synth. Commun. 2005, 35, 3037–3043. [Google Scholar] [CrossRef]
- Hazarkhani, H.; Kumar, P.; Kondiram, K.S.; Shafi Gadwal, I.M. Highly Selective Claisen-Schmidt Condensation Catalyzed by Silica Chloride Under Solvent-Free Reaction Conditions. Synth. Commun. 2010, 40, 2887–2896. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Balooty, L.; Mehregan, M.; Shekouhy, M. Solvent-Free, Cross-Aldol Condensation Reaction Using Silica-Supported, Phosphorus-Containing Reagents Leading to α,α′-Bis(arylidene)cycloalkanones. Synth. Commun. 2010, 40, 3488–3495. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, X.-M.; Pang, L.-L.; Ma, R.; Yue, C.-H.; Yuan, R.; Lin, W.; Yin, W.; Bo, R.-C.; Wu, H. Synthesis and Fluorescence Properties of α,α′-Bis(substituted-benzylidene)cycloalkanones Catalyzed by 1-Methyl-3(2-(sulfooxy)ethyl)-1H-imidazol-3-ium Chloride. Synth. Commun. 2010, 40, 2320–2328. [Google Scholar] [CrossRef]
- Arnold, A.; Markert, M.; Mahrwald, R. Amine-Catalyzed Aldol Condensation in the Presence of Lithium Perchlorate. Synthesis 2006, 7, 1099–1102. [Google Scholar]
- Riadi, Y.; Mamouni, R.; Azzalou, R.; Boulahjar, R.; Abrouki, Y.; Haddad, M.El.; Routier, S. Animal bone meal as an efficient catalyst for crossed-aldol condensation. Tet. Lett. 2010, 51, 6715–6717. [Google Scholar] [CrossRef]
- Yang, S.-D.; Wu, L.-Y.; Yan, Z.-Y.; Pan, Z.-L.; Liang, Y.-M. A novel ionic liquid supported organocatalyst of pyrrolidine amide: Synthesis and catalyzed Claisen-Schmidt. J. Mol. Catal. A Chem. 2007, 268, 107–111. [Google Scholar] [CrossRef]
- Kang, K.-Q.; Song, G.-H.; Wang, J.-Y.; Wei, B.-G. Synthesis of α,α′-Bis(substituted benzylidene)cycloalkanones Catalyzed by Amino-Functionalized Ionic Liquid. J. Chin. Chem. Soc. 2008, 55, 1125–1128. [Google Scholar]
- Solhy, A.; Amer, W.; Karkouri, M.; Tahir, R.; El Bouari, A.; Fihri, A.; Bousmina, M.; Zahouily, M. Bi-functional modified-phosphate catalyzed the synthesis of α-α′-(EE)-bis(benzylidene)-cycloalkanones: Microwave versus conventional-heating. Mol. Catal. A Chem. 2011, 336, 8–15. [Google Scholar] [CrossRef]
- Shrikhande, J.J.; Gawande, M.B.; Jayaram, R.V. Cross-aldol and Knoevenagel condensation reactions in aqueous micellar media. Catal. Commun. 2008, 9, 1010–1016. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, J.; Yadav, A.; Chaturvedi, V.; Bhatnagar, S.; Gaikwad, A.N.; Sinha, S.K.; Kumar, A.; Shukla, P.K.; Tripathi, R.P. A facile synthesis of α,α′-(EE)-bis(benzylidene)-cycloalkanones and their antitubercular evaluations. Eur. J. Med. Chem. 2009, 44, 1705–1709. [Google Scholar] [CrossRef]
- Bigdeli, M.A.; Mahdavinia, G.H.; Jafari, S.; Hazarkhani, H. Wet 2,4,6-trichloro[1,3,5]triazine (TCT) an efficient catalyst for synthesis of α,α′-bis(substituted-benzylidene) cycloalkanones under solvent-free conditions. Catal. Commun. 2007, 8, 2229–2231. [Google Scholar] [CrossRef]
- An, L.T.; Zou, J.P.; Zhang, L.L. Polymer-supported sulphonic acid catalyzed cross-aldol condensation: An expeditious synthesis of α,α′-bis(substituted benzylidene) cycloalkanones. Catal. Commun. 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Bhagat, S.; Sharma, R.; Chakraborti, A.K. Dual-activation protocol for tandem cross-aldol condensation: An easy and highly efficient synthesis of α,α′-bis(aryl/alkylmethylidene)ketones. J. Mol. Catal. A Chem. 2006, 260, 235–240. [Google Scholar] [CrossRef]
- Yi, W.B.; Cai, C. Aldol condensations of aldehydes and ketones catalyzed by rare earth(III) perfluorooctane sulfonates in fluorous solvents. J. Fluorine Chem. 2005, 126, 1553–1558. [Google Scholar] [CrossRef]
- Schriner, L.; Kurosawa, T. Chalcones. ii. decomposition by alkali. J. Am. Chem. Soc. 1930, 52, 2538–2540. [Google Scholar] [CrossRef]
- Dhar, D.N.; Lal, J.B. Chalcones. Condensation of Aromatic Aldehydes with Resacetophenone II. J. Org. Chem. 1958, 23, 1159–1161. [Google Scholar] [CrossRef]
- Hathaway, B.A. An aldol condensation experiment using a number of aldehydes and ketones. J. Chem. Educ. 1987, 64, 367. [Google Scholar] [CrossRef]
- Irie, K.; Watanabe, K. Aldol Condensations with Metal(II) Complex Catalysts. Bull. Chem. Soc. Jpn. 1980, 53, 1366–1371. [Google Scholar] [CrossRef]
- Rahman, M.A.F.M.; Jeong, B.S.; Kim, D.H.; Park, J.K.; Lee, E.S.; Jahng, Y. A facile synthesis of α,α′-bis(substituted-benzylidene)-cycloalkanones and substituted-benzylidene heteroaromatics: utility of NaOAc as a catalyst for aldol-type reaction. Tetrahedron 2007, 63, 2426–2431. [Google Scholar] [CrossRef]
- Kim, D.H.; Rahman, M.A.F.M.; Jeong, B.S.; Lee, E.S.; Jahng, Y. Acetate-Promoted Aldol-Type Reaction: Scope and Reactivity of Acetates and Aldehydes. Bull. Kor. Chem. Soc. 2009, 30, 797–802. [Google Scholar] [CrossRef]
- Garland, C.E.; Reid, E.E. Some new derivatives of cyclohexanone. J. Am. Chem. Soc. 1925, 47, 2333–2340. [Google Scholar] [CrossRef]
- English, J., Jr.; Lamberti, V. Optically Active 1-Cyclohexenyl- and 1-Cyclopentenylmethylcarbinols. J. Am. Chem. Soc. 1952, 74, 1909–1912. [Google Scholar] [CrossRef]
- Murakaiyama, T.; Banno, K.; Narasaka, K. New cross-aldol reactions. Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride. J. Am. Chem. Soc. 1974, 96, 7503–7509. [Google Scholar] [CrossRef]
- Tully, W.; Main, L.; Nicholson, B.K. β-Cyclomanganated 1,5-diphenylpenta-1,4-dien-3-ones and their reactions with alkynes: Routes to η5-pyranyl and η5-oxocycloheptadienylMn(CO)3 complexes. J. Organomet. Chem. 2001, 633, 162–172. [Google Scholar] [CrossRef]
- Vogel, A.I. Practical Organic Chemistry, 3rd ed; Longman: London, UK, 1956; p. 718. [Google Scholar]
- Kossanyi, J.; Furth, B.; Morizur, J.P. Influence de l'insaturation en série bicyclique pontée: Transposition de l'acétate de trans-benzylidène-3 isobornyle. Tetrahedron 1970, 26, 395–409. [Google Scholar] [CrossRef]
- Groselj, U.; Bevk, D.; Jakse, R.; Meden, A.; Stanovnik, B.; Svete, J. Stereoselective additions to the exocyclic C=C bond of some α-alkylidene-(+)-camphor derivatives. Tetrahedron: Asymmetry 2006, 17, 1217–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).