Antimycobacterial Activity of Salicylanilide Benzenesulfonates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
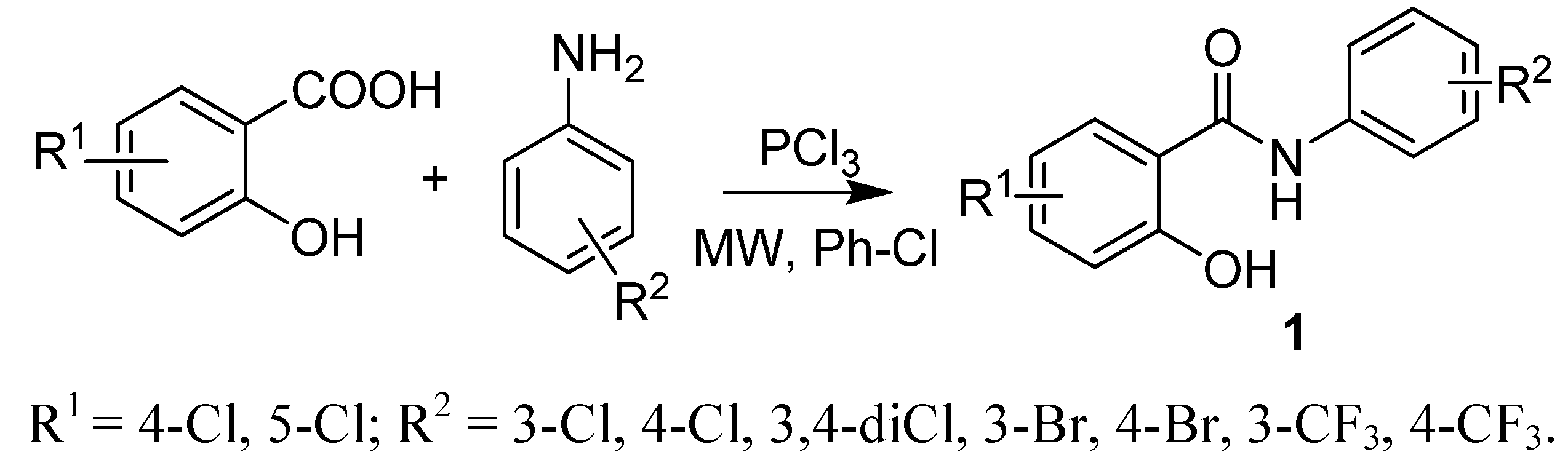
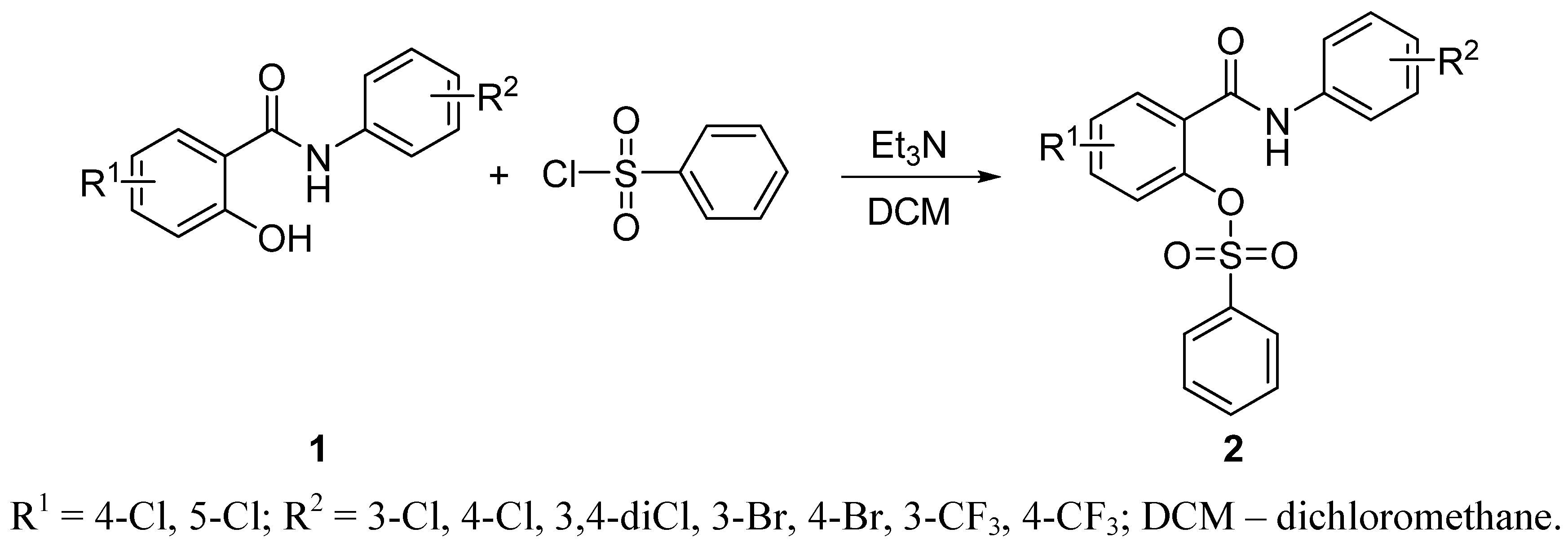
2.2. In Vitro Antimycobacterial Evaluation
| MIC [μmol/L] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | M. tuberculosis 331/88 | M. avium 330/88 | M. kansasii 235/80 | M. kansasii 6509/96 | |||||||
| 14 d | 21 d | 14 d | 21 d | 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | |||
| 2a | 4-Cl | 3-Cl | 4 | 4 | 32 | 32 | 8 | 16 | 16 | 8 | 16 | 16 |
| 2b | 5-Cl | 3-Cl | 16 | 16 | 500 * | 500 * | 16 | 16 | 16 | 125 | 500 | 500 |
| 2c | 4-Cl | 4-Cl | 4 | 4 | 32 | 32 | 8 | 8 | 8 | 8 | 8 | 8 |
| 2d | 5-Cl | 4-Cl | 8 | 8 | 8 | 8 | 8 | 16 | 16 | 8 | 16 | 16 |
| 2e | 4-Cl | 3,4-diCl | 1 | 2 | 16 | 16 | 4 | 8 | 8 | 8 | 8 | 8 |
| 2f | 5-Cl | 3,4-diCl | 4 | 8 | 62.5 | 62.5 | 4 | 8 | 8 | 8 | 8 | 8 |
| 2g | 4-Cl | 3-Br | 8 | 8 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 8 |
| 2h | 5-Cl | 3-Br | 16 | 16 | 250 | 500 * | 16 | 62.5 | 62.5 | 32 | 125 | 125 |
| 2i | 4-Cl | 4-Br | 8 | 8 | 32 | 32 | 8 | 8 | 8 | 8 | 8 | 16 |
| 2j | 5-Cl | 4-Br | 8 | 8 | 8 | 8 | 8 | 16 | 16 | 4 | 8 | 8 |
| 2k | 4-Cl | 3-F | 16 | 16 | 500 * | 500 * | 32 | 32 | 32 | 16 | 32 | 125 |
| 2l | 5-Cl | 3-F | 16 | 16 | 500 | 500 | 16 | 32 | 32 | 16 | 32 | 32 |
| 2m | 4-Cl | 4-F | 8 | 8 | 16 | 32 | 8 | 8 | 8 | 16 | 16 | 16 |
| 2n | 5-Cl | 4-F | 16 | 16 | 32 | 32 | 16 | 32 | 32 | 16 | 16 | 16 |
| 2o | 4-Cl | 3-CF3 | 2 | 4 | 62.5 | 62.5 | 8 | 8 | 8 | 8 | 16 | 16 |
| 2p | 5-Cl | 3-CF3 | 125 * | 125 * | 125 * | 125 * | 125 * | 125 * | 125 * | 125 * | 125 * | 125 * |
| 2q | 4-Cl | 4-CF3 | 1 | 1 | 125 | 125 | 125 * | 125 * | 125 * | 125 * | 125 * | 125 * |
| 2r | 5-Cl | 4-CF3 | 2 | 4 | 250 * | 250 * | 2 | 4 | 4 | 4 | 4 | 4 |
| INH | 0.5 | 0.5 | >250 | >250 | >250 | >250 | >250 | 2 or 4 | 4 | 8 | ||
| PAS | 62.5 | 62.5 | 32 | 125 | 125 | 1000 | >1000 | 250 | 1000 | 1000 | ||
| EMB | 1 | 2 | 16 | 16 | 1 | 2 | 2 | 1 | 2 | 2 | ||
3. Experimental
3.1. General Methods
3.2. Synthesis of Salicylanilide Benzenesulfonates
3.3. Antimycobacterial Susceptibility Testing
4. Conclusions
Conflict of Interest
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- WHO. 2011/2012 Tuberculosis Global Facts. Available online: http://www.who.int/tb/publications/2011/factsheet_tb_2011.pdf (accessed on 28 October 2011).
- Vinsova, J.; Kratky, M. Development of New MDR-Tuberculosis Drugs, 1st ed; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Kratky, M.; Vinsova, J. Advances in the Development of Antituberculotics Acting on Multidrug-Resistant Strains. Chem. Listy 2010, 104, 998–1005. [Google Scholar]
- Sugita, A.; Ogawa, H.; Azuma, M.; Muto, S.; Honjo, A.; Yanagawa, H.; Nishioka, Y.; Tani, K.; Itai, A.; Sone, S. Antiallergic and anti-inflammatory effects of a novel IkB kinase b inhibitor, IMD-0354, in a mouse model of allergic inflammatio. Int. Arch. Allergy Immunol. 2009, 148, 186–198. [Google Scholar] [CrossRef]
- Brown, M.E.; Fitzner, J.N.; Stevens, T.; Chin, W.; Wright, C.D.; Boyce, J.P. Salicylanilides: Selective inhibitors of interleukin-12p40 production. Bioorg.Med. Chem. 2008, 16, 8760–8764. [Google Scholar] [CrossRef]
- Zhong, G.X.; Chen, L.L.; Li, J.; Hu, W.X.; Qi, M.Y. Synthesis and anti-inflammatory-analgesic activity of 2′,4′-difluoro-3-(carbamoyl)biphenyl-4-yl benzoates. Chin. Chem. Lett. 2008, 19, 1419–1422. [Google Scholar] [CrossRef]
- Calderone, V.; Coi, A.; Fiamingo, F.L.; Giorgi, I.; Leonardi, M.; Livi, O.; Martelli, A.; Martinotti, E. Structural modifications of benzanilide derivatives, effective potassium channel openers. X. Eur. J. Med. Chem. 2006, 41, 1421–1429. [Google Scholar] [CrossRef]
- Liechti, C.; Séquin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, J.S.; Cai, L.L.; Zeng, Y.X.; Yang, D. SUCI02 inhibits the erbB-2 tyrosine kinase receptor signaling pathway and arrests the cell cycle in G1 phase in breast cancer cells. Cancer Sci. 2006, 97, 84–89. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J. Antiviral Activity of Substituted Salicylanilides—A Review. Mini-Rev. Med. Chem. 2011, 97, 956–967. [Google Scholar]
- Krátký, M.; Vinšová, J. Salicylanilide Ester Prodrugs as Potential Antimicrobial Agents—A Review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef]
- Hamilton, W.A. Mechanism of the Bacteriostatic Action of Tetrachlorosalicylanilide—A Membrane Active Antibacterial Compound. J. Gen. Microbiol. 1968, 50, 441–458. [Google Scholar] [CrossRef]
- Terada, H.; Goto, S.; Yamamoto, K.; Takeuchi, I.; Hamada, Y.; Miyake, K. Structural requirements of salicylanilides for uncoupling activity in mitochondria: Quantitative analysis of structure-uncoupling relationships. Biochim.Biophys. Acta 1988, 936, 504–512. [Google Scholar] [CrossRef]
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russel, R.K.; Sui, Z.H.; Weidner-Wells, M.A.; Werblood, H.; et al. Substituted Salicylanilides as Inhibitors of Two-Component Regulatory Systems in Bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Buchta, V.; Horvati, K.; Bösze, S.; Stolaříková, J. New amino acid esters of salicylanilides active against MDR-TB and other microbes. Eur. J. Med. Chem. 2010, 45, 6106–6113. [Google Scholar] [CrossRef]
- Glowienke, S.; Frieauff, W.; Allmendinger, T.; Martus, H.J.; Suter, W.; Mueller, L. Structure–activity considerations and in vitro approaches to assess the genotoxicity of 19 methane-, benzene- and toluenesulfonic acid esters. Mutat.Res. 2005, 581, 23–34. [Google Scholar] [CrossRef]
- Imramovský, A.; Vinšová, J.; Férriz, J.M.; Doležal, R.; Jampílek, J.; Kaustová, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg.Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef]
- Vinsova, J.; Imramovsky, A.; Buchta, V.; Ceckova, M.; Dolezal, M.; Staud, F.; Jampilek, J.; Kaustova, J. Salicylanilide Acetates: Synthesis and Antibacterial Evaluation. Molecules 2007, 12, 1–12. [Google Scholar] [CrossRef]
- Imramovský, A.; Férriz, J.M.; Pauk, K.; Krátký, M.; Vinšová, J. Synthetic route for the preparation of 2-hydroxy-N-[1-(2-hydroxyphenylamino)-1-oxoalkan-2-yl]benzamides. J. Comb. Chem. 2010, 12, 414–416. [Google Scholar] [CrossRef]
- Gwaltney, S.L.; Imade, H.M.; Li, Q.; Gehrke, L.; Credo, R.B.; Warner, R.B.; Lee, J.Y.; Kovar, P.; Frost, D.; Ng, S.C.; Sham, H.L. Novel Sulfonate Derivatives: Potent Antimitotic Agents. Bioorg.Med. Chem. Lett. 2001, 11, 1671–1673. [Google Scholar] [CrossRef]
- Waisser, K.; Bureš, O.; Holý, P.; Kuneš, J.; Oswald, R.; Jirásková, L.; Pour, M.; Klimešová, V.; Kubicová, L.; Kaustová, J. Relationship between the structure and antimycobacterial activity of substituted salicylanilides. Arch. Pharm. Pharm. Med. Chem. 2003, 1, 53–71. [Google Scholar]
- Ferriz, J.M.; Vávrová, K.; Kunc, F.; Imramovský, A.; Stolaříková, J.; Vavříková, E.; Vinšová, J. Salicylanilide carbamates: Antitubercular agents active against multidrug-resistant Mycobacterium tuberculosis strains. Bioorg.Med. Chem. 2010, 18, 1054–1061. [Google Scholar] [CrossRef]
- Kaustová, J. Quantitative micromethod for drug susceptibility testing of mycobacteria in Šula´s medium. Klin.Microbiol. Inf. Lek. 1997, 3, 115–124. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krátký, M.; Vinšová, J.; Rodriguez, N.G.; Stolaříková, J. Antimycobacterial Activity of Salicylanilide Benzenesulfonates. Molecules 2012, 17, 492-503. https://doi.org/10.3390/molecules17010492
Krátký M, Vinšová J, Rodriguez NG, Stolaříková J. Antimycobacterial Activity of Salicylanilide Benzenesulfonates. Molecules. 2012; 17(1):492-503. https://doi.org/10.3390/molecules17010492
Chicago/Turabian StyleKrátký, Martin, Jarmila Vinšová, Nabila Guisado Rodriguez, and Jiřina Stolaříková. 2012. "Antimycobacterial Activity of Salicylanilide Benzenesulfonates" Molecules 17, no. 1: 492-503. https://doi.org/10.3390/molecules17010492
APA StyleKrátký, M., Vinšová, J., Rodriguez, N. G., & Stolaříková, J. (2012). Antimycobacterial Activity of Salicylanilide Benzenesulfonates. Molecules, 17(1), 492-503. https://doi.org/10.3390/molecules17010492





