Synthesis and Antimicrobial Activity of Some New Pyrazoles, Fused Pyrazolo[3,4-d]-pyrimidine and 1,2-Dihydroimidazo-[2,1-c][1,2,4]triazin-6-one Derivatives
Abstract
:1. Introduction
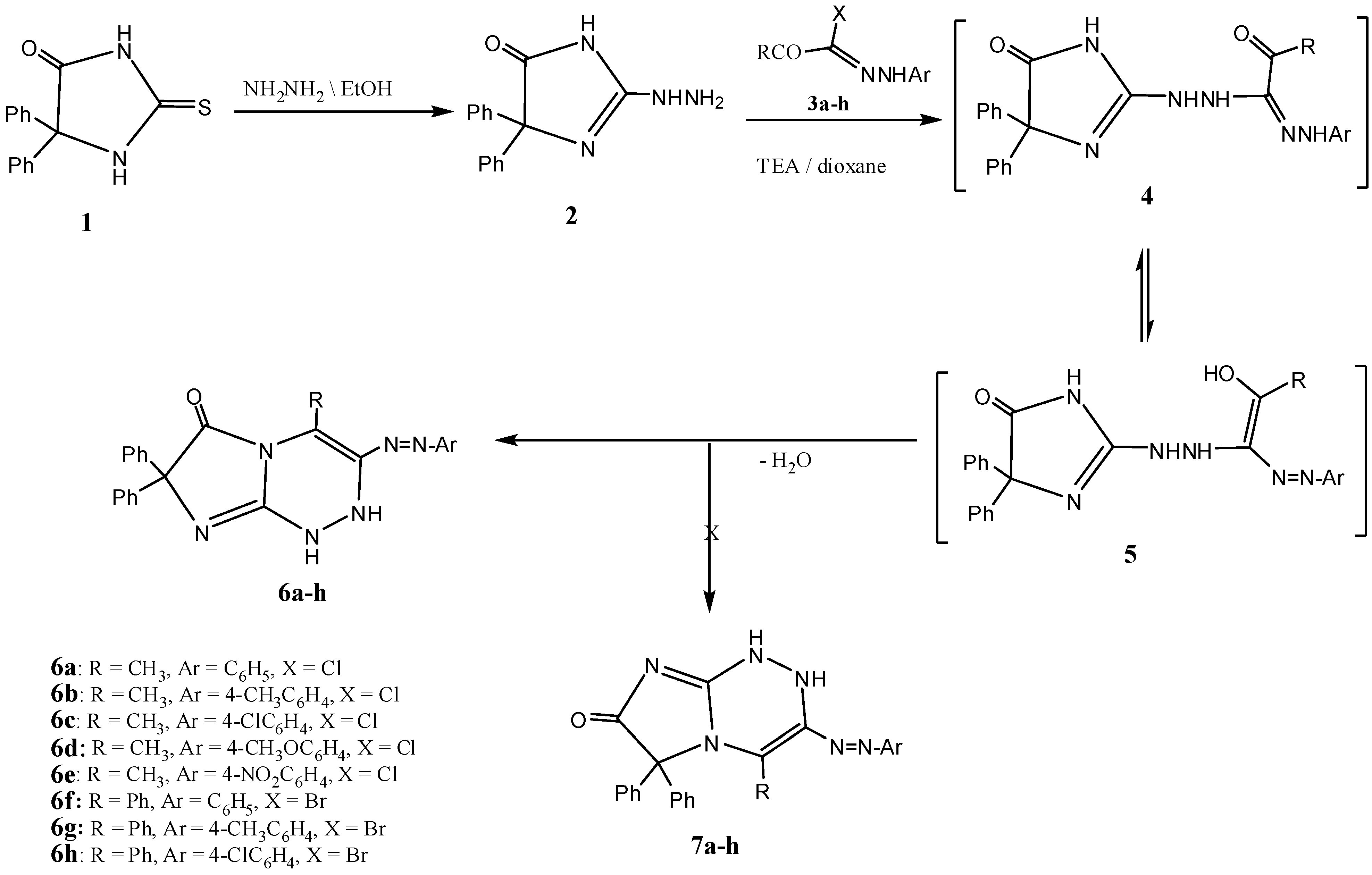
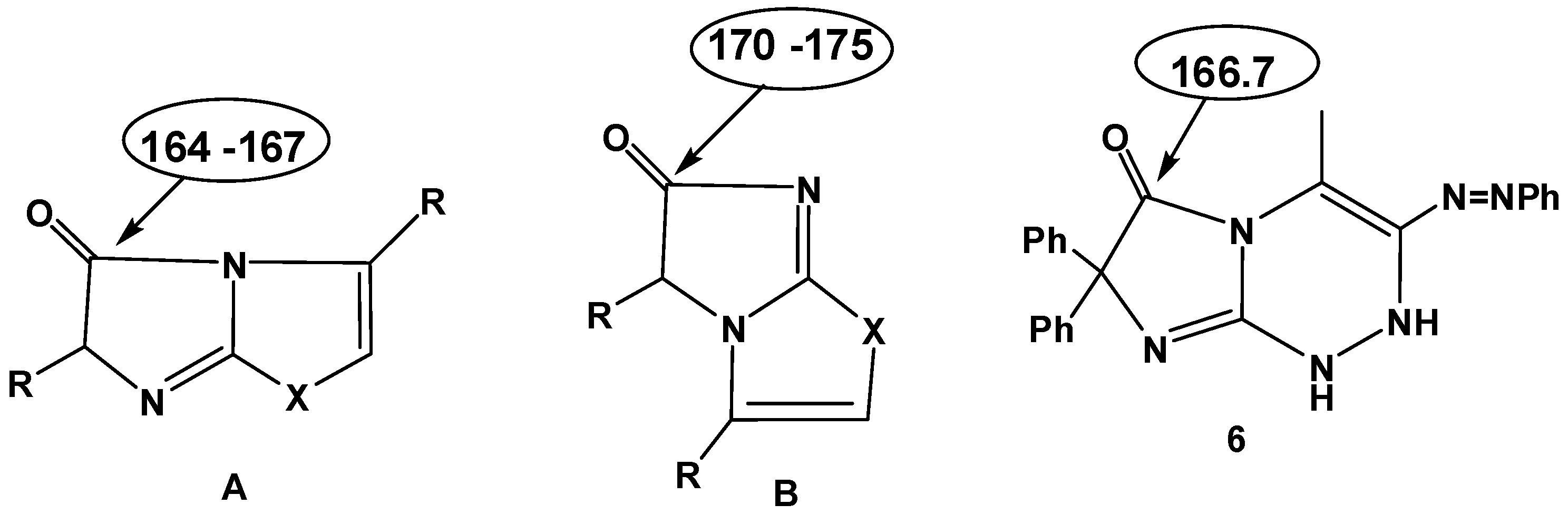
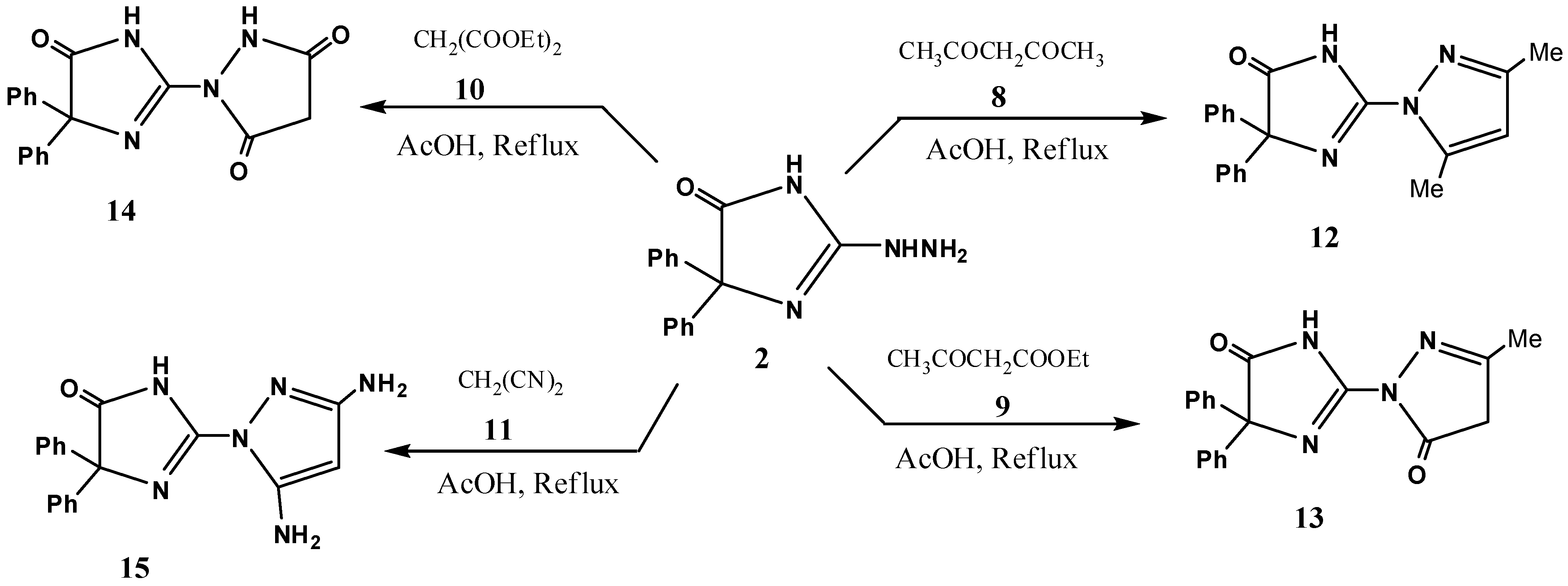
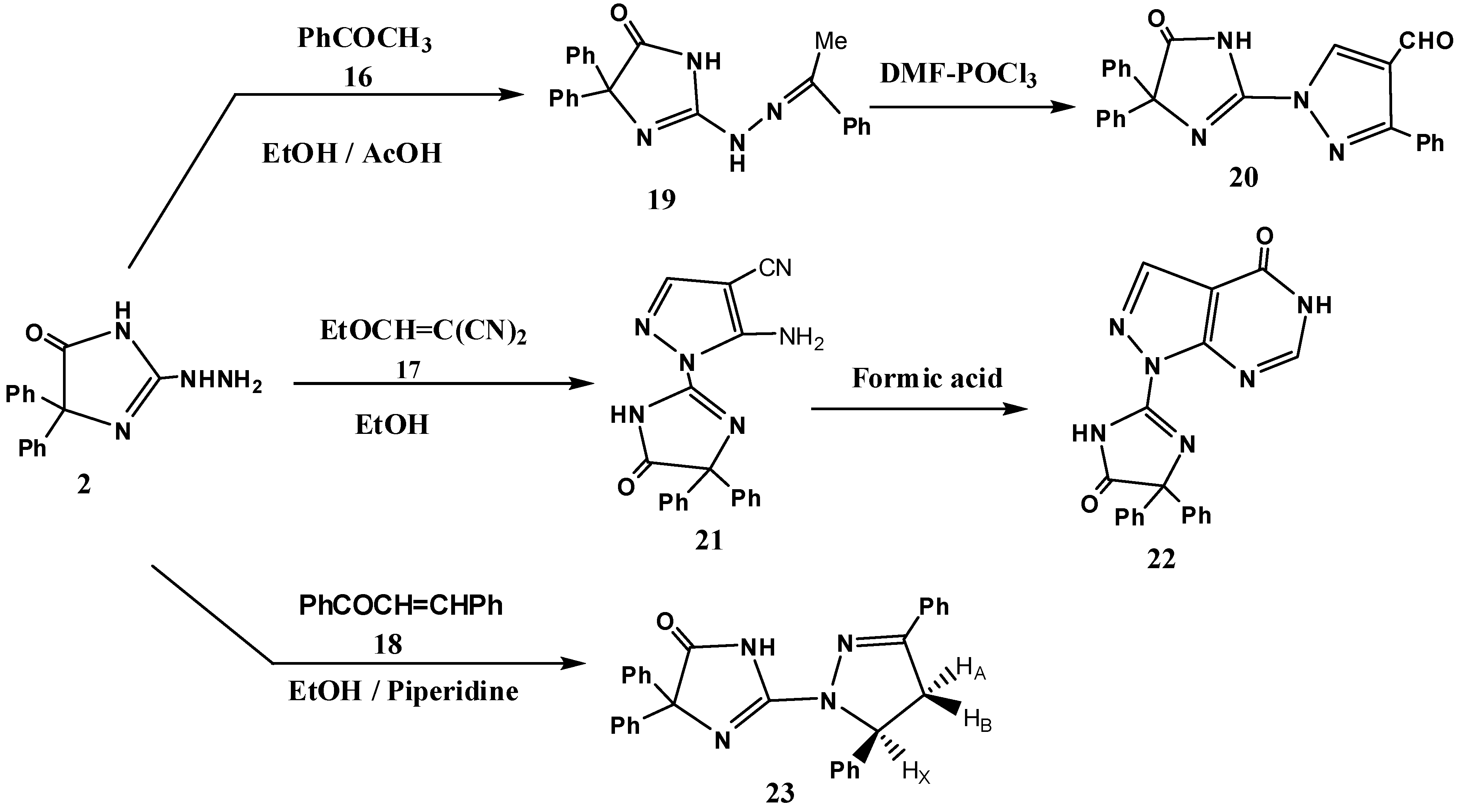
2. Results and Discussion
Antimicrobial Activity
| Sample No. | Inhibition zone diameter (mm/mg sample) | |||
|---|---|---|---|---|
| E. coli (G−) | S. aureus (G+) | Fungus | ||
| A. flavus | C. albicans | |||
| 6a | 22 | 16 | -- | -- |
| 6c | 21 | 20 | 10 | 15 |
| 6f | 14 | 16 | -- | -- |
| 6h | 15 | 13 | 9 | 13 |
| 12 | 18 | 15 | -- | -- |
| 13 | 24 | 12 | -- | -- |
| 14 | 16 | 19 | -- | -- |
| 15 | 18 | 14 | -- | -- |
| 20 | 12 | 18 | 11 | 14 |
| 22 | 18 | 22 | -- | -- |
| 23 | 14 | 23 | -- | -- |
| Tetracycline | 30 | 30 | -- | -- |
| Amphotricine | -- | -- | 18 | 21 |
3. Experimental
3.1. General
3.2. 2-Hydrazinyl-4,4-diphenyl-1H-imidazol-5(4H)-one (2)
3.3. 3,4-Disubstituted 7,7-diphenyl-1,2-dihydroimidazo[2,1-c][1,2,4]triazin-6(7H)-ones 6a–h
3.4. Reaction of 2 with Active Methylene Compounds
3.5. Preliminary Antimicrobial Screening
4. Conclusions
References and Notes
- Lombardino, J.G.; Wiseman, E.H. Preparation and antiinflammatory activity of some non-acidic trisubstituted imidazoles. J. Med. Chem. 1974, 17, 1182–1188. [Google Scholar]
- Gauthier, M.P.; Michaux, C.; Rolin, S.; Vastersaegher, C.; Leval, X.; Julémont, F.; Pochet, L.; Masereel, B. Synthesis, molecular modelling and enzymatic evaluation of (±)3,5-diphenyl-2-thioxoimidazolidin-4-ones as new potential cyclooxygenase inhibitors. Bioorg. Med. 2006, 14, 918–927. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Telkar, S.; Peethambar, S.K.; Rai, S.; Isloor, N. Synthesis, characterization and antimicrobial studies of some new pyrazole incorporated imidazole derivatives. Eur. J. Med. Chem. 2011, 46, 3531–3536. [Google Scholar] [CrossRef]
- Pozherskii, A.F.; Soldatenkov, A.T.; Katritzky, A.R. Heterocycles in Life and Society; Wiley: New York, NY, USA, 1997; p. 179. [Google Scholar]
- Daidone, G.; Maggio, B.; Raffa, D.; Plescia, S.; Schillaci, D.; Raimondi, M.V. Synthesis and in vitro antileukemic activity of new 4-triazenopyrazole derivatives. Ⅱ Farmaco 2004, 59, 413–417. [Google Scholar] [CrossRef]
- Manetti, F.; Brullo, C.; Magnani, M.; Mosci, F.; Chelli, B.; Crespan, E.; Schenone, S.; Naldini, A.; Bruno, O.; Trincavelli, M.L.; et al. Structure-based optimization of pyrazolo[3,4-d]pyrimidines as abl inhibitors and antiproliferative agents toward human leukemia cell lines. J. Med. Chem. 2008, 51, 1252–1259. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, Z.-W.; Zhao, B.-X.; Ge, X.; Meng, N.; Shin, D.-S.; Miao, J.-Y. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg. Med. Chem. 2007, 15, 6893–6899. [Google Scholar]
- Farag, A.M.; Mayhoub, A.S.; Barakat, S.E.; Bayomi, A.H. Regioselective synthesis and antitumor screening of some novel N-phenylpyrazole derivatives. Bioorg. Med. Chem. 2008, 16, 881–889. [Google Scholar] [CrossRef]
- Daidone, G.; Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Schillaci, D. Synthesis and antiproliferative activity of triazenoindazoles and triazenopyrazoles: A comparative study. Eur. J. Med. Chem. 2004, 39, 219–224. [Google Scholar]
- Zhu, G.-D.; Gong, J.; Gandhi, V.B.; Woods, K.; Luo, Y.; Liu, X.; Guan, R.; Klinghofer, V.; Johnson, E.F.; Stoll, V.S.; et al. Design and synthesis of pyridine-pyrazolopyridine-based inhibitors of protein kinase B/Akt. Bioorg. Med. Chem. 2007, 15, 2441–2452. [Google Scholar]
- Fylaktakidou, C.K.; Hadjipavlou, J.D.; Litinas, E.K.; Nicolaides, N.D. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Jung, J.C.; Watkins, E.B.; Avery, M.A. Synthesis and cyclization reaction of pyrazolin-5-one derivatives. Heterocycles 2005, 65, 77. [Google Scholar]
- Anderson, J.D.; Cottam, H.B.; Larson, S.B.; Nord, L.D.; Revankar, G.R.; Robins, R.K. Synthesis of certain pyrazolo[3,4-d]pyrimidin-3-one nucleosides. J. Heterocycl. Chem. 1990, 27, 439–453. [Google Scholar] [CrossRef]
- Ballell, L.; Field, R.A.; Chung, G.A.C.; Young, R. New thiopyrazolo[3,4-d]pyrimidine derivatives as anti-mycobacterial agents. J. Bioorg. Med. Chem. Lett. 2007, 17, 1736–1740. [Google Scholar] [CrossRef]
- Husain, S.; Shearer, T.W.; Crosson, C.E. Mechanisms linking adenosine A1 receptors and extracellular signal-regulated kinase 1/2 activation in human trabecular meshwork cells. J. Pharm. Exp. Ther. 2007, 320, 258–265. [Google Scholar]
- Pittaluga, A.; Feligioni, M.; Longordo, F.; Arvigo, M.; Raiteri, M. Somatostatin-induced activation and up-regulation of N-Methyl-D-aspartate receptor function: Mediation through calmodulin-dependent protein kinase II, phospholipase C, protein kinase C, and tyrosine kinase in hippocampal noradrenergic nerve endings. J. Pharm. Exp. Ther. 2005, 313, 242–249. [Google Scholar]
- Doolen, S.; Zahniser, N.R. Protein tyrosine kinase inhibitors alter human dopamine transporter activity in Xenopus oocytes. J. Pharm. Exp. Ther. 2001, 296, 931–938. [Google Scholar]
- Halazy, S. Designing heterocyclic selective kinase inhibitors: From concept to new drug candidates. ARKIVOC 2006, vii, 496–508. [Google Scholar]
- Hirst, G.C.; Rafferty, P.; Ritter, K.; Calderwood, D.; Wishart, N.; Arnold, L.D.; Friedman, M.M. U.S. U.S. Patent 663,780, 2002. Chem. Abstr. 2002, 137, 310930. [Google Scholar]
- Vicentini, C.B.; Forlani, G.; Manfrini, M.; Romagnoli, C.; Mares, D. Development of new fungicides against magnaporthe grisea: Synthesis and biological activity of pyrazolo[3,4-d][1,3]thiazine, pyrazolo[1,5-c][1,3,5]thiadiazine, and pyrazolo[3,4-d]pyrimidine derivatives. J. Agric. Food Chem. 2002, 50, 4839–4845. [Google Scholar] [CrossRef]
- Quintela, J.M.; Peinador, C.; Moreira, M.J.; Alfonso, A.; Botana, L.M.; Riguera, R. Pyrazolopyrimidines: Synthesis, effect on histamine release from rat peritoneal mast cells and cytotoxic activity. Eur. J. Med. Chem. 2001, 36, 321–332. [Google Scholar]
- Larsen, S.D.; Connell, M.A.; Cudahy, M.M.; Evans, B.R.; May, P.D.; Meglasson, M.D.; O'Sullivan, T.J.; Schostarez, H.J.; Sih, J.C.; Stevens, F.C.; et al. Synthesis and biological activity of analogues of the antidiabetic/antiobesity agent 3-guanidinopropionic acid: Discovery of a novel aminoguanidinoacetic acid antidiabetic agent. J. Med. Chem. 2001, 44, 1217–1230. [Google Scholar] [CrossRef]
- Bhat, G.A.; Montero, J.L.G.; Panzica, R.P.; Wotring, L.L.; Townsend, L.B. Pyrazolo-pyrimidine nucleosides: Synthesis and biological activity of certain pyrazolo[3,4-d]pyrimidine nucleosides related to adenosine. J. Med. Chem. 1981, 24, 1165–1172. [Google Scholar]
- Trivedi, A.R.; Siddiqui, A.B.; Shah, V.H. Design, synthesis, characterization and antitubercular activity of some 2-heterocycle-substituted phenothiazines. ARKIVOC 2008, ii, 210–217. [Google Scholar]
- Mansour, A.K.; Eid, M.M.; Khalil, N.S.A.M. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- Carraro, F.; Naldini, A.; Pucci, A.; Locatelli, G.A.; Maga, G.; Schenone, S.; Brullo, C.; Fossa, P.; Menozzi, G.; Mosti, L.; et al. Pyrazolo[3,4-d]pyrimidines as potent antiproliferative and proapoptotic agents toward A431 and 8701-BC cells in culture via inhibition of c-Src phosphorylation. J. Med. Chem. 2006, 49, 1549–1561. [Google Scholar] [CrossRef]
- Kumar, R.; Joshi, Y.C. Synthesis, spectral studies and biological activity of 3H-1,5-benzodiazepine derivatives. ARKIVOC 2007, xiii, 142. [Google Scholar]
- Abdallah, M.A.; Riyadh, S.M.; Abbas, I.M.; Gomha, S.M. Synthesis and biological activities of 7-arylazo-7H-pyrazolo[5,1-c] [1,2,4] triazolo-6(5H)-ones and 7-arylhydrazono-7H-[1,2,4]triazolo-[3,4-b][1,3,4] thiadiazines. J. Chin. Chem. Soc. 2005, 52, 987–994. [Google Scholar]
- Abdallah, M.A.; Riyadh, S.M.; Abbas, I.M.; Gomha, S.M. Synthesis and antimicrobial activity of 3,5-disubstituted-1,3,4-thiadiazol-2-yliden by the intramolecular ring transformation of 1,3,4-oxadiazole. Int. J. Pure Appl. Chem. 2006, 1, 265–271. [Google Scholar]
- Gomha, S.M. A Facile one-pot synthesis of 4,5,6,7-tetrahydro-benzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-one. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Hassaneen, H.M.E.; Mohammed, Y.S.; Pagni, R.M. Synthesis, Reactions and Antibacterial activity of 3-acetyl[1,2,4]triazolo[3,4-a]isoquinoline derivatives using Chitosan as heterogeneous catalyst under microwave irradiation. Z. Naturforsch. 2011, 66b, 299–310. [Google Scholar]
- Muccioli, G.G.; Poupaert, J.H.; Wouters, J.; Norberg, B.; Poppitz, W.; Gerhard, K.; Lamberta, D.M. A rapid and efficient microwave-assisted synthesis of hydantoins and thiohydantoins. Tetrahedron 2003, 59, 1301–1307. [Google Scholar] [CrossRef]
- Bedford, G.; Taylor, J.; Webb, G.A. 15N-NMR studies of guanidines. II-the fused-in guanidine unit of some oxoheterocycles: A combined 15N-NMR, 13C-NMR and IR study. Mag. Res. Chem. 1995, 33, 389–394. [Google Scholar] [CrossRef]
- Gaudarshivannanavar, B.C.; Jayadevappa, H.; Mahadevan, K.M. A convenient synthesis of 2(2-benz[b]furo)indoles and benzofuropyrazoles. Indian J. Chem. 2009, 48B, 1419–1423. [Google Scholar]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles. Part II new routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdelhamid, A.O. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–327. [Google Scholar] [CrossRef]
- Grayer, R.J.; Harborne, J.B. A survey of antifungal compounds from higher plants. Phytochemistry 1994, 37, 19–42. [Google Scholar]
- Irob, O.N.; Moo-Young, M.; Anderson, W.A. Antimicrobial activity of annatto extract. Int. J. Pharm. 1996, 34, 87–90. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2–23 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gomha, S.M.; Hassaneen, H.M.E. Synthesis and Antimicrobial Activity of Some New Pyrazoles, Fused Pyrazolo[3,4-d]-pyrimidine and 1,2-Dihydroimidazo-[2,1-c][1,2,4]triazin-6-one Derivatives. Molecules 2011, 16, 6549-6560. https://doi.org/10.3390/molecules16086549
Gomha SM, Hassaneen HME. Synthesis and Antimicrobial Activity of Some New Pyrazoles, Fused Pyrazolo[3,4-d]-pyrimidine and 1,2-Dihydroimidazo-[2,1-c][1,2,4]triazin-6-one Derivatives. Molecules. 2011; 16(8):6549-6560. https://doi.org/10.3390/molecules16086549
Chicago/Turabian StyleGomha, Sobhi Mohamed, and Huwaida M.E. Hassaneen. 2011. "Synthesis and Antimicrobial Activity of Some New Pyrazoles, Fused Pyrazolo[3,4-d]-pyrimidine and 1,2-Dihydroimidazo-[2,1-c][1,2,4]triazin-6-one Derivatives" Molecules 16, no. 8: 6549-6560. https://doi.org/10.3390/molecules16086549
APA StyleGomha, S. M., & Hassaneen, H. M. E. (2011). Synthesis and Antimicrobial Activity of Some New Pyrazoles, Fused Pyrazolo[3,4-d]-pyrimidine and 1,2-Dihydroimidazo-[2,1-c][1,2,4]triazin-6-one Derivatives. Molecules, 16(8), 6549-6560. https://doi.org/10.3390/molecules16086549






