Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables
Abstract
:1. Introduction
2. Results and Discussion
2.1. Brassica bioactive compounds
| Botanical group | Year | Climate season | Aliphatic | Aromatic | Indole | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GIB | PROG | SIN | GNAP | GBRASSNAP | GRAF | GNAST | 4-OH | GBRASS | 4-METHOX | NGB | Total GLS | |||
| Broccoli | 2005-2006 | SS | 120 ± 13 | ND | ND | ND | ND | 958 ± 68 | ND | ND | 175 ± 19 | 30 ± 3 | 579 ±132 | 1862 ± 182 |
| SW | ND | ND | ND | ND | ND | 2152 ± 157* | ND | ND | 529 ± 28* | 173 ± 19* | 273 ± 20* | 3128 ± 124* | ||
| 2006-2007 | SS | 132 ± 4* | ND | ND | ND | ND | 1154 ± 37 | ND | 64 ± 2 * | 350 ± 22 | 547 ± 17* | 1694 ± 78* | 3942 ± 159* | |
| SW | 110 ± 5 | ND | ND | ND | ND | 1222 ± 65 | ND | 56 ± 1 | 589 ± 65* | 434 ± 6 | 274 ± 34 | 2686 ± 50 | ||
| Portuguese kale | 2005-2006 | SS | 679 ± 88* | ND | 368 ± 24 | ND | ND | ND | ND | ND | 272 ± 44 | ND | 47 ± 4 * | 1366 ± 103* |
| SW | 101 ± 7 | 17 ± 3 | 284 ± 20 | ND | ND | 25 ± 2 | ND | ND | 187 ± 10 | 34 ± 2 | 30 ± 1 | 676 ± 13 | ||
| 2006-2007 | SS | 732 ± 10* | 17 ± 5 | 328 ± 34* | ND | ND | 43 ± 5 | ND | 12 ± 1 | 608 ± 73* | 71 ± 12 | 45 ± 10* | 1855 ± 112* | |
| SW | 68 ± 22 | ND | 21 ± 11 | ND | ND | 60 ± 7 | ND | 34 ± 7 * | 107 ± 15 | 69 ± 10 | 8 ± 4 | 369 ± 53 | ||
| Savoy cabbage | 2005-2006 | SS | 718 ± 76* | 59 ± 7 | 236 ± 18 | ND | ND | ND | ND | ND | 210 ± 28 | ND | ND | 1224 ± 85 |
| SW | 355 ± 24 | 75 ± 4 | 329 ± 12* | ND | ND | 222 ± 19 | ND | ND | 320 ± 2* | 203 ± 11 | 11 ± 0* | 1515 ± 35* | ||
| 2006-2007 | SS | 932 ± 58* | 87 ± 6 | 149 ± 10 | 21 ± 1 | ND | 396 ± 41 | ND | 73 ± 7* | 1144 ± 37* | 611 ± 31 | 33 ± 5 | 3447 ± 51 | |
| SW | 865 ± 105 | 96 ± 22 | 290 ± 72 | 33 ± 5 | ND | 314 ± 54 | ND | 21 ± 6 | 832 ± 77 | 559 ± 24 | 18 ± 3 | 3030 ± 194 | ||
| White cabbage | 2005-2006 | SS | 106 ± 18 | 17 ± 2 | 106 ± 23 | ND | ND | ND | ND | ND | 20 ± 2 | 5 ± 0 | 2 ± 1 | 256 ± 46 |
| SW | 174 ± 33 | 172 ± 14* | 709 ± 38* | ND | ND | 50 ± 8 | ND | ND | 219 ± 24* | 119 ± 15* | 16 ± 1* | 1460 ± 18* | ||
| 2006-2007 | SS | 217 ± 15 | 33 ± 1 | 199 ± 23* | 16 ± 2 | ND | 32 ± 3 | ND | 6 ± 2 | 137 ± 10 | 163 ± 28 | 26 ± 9 | 828 ± 57 | |
| SW | 452 ± 17* | 236 ± 9* | 63 ± 4 | 94 ± 20* | ND | 958 ± 11* | ND | 67 ± 6 * | 428 ± 25* | 417 ± 19* | 18 ± 3 | 2731 ± 33* | ||
| Portuguese tronchuda cabbage | 2005-2006 | SS | 235 ± 19 | ND | 89 ± 12 | ND | ND | ND | ND | ND | 159 ± 27 | 6 ± 1 | 54 ± 6 | 543 ± 25 |
| SW | 163 ± 32 | 23 ± 12 | 305 ± 50* | ND | ND | 49 ± 19 | ND | ND | 252 ± 15* | ND | 44 ± 2 | 836 ± 80* | ||
| 2006-2007 | SS | 571 ± 57 | 28 ± 5 | 232 ± 62 | ND | ND | 68 ± 13 | ND | 23 ± 2 | 537 ± 81 | 124 ± 6 | 135 ± 5 | 1718 ± 84 | |
| SW | 503 ± 76 | 36 ± 19 | 75 ± 41 | ND | ND | 289 ± 63* | ND | 44 ± 3 * | 799 ± 34* | 397 ± 36* | 137 ± 12 | 2280 ± 52* | ||
| Turnip leaves | 2005-2006 | SS | ND | ND | ND | 921 ± 41 | 876 ± 30 | ND | 71 ± 11 | ND | 16 ± 4 | ND | 54 ± 9 | 1937 ± 17 |
| SW | ND | ND | ND | 848 ± 69 | 1067 ± 86 | ND | 71 ± 5 | ND | 62 ± 10* | 96 ± 3 | 127 ± 4* | 2270 ± 164 | ||
| 2006-2007 | SS | ND | ND | ND | 1190 ± 40 | 1005 ± 100 | ND | 120 ± 11 | 9 ± 1 | 30 ± 2 | ND | 252 ± 5* | 2606 ± 129 | |
| SW | ND | ND | ND | 2075 ± 568 | 1344 ± 101 | ND | 395 ± 58* | 36 ± 16* | 55 ± 11 | ND | 85 ± 24 | 3990 ± 403* | ||
| Turnip roots | 2005-2006 | SS | ND | ND | ND | 538 ± 78 | 323 ± 57 | ND | 399 ± 69 | ND | 17 ± 3 | ND | 37 ± 8 | 1314 ± 212 |
| SW | ND | ND | ND | 507 ± 68 | 583 ± 64* | ND | 673 ± 104* | 55 ± 11 | 34 ± 1* | ND | 133 ± 6* | 1984 ± 131 | ||
| 2006-2007 | SS | ND | ND | ND | 394 ± 22 | 235 ± 28 | ND | 306 ± 14 | 57 ± 9 | 99 ± 11 | 134 ± 40 | 221 ± 13 | 1446 ± 109* | |
| SW | ND | ND | ND | 872 ± 46* | 572 ± 20* | ND | 496 ± 20* | 137 ± 2* | 96 ± 10 | 1233 ± 74 * | 206 ± 20 | 3613 ± 103 | ||
| Botanical group | Year | Climate season | Total phenolics (mg.g–1 GAE) | Total Flavonoids (mg.g–1 CAE) | L-ascorbic acid (mg.g–1 LACE) |
|---|---|---|---|---|---|
| Broccoli | 2005-2006 | SS | 15.3 ± 0.8 | 7.7 ± 0.02 * | 98.2 ± 0.20 * |
| SW | 13.5 ± 0.3 | 4.9 ± 0.10 | 97.3 ± 0.07 | ||
| 2006-2007 | SS | 21.1 ± 0.3 | 12.1 ± 0.02 * | 96.3 ± 0.20 * | |
| SW | 24.3 ± 1.1 * | 3.3 ± 0.01 | 95.5 ± 0.05 | ||
| Portuguese kale | 2005-2006 | SS | 23.0 ± 1.0 * | 9.3 ± 0.02 * | 139.6 ± 0.06 * |
| SW | 18.8 ± 0.7 | 7.4 ± 0.62 | 139.3 ± 0.04 | ||
| 2006-2007 | SS | 27.4 ± 3.6 | 12.0 ± 0.02 * | 139.0 ± 0.06 * | |
| SW | 20.7 ± 0.5 | 8.1 ± 0.02 | 138.8 ± 0.01 | ||
| Savoy cabbage | 2005-2006 | SS | 9.9 ± 0.5 | 2.7 ± 0.28 | 107.5 ± 0.17 * |
| SW | 12.9 ± 2.3 | 3.1 ± 0.20 | 106.7 ± 0.04 | ||
| 2006-2007 | SS | 13.6 ± 0.1 | 4.1 ± 0.01 | 105.9 ± 0.17 * | |
| SW | 16.1 ± 1.4 | 4.1 ± 0.02 | 105.3 ± 0.06 | ||
| White cabbage | 2005-2006 | SS | 11.1 ± 1.5 | 3.0 ± 0.02 | 70.2 ± 0.09 |
| SW | 13.0 ± 1.1 | 6.5 ± 0.01 * | 71.6 ± 0.30 * | ||
| 2006-2007 | SS | 8.7 ± 0.1 | 2.5 ± 0.02 | 67.7 ± 0.08 | |
| SW | 14.8 ± 0.7 * | 3.2 ± 0.00 * | 68.8 ± 0.30 * | ||
| Portuguese tronchuda cabbage | 2005-2006 | SS | 15.7 ± 1.1 | 2.1 ± 0.01 | 138.9 ± 0.03 |
| SW | 19.0 ± 0.9 | 6.9 ± 0.02 * | 139.1 ± 0.06 * | ||
| 2006-2007 | SS | 24.8 ± 0.8 * | 10.5 ± 0.02 * | 138.6 ± 0.06 * | |
| SW | 18.6 ± 0.5 | 5.0 ± 0.02 | 138.4 ± 0.03 | ||
| Turnip leaves | 2005-2006 | SS | 15.3 ± 1.0 | 3.5 ± 0.01 | 132.3 ± 0.04 |
| SW | 19.5 ± 0.7 * | 5.6 ± 0.11 * | 132.7 ± 0.08 | ||
| 2006-2007 | SS | 17.1 ± 0.5 | 5.5 ± 0.02 | 131.6 ± 0.02 | |
| SW | 15.8 ± 8.6 | 8.7 ± 0.01 * | 131.9 ± 0.08 | ||
| Turnip roots | 2005-2006 | SS | 8.8 ± 1.1 | 0.7 ± 0.06 | 22.9 ± 0.13 |
| SW | 11.8 ± 0.3 | 7.6 ± 0.06 * | 25.0 ± 0.46 * | ||
| 2006-2007 | SS | 7.3 ± 0.2 | 1.6 ± 0.01 | 18.9 ± 0.13 | |
| SW | 10.5 ± 0.3 * | 2.0 ± 0.01 * | 20.6 ± 0.46 * |
| Month | 1961–1990 | 2005–2006 | 2006–2007 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T min (°C) | T max (°C) | T mean (°C) | R (mm) | T min (°C) | T max (°C) | T mean (°C) | R (mm) | T min (°C) | T max (°C) | T mean (°C) | R (mm) | |
| March | 4.8 | 14.4 | 9.6 | 3.1 | 5.3 | 15.7 | 10.5 | 1.8 | 6.2 | 14.1 | 10.2 | 5.3 |
| April | 6.4 | 16.5 | 11.4 | 2.9 | 7.3 | 17.1 | 12.2 | 1.7 | 8.2 | 19.5 | 13.8 | 2.0 |
| May | 8.9 | 20.1 | 14.5 | 2.3 | 9.7 | 21.8 | 15.7 | 1.4 | 10.3 | 23.7 | 17.0 | 0.2 |
| June | 12.4 | 25.0 | 18.7 | 1.8 | 15.0 | 28.4 | 21.7 | 0.1 | 13.8 | 27.2 | 20.5 | 2.5 |
| July | 14.3 | 28.8 | 21.6 | 0.5 | 15.0 | 28.9 | 22.0 | 0.3 | 15.8 | 30.3 | 23.1 | 0.9 |
| August | 13.8 | 28.7 | 21.3 | 0.5 | 16.3 | 31.4 | 23.8 | 0.1 | 15.4 | 29.7 | 22.6 | 1.0 |
| September | 12.6 | 25.7 | 19.2 | 1.6 | 12.8 | 25.9 | 19.3 | 0.8 | 13.7 | 26.1 | 19.9 | 3.1 |
| October | 9.4 | 19.5 | 14.4 | 3.5 | 10.9 | 19.8 | 15.4 | 4.6 | 11.8 | 19.7 | 15.7 | 6.1 |
| November | 5.4 | 13.5 | 9.5 | 4.2 | 5.0 | 12.5 | 8.8 | 2.2 | 8.6 | 15.0 | 11.8 | 7.2 |
| December | 3.3 | 10.0 | 6.7 | 5.2 | 2.8 | 10.3 | 6.6 | 3.8 | 3.0 | 9.9 | 6.5 | 3.9 |
| January | 2.6 | 9.7 | 6.2 | 5.2 | 1.6 | 8.6 | 5.1 | 1.0 | 3.3 | 10.5 | 6.9 | 0.6 |
2.2. Effect of climate and bioactive components on antioxidant activity
| Plant extract | Year | Climate season | % Inhibition DPPH radicals | IC50 (mg·mL-1) |
|---|---|---|---|---|
| Broccoli | 2005-2006 | SS | 89.3* | 1.47 |
| SW | 84.9 | 2.56 | ||
| 2006-2007 | SS | 70.2 | 1.6 | |
| SW | 83.0* | 2.23 | ||
| Portuguese kale | 2005-2006 | SS | 91.6* | 1.10 |
| SW | 84.2 | 1.10 | ||
| 2006-2007 | SS | 83.8* | 1.65 | |
| SW | 62.7 | 2.14 | ||
| Savoy cabbage | 2005-2006 | SS | 54.0 | 4.61 |
| SW | 68.9* | 3.49 | ||
| 2006-2007 | SS | 47.9 | 5.48 | |
| SW | 59.8* | 4.4 | ||
| White cabbage | 2005-2006 | SS | 27.2 | 8.14 |
| SW | 37.9* | 6.95 | ||
| 2006-2007 | SS | 33.6 | >4.27 | |
| SW | 68.1* | 3.74 | ||
| Portuguese tronchuda cabbage | 2005-2006 | SS | 88.8 | 1.48 |
| SW | 89.8* | 2.12 | ||
| 2006-2007 | SS | 79.1* | 1.34 | |
| SW | 71.1 | 2.93 | ||
| Turnip leaves | 2005-2006 | SS | 77.2 | 2.22 |
| SW | 91.2* | 1.32 | ||
| 2006-2007 | SS | 46.4 | 9.19 | |
| SW | 74.7* | 2.12 | ||
| Turnip roots | 2005-2006 | SS | 14.1 | 9.18 |
| SW | 28.8* | 7.98 | ||
| 2006-2007 | SS | 26.5 | >4.82 | |
| SW | 49.3* | 5.68 | ||
| Control | Trolox | 89.9 | 0.24 |
| Compounds | Pearson’s correlation coefficients |
|---|---|
| Total phenolics vs DPPH | 0.6400 ** |
| Total flavonoids vs DPPH | 0.4580 ** |
| L-ascorbic acid vs DPPH | 0.7620 ** |
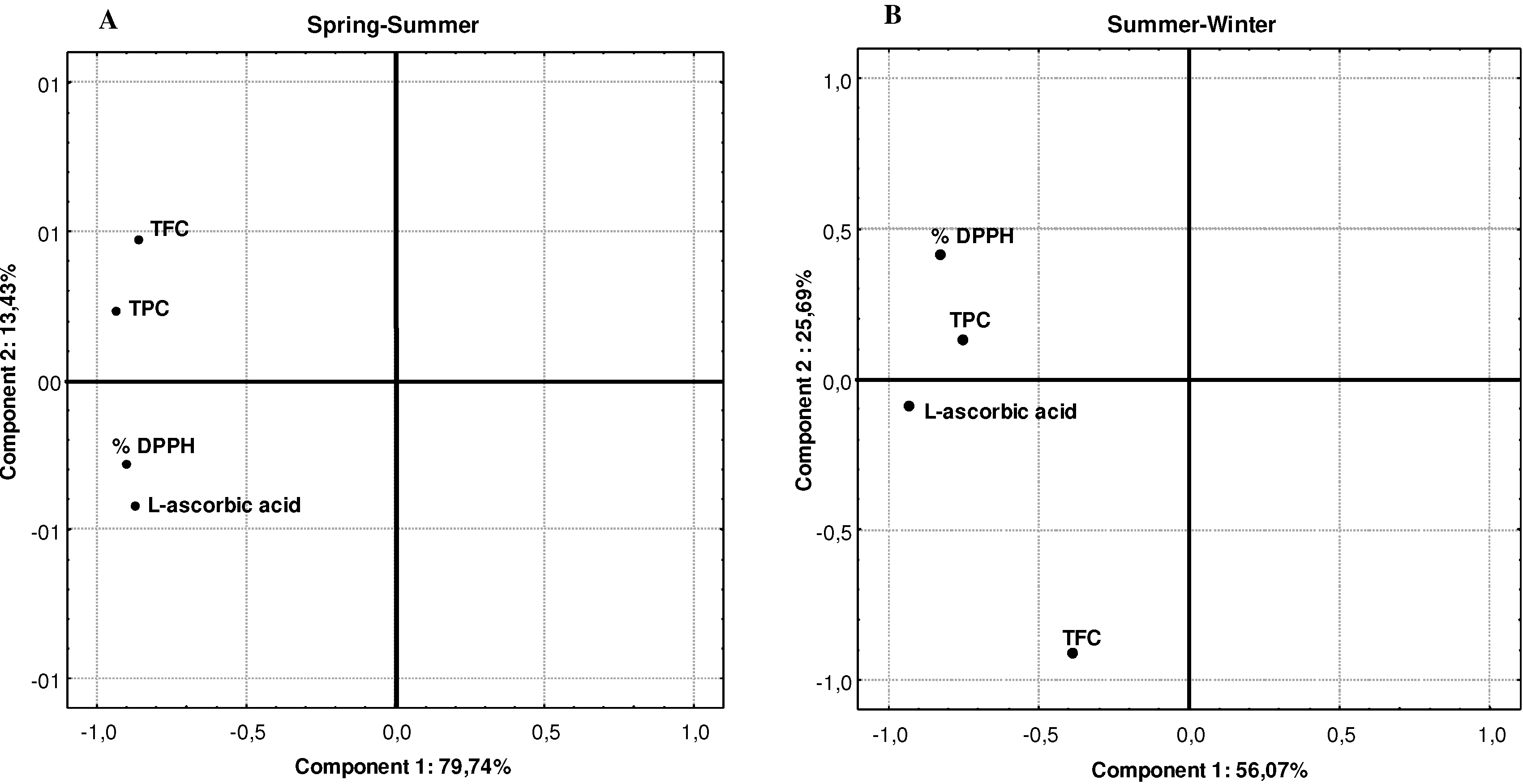
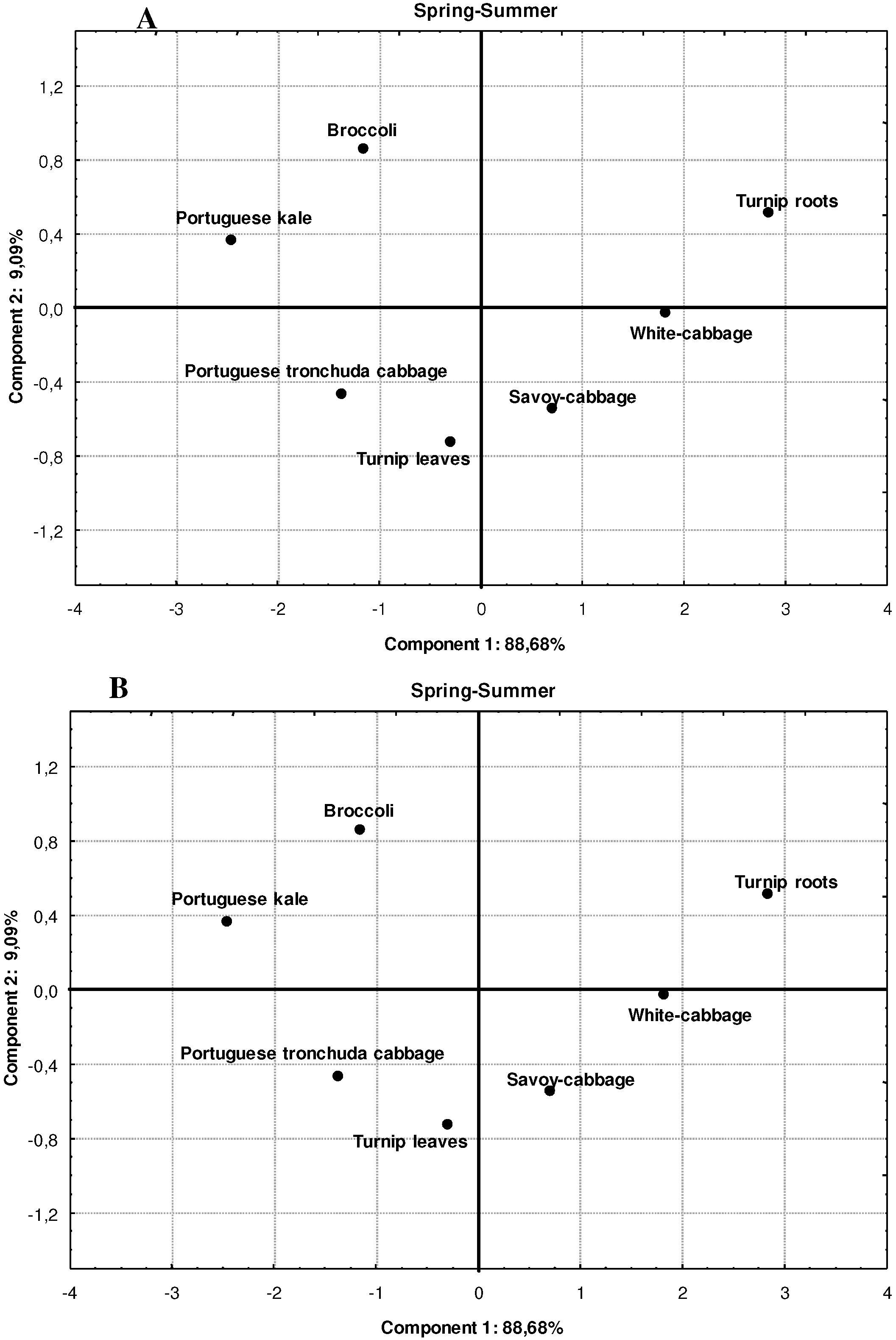
3. Experimental
3.1. Plant material
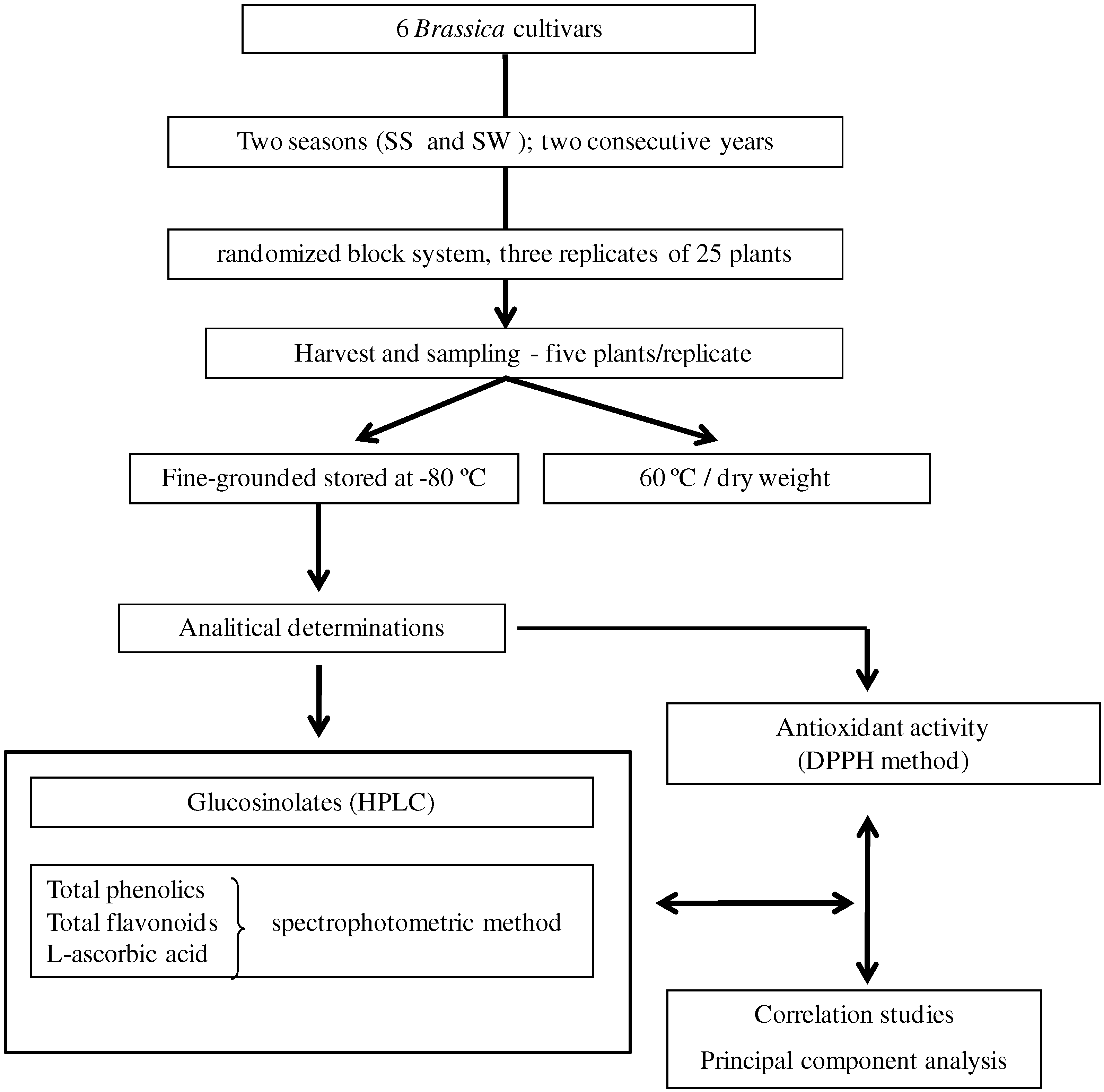
3.2. Determination of bioactive compounds
3.2.1. Glucosinolates
3.2.2. Total phenolics (TP) and total flavonoid contents (TF)
3.2.3. L-Ascorbic acid
3.3. Determination of antioxidant activity
3.4. Statistical analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Wu, X.; Beecher, G.R.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophylic and hidrophylic antioxidant capacities of common foods in United states. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Powers, S.K.; DeRuisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercice. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Lölinger, J.; Aruoma, O.I. The characterization on antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Lichtenthaler, R.; Marx, F. Total oxidant scavenging capacities of common European fruits and vegetables juices. J. Agric. Food Chem. 2005, 53, 103–110. [Google Scholar] [CrossRef]
- Tatsione, A.; Bonitsis, N.G.; Ioannidis, J.P.A. Presistence of contradicted claims in the literature. JAMA 2007, 298, 2517–2526. [Google Scholar] [CrossRef]
- Ysuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Seight, P. Vitamin E supplementation and cardiovascular events in high-risk patients: The heart outcomes prevention evaluation study investigators. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [CrossRef]
- Eichholzer, M.; Luthy, J.; Gutzwiller, F.; Stahelin, H.B. The role of folate, antioxidant vitamins and other constituents in fruit and vegetables in the prevention of cardiovascular disease: the epidemiological evidence. Int. J. Vitam. Nutr. Res. 2001, 71, 5–17. [Google Scholar] [CrossRef]
- Vogel, J.H.; Bolling, S.F.; Costello, R.B. Integrating a complementary medicine into cardiovascular medicine: a report of the American college of cardiology foundation task force on clinical expert consensus documents. J. Am. Coll. Cardiol. 2005, 46, 184–221. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant activity supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Komatsu, F.; Kagawa, Y.; Sakuma, M. Investigation of oxidative stress and dietary habits in Mongolian people, compared to Japanese people. Nutr. Metab. 2006, 3, 21. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Grossi, M.G.; Renzo, A.; Errichi, S.; Schönlau, F.; Wilmer, M.L.; Blumendeld, J. Accelerated antioxidant bioavalability of OPC-3® bioflavonoids administered as isotonic solution. Phytother. Res. 2009, 23, 775–777. [Google Scholar] [CrossRef]
- Ciska, E.; Martyniak-Przybysewska, B.; Kozlowska, H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J. Agric. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; de Haro, A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry 2008, 68, 403–410. [Google Scholar]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate ad genotype to seasonal variation in the glucosinolate-mirosinase system I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; Benavente-García, A.G.; García-Viguera, C. Total and individual glucosinolate contents in inflorescences of eight broccoli cultivars grown under various climatic and fertilization conditions. J. Sci. Food Agric. 2003, 83, 307–313. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar] [CrossRef]
- Sikora, E.; Cieślik, E.; Leszcyńskab, T.; Filipiak-Florkiewicz, A.; Pisulewski, P.M. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem. 2008, 107, 55–59. [Google Scholar] [CrossRef]
- Yao, L.; Caffin, N.; D´Arcy, B.; Jiang, Y.; Shi, J.; Singanusong, R.; Liu, X.; Datta, N.; Kakuda, Y.; Xu, Y. Seasonal variations of phenolic compounds in Australia-grown tea (Camellia sinensis). J. Agric. Food Chem. 2005, 53, 6477–6483. [Google Scholar]
- Hakala, M.; Lapvetelainen, A.; Huopalahti, R.; Kallio, H.; Tahvonen, R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Comp. Anal. 2003, 16, 67–80. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E. Patterns of pigment changes in Apple fruits during adaptation to high sunlight and sunscald development. Plant Biochem. Phsysiol. 2002, 40, 679–684. [Google Scholar] [CrossRef]
- Nilsson, J.; Olsson, K.; Engqvist, G.; Ekvall, J.; Olsson, M. Nyman M and Akesson B. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates in Brassica vegetables. J. Sci. Food Agric. 2006, 86, 528–538. [Google Scholar] [CrossRef]
- Plumb, W.; Lambert, N.; Chambers, S.J.; Wanigatunga, S.; Heaney, R.K.; Plumb, J.A.; Aruoma, O.I.; Halliwell, B.; Miller, N.J.; Williamson, G. Are whole extracts and purified glucosinolates from Cruciferous vegetables antioxidants? Free Radic. Res. 1996, 25, 75–86. [Google Scholar] [CrossRef]
- Valgimigli, L.; Iori, R. Antioxidant and Pro-oxidant capacities of ITCs. Environ. Mol. Mutagen 2009, 50, 222–237. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Papi, A.; Orlandi, M.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Fumo, M.G.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef]
- Barillari, J.; Iori, R.; Papi, A.; Orlandi, M.; Bartolini, G.; Gabbanini, S.; Pedulli, G.F.; Valgimigli, L. Kaiware Daikon (Raphanus sativus L.) extract: A naturally multi-potent chemopreventive agent. J. Agric. Food Chem. 2008, 56, 7823–7830. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar]
- Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Screening crucifer seeds as sources of specific intact glucosinolates using ion-pair HPLC negative ion electrospray mass spectrometry. J. Agric. Food Chem. 2003, 52, 428–438. [Google Scholar]
- Bennett, R.N.; Rosas, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, phenolics, flavonoids and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in colorado. LWT-Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchioca, M. Antioxidant capacity of vegetables, spices and dressing relevant to nutrition. Brit. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- Llorach, R.; Espian, J.C.; S-Barberaan, F.A.T.; Ferreres, F. Valorization of Cauliflower (Brassica oleracea L. var. botrytis) By-Products as a Source of Antioxidant Phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jefferey, E.H.; Kushad, M.; Wallig, M.A.; Juvic, J.A. Carotene, tocopherol and ascorbate contents in subspecies of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1576–1581. [Google Scholar]
- Rosa, E.A.S. Glucosinolates variation between leaves and roots of cabbage seedlings. In Proceedings of ISHS symposium on Brassicas/Ninth Crucifer Genetics Workshop, Lisbon, Portugal, 15-18 November 1994; Dias, J.S., Crute, I., Monteiro, A.A., Eds.; ISHS: Leiden, The Netherlands, 1996. [Google Scholar]
- Pereira, F.M.V.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef]
- Heaney, R.K.; Fenwick, G.R. Glucosinolates in Brassica vegetables, Analysis of 22 varieties of Brussels sprouts (Brassica oleracea var gemmifera). J. Sci. Food Agric. 1980, 18, 492–495. [Google Scholar]
- Spinks, E.A.; Sones, K.; Fenwick, G.R. The quatitative analysis of glucosinolates in cruciferous vegetables, oilseed and forages using high-performance liquid chromatography. Fette Seifen Anstrichm 1984, 86, 228–231. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum acessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Chun, K.O.; Smith, N.; Sakagawa, A.; Lee, C.Y. Antioxidant properties of raw and processed cabbages. Int. J. Food Sci. Nutr. 2004, 55, 191–199. [Google Scholar] [CrossRef]
- Sousa, J.A. Aulas Práticas de Química e Bioquímica dos Alimentos (1° Módulo). Licenciatura em Tecnologia e Segurança Alimentar; University Nova de Lisboa Press: Lisbon, Portugal, 2005; pp. 1–16. [Google Scholar]
- Siddhraju, P.; Becker, K. Antioxidant properties of various solvents extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A.S. Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables. Molecules 2011, 16, 6816-6832. https://doi.org/10.3390/molecules16086816
Aires A, Fernandes C, Carvalho R, Bennett RN, Saavedra MJ, Rosa EAS. Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables. Molecules. 2011; 16(8):6816-6832. https://doi.org/10.3390/molecules16086816
Chicago/Turabian StyleAires, Alfredo, Conceição Fernandes, Rosa Carvalho, Richard N. Bennett, Maria J. Saavedra, and Eduardo A.S. Rosa. 2011. "Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables" Molecules 16, no. 8: 6816-6832. https://doi.org/10.3390/molecules16086816
APA StyleAires, A., Fernandes, C., Carvalho, R., Bennett, R. N., Saavedra, M. J., & Rosa, E. A. S. (2011). Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables. Molecules, 16(8), 6816-6832. https://doi.org/10.3390/molecules16086816







